INTRODUCTION
Bioeroding sponges inhabit and corrode calcium carbonate materials and can significantly affect the carbonate balance of many marine habitats (Schönberg, Reference Schönberg, Wisshak and Tapanila2008; Wisshak et al., Reference Wisshak, Schönberg, Form and Freiwald2014). Within the family Clionaidae (Demospongiae, Clionaida) the genus Cliona Grant, 1826 is best studied and comprises mostly shallow-water marine sponges (Rützler, Reference Rützler, Hooper and Van Soest2002). Cliona species occur worldwide (Van Soest et al., Reference Van Soest, Boury-Esnault, Hooper, Rützler, De Voogd, Alvarez de Glasby, Hajdu, Pisera, Manconi, Schönberg, Janussen, Tabachnick, Klautau, Picton, Kelly, Vacelet, Dohrmann, Díaz and Cárdenas2015), in warm waters often representing the dominant endolithic organisms (e.g. MacGeachy, Reference MacGeachy and Taylor1977; Mallela & Perry, Reference Mallela and Perry2007). Their bioerosion activity reworks especially skeletons of molluscs and reef-building corals (Schönberg, Reference Schönberg, Wisshak and Tapanila2008).
Their important ecological role requires a sound understanding of their distributions and abundances, and in that context knowledge about local biodiversities and faunistics is essential (e.g. Carballo et al., Reference Carballo, Sánchez-Moyano and García-Gómez1994). Unfortunately, many bioeroding sponges are challenging to identify (e.g. Rosell, Reference Rosell1994; Barbieri et al., Reference Barbieri, Bavestrello and Sarà1995; Bavestrello et al., Reference Bavestrello, Calcinai, Cerrano, Pansini and Sarà1996a; Schönberg & Beuck, Reference Schönberg and Beuck2007). The most important features for differentiating Cliona species are growth forms (alpha: endolithic-papillate, beta: endolithic-encrusting, gamma: free-living massive; Schönberg, Reference Schönberg, Wisshak and Tapanila2008), papillar characters, the size and morphology of spicules and excavation patterns (e.g. Pang, Reference Pang1973; Rosell, Reference Rosell1994; Rosell & Uriz, Reference Rosell and Uriz1997; Schönberg, Reference Schönberg2001, Reference Schönberg, Wisshak and Tapanila2008; Rützler, Reference Rützler, Hooper and Van Soest2002), but there are variable characters and similarities between species. For example, sponges are well-known for their high degree of phenotypic plasticity, creating morphological dissimilarities in the same and similarities between different species, and clionaid sponges are no exception (e.g. Rützler, Reference Rützler1974; Wiedenmayer, Reference Wiedenmayer1977; Hill, Reference Hill1999; Bell et al., Reference Bell, Barnes and Turner2002; Hill & Hill, Reference Hill and Hill2002). Moreover, spicule dimensions overlap between species belonging to Clionaidae, and the size and the shape of their tylostyles and abundance of microscleres can occasionally vary with individual specimens, which apparently can be caused by environmental conditions, patchy distribution in the tissues, developmental stages, cryptic speciation, misidentification or contamination (e.g. Rosell & Uriz, Reference Rosell and Uriz2002; Xavier et al., Reference Xavier, Rachello-Dolmen, Parra-Velandia, Schönberg, Breeuwer and Van Soest2010; De Paula et al., Reference De Paula, Zilberberg, Hajdu and Lôbo-Hajdu2012). Identification is especially difficult in species in which microscleres are rare or absent (Rützler, Reference Rützler, Hooper and Van Soest2002; Schönberg et al., Reference Schönberg, Grass and Heiermann2006). At genus or family level some distinguishing information can be obtained from erosion scars on the substratum (Calcinai et al., Reference Calcinai, Arillo, Cerrano and Bavestrello2003, Reference Calcinai, Bavestrello and Cerrano2004, but see Schönberg, Reference Schönberg, Wisshak and Tapanila2008), but size and shape of galleries and sponge chips resulting from the boring activity may be affected by the nature of substratum and the state of advance into the substrate (Schönberg, Reference Schönberg, Wisshak and Tapanila2008), and their usefulness in taxonomy was occasionally questioned (e.g. Schönberg, Reference Schönberg2000a; Fromont et al., Reference Fromont, Craig, Rawlinson and Alder2005; Calcinai et al., Reference Calcinai, Azzini, Bavestrello, Gaggero, Cerrano, Custódio, Hajdu, Lôbo-Hajdu and Muricy2007). Taxonomic studies on bioeroding sponges are further hampered by poor species descriptions, scarcity of comparative material in scientific collections or their inaccessibility, and difficulties with usual methods of collection (e.g. Schönberg & Beuck, Reference Schönberg and Beuck2007).
As a result the systematics of the Clionaidae is partly confused, and their biodiversity is underestimated at many locations (e.g. Xavier et al., Reference Xavier, Rachello-Dolmen, Parra-Velandia, Schönberg, Breeuwer and Van Soest2010; De Paula et al., Reference De Paula, Zilberberg, Hajdu and Lôbo-Hajdu2012). Currently there are 77 accepted species in the genus Cliona (Van Soest et al., Reference Van Soest, Boury-Esnault, Hooper, Rützler, De Voogd, Alvarez de Glasby, Hajdu, Pisera, Manconi, Schönberg, Janussen, Tabachnick, Klautau, Picton, Kelly, Vacelet, Dohrmann, Díaz and Cárdenas2015). Cliona species are fairly well known from the Mediterranean Sea and neighbouring areas of the Eastern Atlantic (14 species: Von Lendenfeld, Reference Von Lendenfeld1897; Rützler & Bromley, Reference Rützler and Bromley1981; Topsent, Reference Topsent1932; Van Soest, Reference Van Soest1993; Carballo et al., Reference Carballo, Sánchez-Moyano and García-Gómez1994, Reference Carballo, Naranjo and García-Gómez1997; Corriero & Scalera-Liaci, Reference Corriero and Scalera-Liaci1997; Rosell & Uriz, Reference Rosell and Uriz2002; Corriero & Nonnis Marzano, Reference Corriero and Nonnis Marzano2006; Calcinai et al., Reference Calcinai, Bavestrello, Cuttone and Cerrano2011), Australia (15 species: Carter, Reference Carter1886; Topsent, Reference Topsent1888, Reference Topsent1932; Hooper & Wiedenmayer, Reference Hooper, Wiedenmayer and Wells1994; Schönberg, Reference Schönberg2000a; Fromont et al., Reference Fromont, Craig, Rawlinson and Alder2005; Schönberg et al., Reference Schönberg, Grass and Heiermann2006; Van Soest et al., Reference Van Soest, Boury-Esnault, Hooper, Rützler, De Voogd, Alvarez de Glasby, Hajdu, Pisera, Manconi, Schönberg, Janussen, Tabachnick, Klautau, Picton, Kelly, Vacelet, Dohrmann, Díaz and Cárdenas2015), and best-studied in the Tropical Western Atlantic, especially in the Caribbean region (24 accepted species: De Laubenfels, Reference De Laubenfels1950; Pang, Reference Pang1973; Rützler, Reference Rützler1974; Duchassaing & Michelotti, Reference Duchassaing and Michelotti1864; Sollas, Reference Sollas1878; Carter, Reference Carter1882; Topsent, Reference Topsent1888; Leidy, Reference Leidy1889; Van Soest, Reference Van Soest1993; Holmes, Reference Holmes2000; Zea & Weil, Reference Zea and Weil2003; Miloslavich et al., Reference Miloslavich, Díaz, Klein, Alvarado, Díaz, Gobin, Escobar-Briones, Cruz-Motta, Weil, Cortés, Bastidas, Robertson, Zapata, Martín, Castill, Kazandjian and Ortiz2010; Friday et al., Reference Friday, Poppel and Hill2013; Van Soest et al., Reference Van Soest, Boury-Esnault, Hooper, Rützler, De Voogd, Alvarez de Glasby, Hajdu, Pisera, Manconi, Schönberg, Janussen, Tabachnick, Klautau, Picton, Kelly, Vacelet, Dohrmann, Díaz and Cárdenas2015), while subareas of the above and other regions are under-represented. Nine Cliona spp. were reported for Brazil and neighbouring waters from early expeditions such as the ‘HMS Alert’ (Hechtel, Reference Hechtel, Harrison and Cowden1976; Ridley, Reference Ridley and Günther1881) and the ‘Calypso’ (Boury-Esnault, Reference Boury-Esnault1973) and contemporarily, by Brazilian researchers (e.g. Muricy & Hajdu, Reference Muricy and Hajdu2006; Muricy et al., Reference Muricy, Esteves, Moraes, Santos, Silva, Almeida, Klautau and Lanna2008).
Species united in the Cliona viridis (Schmidt, 1862) species complex are difficult to distinguish from each other and have historically caused much confusion (e.g. Bavestrello et al., Reference Bavestrello, Calcinai, Cerrano, Pansini and Sarà1996a, Reference Bavestrello, Calcinai and Saràb; Schönberg, Reference Schönberg, Moosa, Soegiarto, Romimohtarto, Nontji, Soekarno and Suharsono2000b; Zea & Weil, Reference Zea and Weil2003). Schönberg (Reference Schönberg, Moosa, Soegiarto, Romimohtarto, Nontji, Soekarno and Suharsono2002) defined C. viridis species complex species by having tylostyles and mostly delicate spirasters, and being in symbiosis with dinoflagellate zooxanthellae that cause a brownish colour in many species. Members of this species complex that can develop to beta or gamma growth forms have been recognized as very good space competitors that can aggressively invade and kill live corals (e.g. Schönberg & Wilkinson, Reference Schönberg and Wilkinson2001; López-Victoria et al., Reference López-Victoria, Zea and Weil2006; Márquez & Zea, Reference Márquez and Zea2012), and that they will acutely react to ocean acidification with accelerated bioerosion rates (Wisshak et al., Reference Wisshak, Schönberg, Form and Freiwald2012, Reference Wisshak, Schönberg, Form and Freiwald2013, Reference Wisshak, Schönberg, Form and Freiwald2014; Fang et al., Reference Fang, Athayde, Schönberg, Kline, Hoegh-Guldberg and Dove2013; Stubler et al., Reference Stubler, Furman and Peterson2014). They can increase in abundance after disturbance events or with decreasing water quality (e.g. Schönberg & Ortiz, Reference Schönberg and Ortiz2009), and because the most destructive C. viridis complex species share a global distribution with reef-forming corals they have been recommended to be monitored at regular intervals (Schönberg, Reference Schönberg2015). This highlights the importance of faunistic and eco-physiological knowledge about this group.
Recent expeditions carried out to Maricás Archipelago, central coast of Rio de Janeiro, Brazil, have yielded specimens of sponges of C. viridis complex very similar to those described for its type locality in the Mediterranean Sea. This work identifies and describes the sampled material using morphological and molecular approaches and will discuss findings in context of present knowledge of the C. viridis species complex.
MATERIALS AND METHODS
Study area
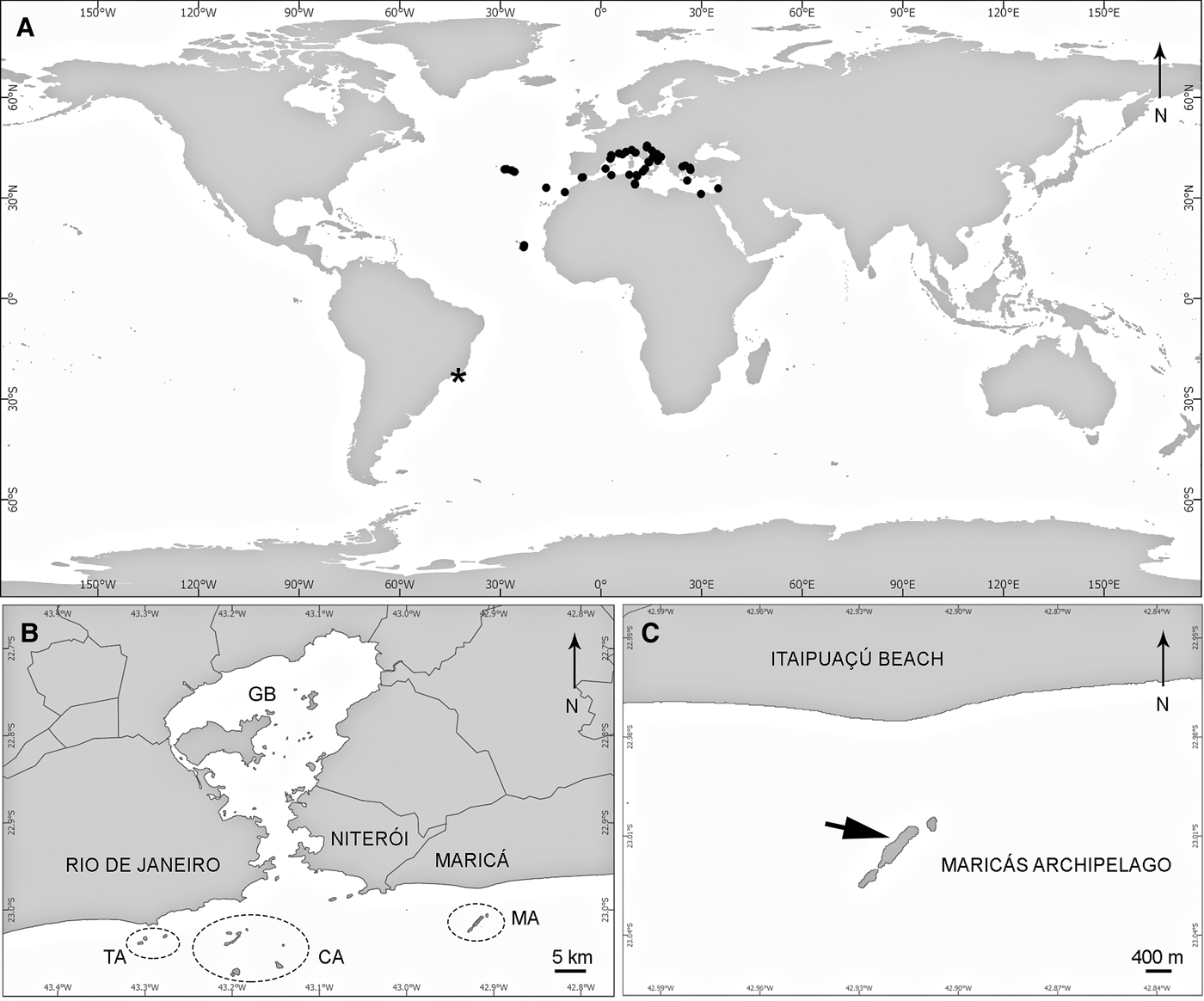
Maricás Archipelago is located 30 km east of Rio de Janeiro city and 3.6 km off mainland (23°00′S 42°55′E), on the central coast of Rio de Janeiro state, south-east Brazil (Figure 1). The archipelago is formed by Maricá Island (the largest island, 1.6 km long by 300 m wide) and two smaller rocky islets aligned in a SW–NE direction, with the mean depth in this area being 17 m (Monteiro-Neto et al., Reference Monteiro-Neto, Bertoncini, Chaves, Noguchi, Mendonça-Neto and Rangel2013). The rocky bottom is composed of granitic boulders of varying size (Monteiro-Neto et al., Reference Monteiro-Neto, Bertoncini, Chaves, Noguchi, Mendonça-Neto and Rangel2013). A preliminary survey at Maricás Archipelago conducted by the first and last authors in 2010 recorded 15 sponge species (E. Esteves, personal observation). Water quality is poor in this area due to the effect of untreated domestic and industry sewage from Guanabara Bay (Kjerfv et al., Reference Kjerfv, Ribeiro, Dias, Filippo and Quaresma1997) and runoff from small estuaries nearby. Maricás Archipelago is also subject to introduction of exotic species due to intense traffic related to oil platforms next to the Guanabara Bay entrance (Ignacio et al., Reference Ignacio, Julio, Junqueira and Ferreira-Silva2010). The area experiences common upwelling events, occasionally reducing temperatures to <20°C (Campos et al., Reference Campos, Velhote and da Silveira2000).

Fig. 1. Geographic distribution of C. viridis (Schmidt, 1862): (A) map with the confirmed distribution of C. viridis in the world, according to Van Soest et al. (Reference Van Soest, Boury-Esnault, Hooper, Rützler, De Voogd, Alvarez de Glasby, Hajdu, Pisera, Manconi, Schönberg, Janussen, Tabachnick, Klautau, Picton, Kelly, Vacelet, Dohrmann, Díaz and Cárdenas2015) (black circles) and the new record of this species from Brazil (asterix); (B) map of the central coast of Rio de Janeiro state and continental islands in its surroundings; (C) precise collection locality of C. viridis in Maricás Archipelago, SE Brazil (arrow). CA, Cagarras Archipelago; GB, Guanabara Bay; MA, Maricás Archipelago; TA, Tijucas Archipelago.
Sampling and morphological analysis
Sponge specimens were collected on Maricás Archipelago on 26 September 2010. Specimens were photographed in situ prior to collections with an 8MP digital camera (Sealife DC800, Moorestown). Oscula and ostial papillae measurements were estimated from digital underwater photos of specimens of the new Brazilian material. Fragments of three specimens were collected by knife on scuba in a depth of 10–12 m. Samples were fixed in 80% ethanol and deposited in the Porifera Collection of Museu Nacional, Universidade Federal do Rio de Janeiro (MNRJ) after respective analyses. Comparative material was obtained for morphological analysis. A schizolectotype of Cliona varians (Duchassaing & Michelotti, Reference Duchassaing and Michelotti1864) from the British Museum of Natural History, London (BMNH) was examined, as well as a fragment of an unpublished specimen of C. viridis collected in the Mediterranean Sea, deposited in the MNRJ collection. Procedures for preparing slides of spicules followed Rützler (Reference Rützler, Stoddart and Johannes1978). Cross sections were obtained with a diamond saw from epoxy resin-embedded fragments of specimens and substrate. Thirty spicules for each specimen were randomly selected and measured at magnification 40–400× under an Olympus BX 50 light microscope equipped with an eyepiece micrometer. Bioerosion traces and spicules were studied by scanning electron microscopy (SEM microscope JEOL JSM6390LV). Spicule and bioerosion sponge scars dimensions are presented in micrometres as minimum–mean–maximum (±standard deviation).
Molecular analysis
DNA extractions were conducted as described by De Paula et al. (Reference De Paula, Zilberberg, Hajdu and Lôbo-Hajdu2012). PCR amplifications of the D1–D2 region of the 28S rRNA gene (~800 bp) were carried out following protocol employed by Morrow et al. (Reference Morrow, Picton, Erpenbeck, Boury-Esnault, Maggs and Allcock2012), using the primers Por28S-15F and Por28S-878R. PCR products were sequenced by third party service (Macrogen Inc., South Korea) in both directions. The sequences were aligned with MAFFT v6.935 (Katoh et al., Reference Katoh, Kuma, Toh and Miyata2005) using the E-INS-i algorithm for local alignment searches with default parameters, and positions containing gaps or missing data were excluded from the phylogenetic analyses. The phylogenetic reconstruction was performed through the Maximum Likelihood (ML) method, implementing the CAT-GTR model. The analysis was conducted with RAxML v7.0.4 (Stamatakis, Reference Stamatakis2006), and the best-scoring ML tree was inferred after rapid Bootstrap analysis with 1000 replicates. Specimens from Cliona aprica Pang, Reference Pang1973 (MNRJ 15685; the Caribbean) and C. varians (MNRJ 10724; North-east Brazil) were included for comparison, in addition to the following sequences obtained from GenBank (accession numbers in parentheses): C. viridis (AF062606 and AM293637) and its junior synonym C. nigricans (AM293635 and AM293636); C. jullieni Topsent, 1891 (AM293625, AM293626 and AM293627); C. schmidti (Ridley, Reference Ridley and Günther1881) (AM293632 and AM293633); and Spirastrella hartmani Boury-Esnault et al., 1999 (KC869504), used as outgroup. All sequences generated in this work were deposited in GenBank under accession numbers KP400590–400594.
RESULTS
SYSTEMATICS
Class DEMOSPONGIAE Sollas, 1885
Subclass HETEROSCLEROMORPHA Cárdenas et al., Reference Cárdenas, Pérez and Boury-Esnault2012
Order CLIONAIDA Morrow & Cárdenas, Reference Morrow and Cárdenas2015
Family CLIONAIDAE D'Orbigny, 1851
Genus Cliona Grant, 1826
DIAGNOSIS
Clionaidae in alpha, beta or gamma growth forms. Megascleres tylostyles or subtylostyles, and raphides in some species as accessory spicules. Microscleres spirasters and derivatives, as straight, bent, kinked, helical, spiny or rarely smooth rhabds, including amphiastrose forms supposedly derived from true spirasters. Microscleres occasionally rare or entirely absent (Rützler, Reference Rützler, Hooper and Van Soest2002). Type species: Cliona celata Grant, 1826.
Cliona viridis (Schmidt, 1862)
(Figures 1–4; Table 1)

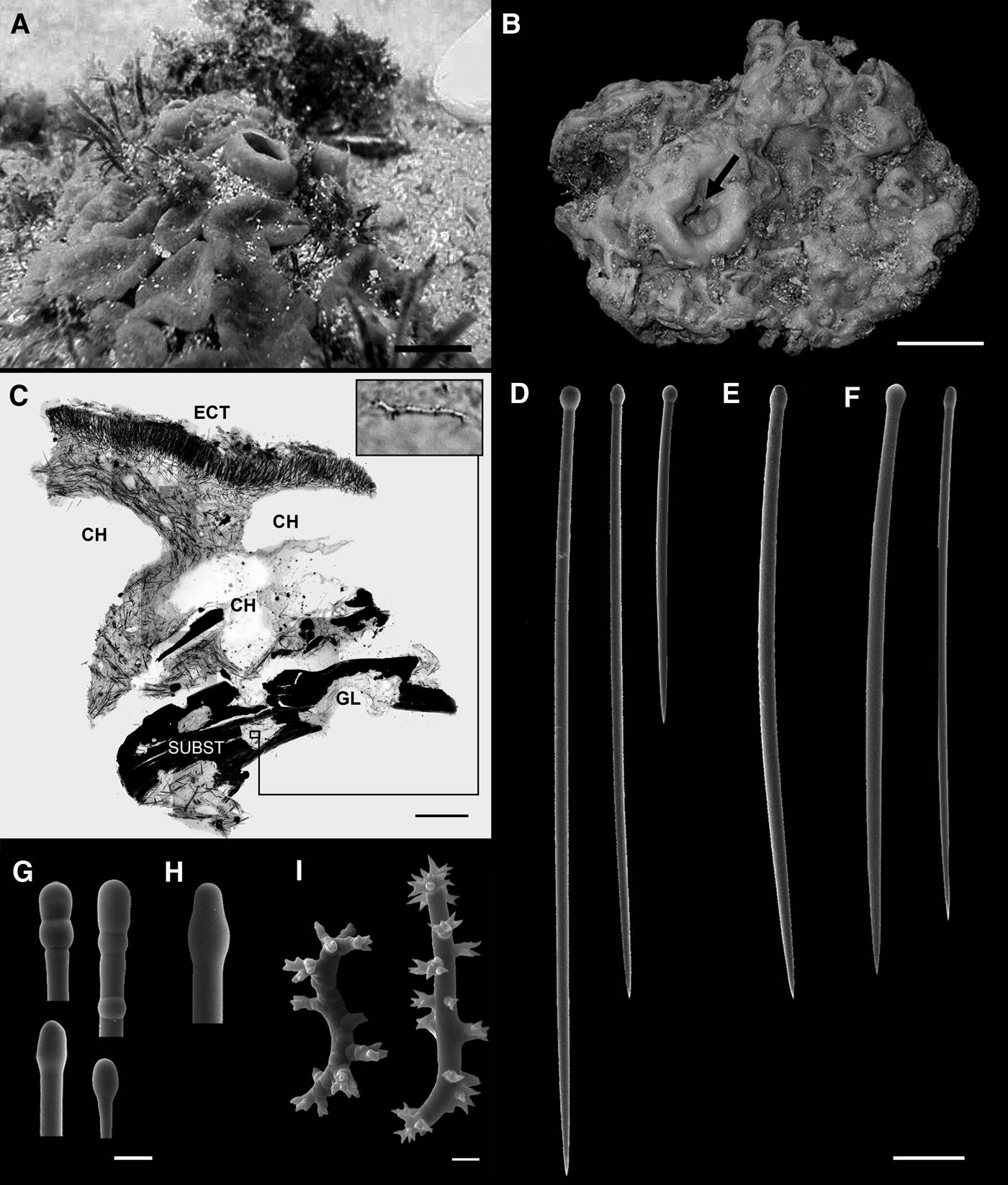
Fig. 2. Cliona viridis (Schmidt, 1862) from Maricás Archipelago, south-eastern Brazil: (A) photo in situ; (B) fixed specimen; note the markedly undulated surface due to the contraction of conspicuous irregular ostial papillae and an oscule (arrow); (C) cross-section of skeleton obtained with a diamond saw with a spiraster 28 µm long in detail; (D–E) tylostyles of a specimen from Maricás Archipelago, south-eastern Brazil; (F) Tylostyles of a specimen from the Mediterranean Sea; (G–H) base of tylostyles of specimens from Maricás Archipelago in detail; (I) Spirasters from a specimen in beta growth form from Maricás Archipelago. Scale bars: A, 2 cm; B, 3 cm; C, 1 mm; D–F, 50 µm; G–H, 10 µm; I, 2 µm. (A, E, H) MNRJ 14019; (B, D, G) MNRJ 14016; (C, I) MNRJ 14017; (F) MNRJ 13458. CH, channels; ECT, ectosome; GL, gallery; SUBST, substrate.

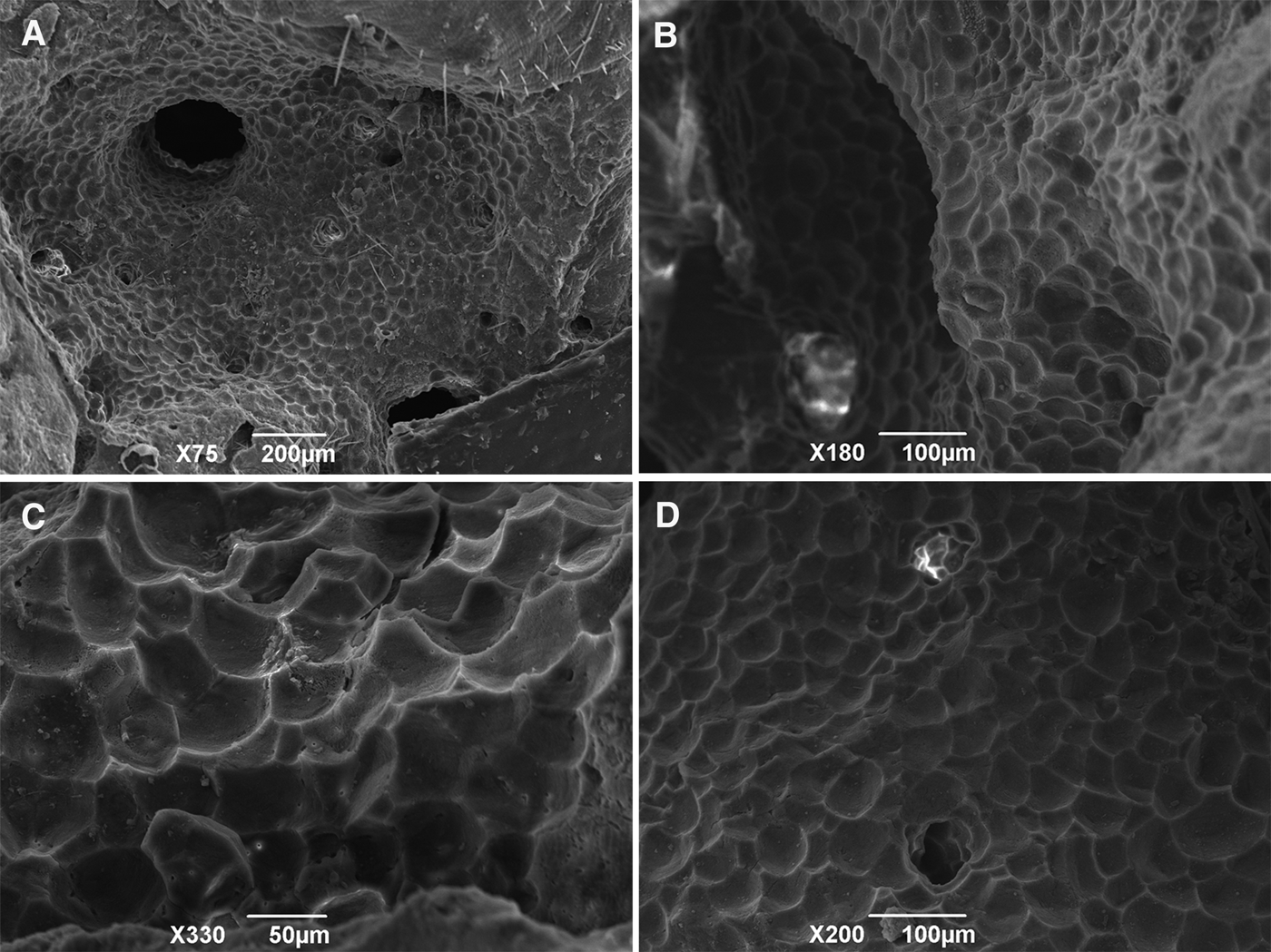
Fig. 3. Scanning electron micrographs of sponge-generated bioerosion chambers found in substrate inhabited by C. viridis (Schmidt, 1862) from Maricás Archipelago, south-eastern Brazil: (A–B, D) Chambers and apertures; (C) Pentagonal and hexagonal pits, the so-called ‘sponge scars’, on the substratum walls resulting from the excavation activity. All micrographs were taken from the substratum excavated by the specimen in beta growth form (MNRJ 14017).

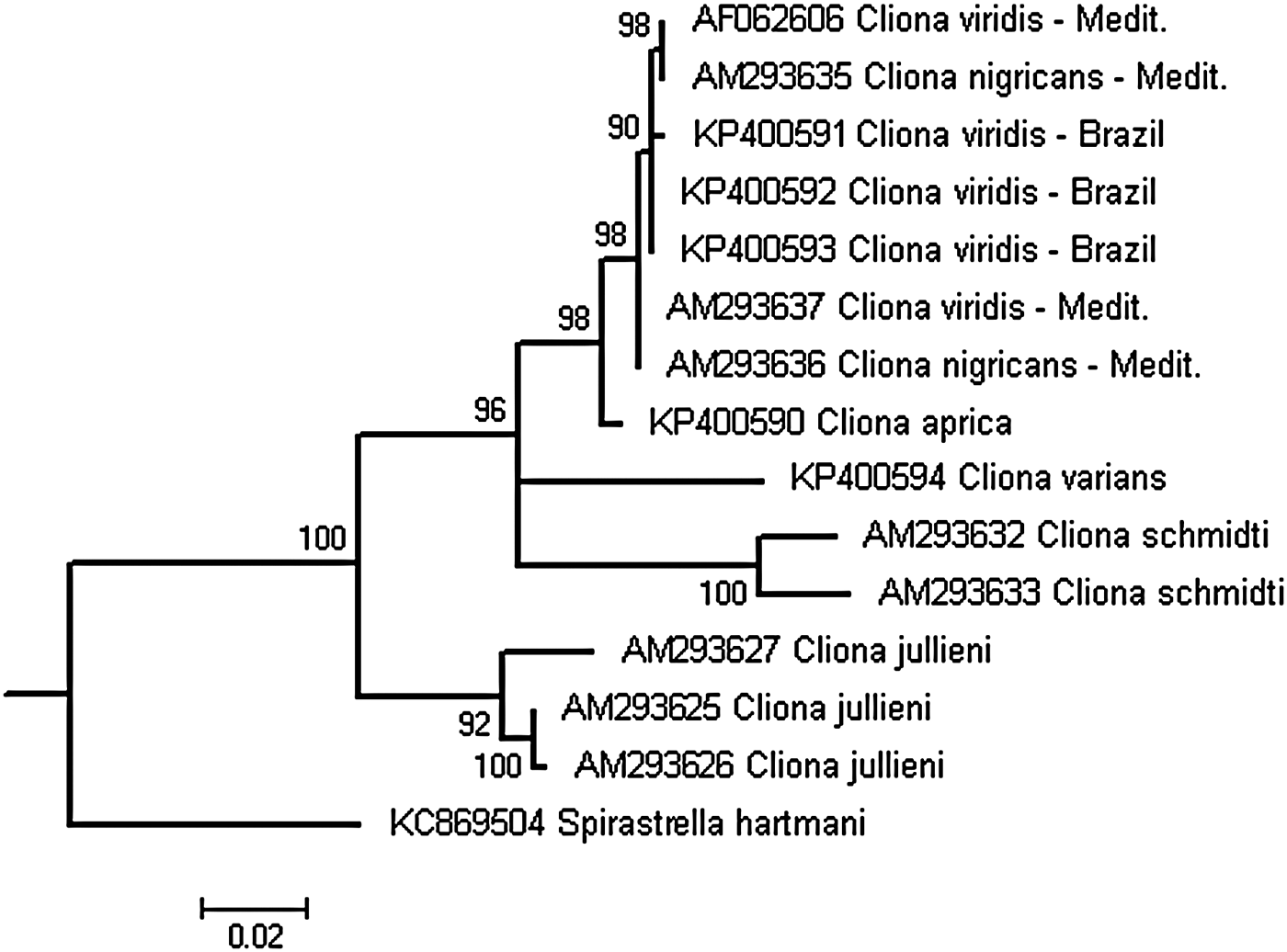
Fig. 4. Cladogram reconstructed from partial 28S sequences of species belonging to the C. viridis complex. Tree topology recovered through the Maximum Likelihood method, implementing the CAT-GTR model. Numbers above branches are the support values obtained by 1000 rapid bootstrap replicates. Sequences are listed by their accession numbers, followed by taxon name and locality. See text for details.
Table 1. Dimensions of spicules of C. viridis in micrometres from Maricás Archipelago, Rio de Janeiro State, south-eastern Brazil. Values are presented as minimum–mean–maximum (±standard deviation). N = 30 per specimen.

* Measurements of microscleres taken from cross sections.
SYNONYMY
Vioa viridis Schmidt, 1862, p. 77
Papillina nigricans Schmidt, 1862, p. 69
Papillina suberea Schmidt, 1862, p. 69
Osculina polystomella Schmidt, 1868, p. 3
Cliona copiosa Sarà, 1959, p. 8
Cliona tremitensis Sarà, 1961, p. 38
For additional synonyms see: Van Soest et al. (Reference Van Soest, Boury-Esnault, Hooper, Rützler, De Voogd, Alvarez de Glasby, Hajdu, Pisera, Manconi, Schönberg, Janussen, Tabachnick, Klautau, Picton, Kelly, Vacelet, Dohrmann, Díaz and Cárdenas2015).
diagnosis (based on Carballo et al., Reference Carballo, Sánchez-Moyano and García-Gómez1994; Rosell & Uriz, Reference Rosell and Uriz2002; Schönberg, Reference Schönberg, Moosa, Soegiarto, Romimohtarto, Nontji, Soekarno and Suharsono2000b).
Cliona species occurring in all three growth forms: alpha, beta and gamma. Moss green, brown, blackish or whitish colour in life. Specimens in alpha or beta growth form with tiny papillae, up to 4 mm in diameter, circular to oval in shape. Specimens in gamma growth form exhibiting circular, oval or irregular ostial papillae and fleshy oscules over 2 cm wide. Spicules: megasclere tylostyles up to 400 µm long in alpha and beta growth forms, up to 600 µm long in gamma form sponges. Two categories of spirasters in all growth forms: spiraster I, with straight shaft 13–30 µm long and relatively large spines concentrated terminally, resembling amphiasters; and spiraster II, with 2–5 bends, 10–50 µm long. Type locality: Zadar Channel, Adriatic Sea (Schmidt, 1862).
MATERIAL EXAMINED
MNRJ 14016, 14017 and 14019, Maricás Archipelago, next to the ‘Moreno’ shipwreck (23°00′41″S 042°55′11″W), Maricá, Rio de Janeiro, south-eastern coast of Brazil, 10–12 m depth, E. L. Esteves coll., 26 September 2010.
COMPARATIVE MATERIAL FOR MORPHOLOGICAL STUDIES
MNRJ 13458 (unpublished specimen of C. viridis), Mediterranean Sea, S. Ribeiro and A. Villamor colls. Date of collection and precise collection locality not available.
BMNH 1928.11.12.49 (schizolectotype of C. varians (Duchassaing & Michelotti, Reference Duchassaing and Michelotti1864)), St. Thomas, US Virgin Islands. Date of collection and collector not available.
DESCRIPTION
External morphology
Thickly encrusting (beta growth form) to massive (gamma growth form), forming patches of over 20 cm in diameter and up to 5 cm thickness. Ostial papillae irregular to oval, numerous, 2 cm wide on average in vivo (Figure 2A), barely distinguishable after fixation in ethanol (Figure 2B). Oscular papillae more pronounced than ostial ones, slightly elevated, 3 cm in diameter, oval in shape (Figure 2A). Colour in life moss green externally and internally, changing to brownish green after fixation in ethanol. Surface rough by large amounts of encrusting and embedded sand debris on ectosome, markedly undulated after fixation in ethanol due to papillar contraction. Consistency firm, only slightly compressible.
Internal anatomy
Ectosomal skeleton composed of tylostyle palisade (Figure 2C). Choanosome cavernous with wide channels and abundant coarse calcareous debris loosely incorporated in inner parts and close to ectosome. Choanosomal skeleton formed by subtylostyles disorganized in inner parts and arranged in bundles close to ectosome (Figure 2C). Spirasters, when present, dispersed in the choanosome (Figure 2C).
Spicules
Megascleres: No apparent size classes, but tylostyles to subtylostyles size-variable, straight or slightly curved, with rounded, elongated or occasionally subterminal tyle, and sharply pointed (Table 1; Figure 2D–E, G–H). Microscleres: spirasters observed in two specimens in beta and gamma growth forms, with 1–5 twists, the smaller with stout and almost straight axis and with scarce spines, the larger with up to five twists and slender axis, with abundant and bifurcated spines (Table 1; Figure 2I).
Excavation pattern
Bioerosion sponge scars on calcareous substrate in erosion chambers made in encrusting coralline algae convex, pentagonal or hexagonal depressions (Figure 3A–D) measuring 42.0–55.0–65.0 (±1.8) μm in width (N = 30). Chambers connected by apertures ~300 µm wide.
Ecology
Cliona viridis was one of the most common sponge species in the north-western portion of the Maricás Archipelago during a visit in September 2010 and could not be relocated at the same locality in February 2013 and 2015 (E. Esteves, personal observation). Putative specimens of C. viridis were observed and collected in July 2015 at Maricás and Cagarras Archipelago (Figure 1B), but their identification needs to be confirmed (F. Moraes, personal communication). Cliona viridis is a photosymbiotic species, covering the uppermost portion of rocks, and was found excavating crusts of calcareous algae. Cliona viridis was observed frequently associated with epizoic green macroalgae and was usually covered by coarse sand (Figure 2A).
GEOGRAPHIC AND BATHYMETRIC DISTRIBUTION
Cliona viridis is distributed in the Mediterranean and Eastern Atlantic, occurring from zero to 365 m depth (Carballo et al., Reference Carballo, Sánchez-Moyano and García-Gómez1994; Rosell & Uriz, Reference Rosell and Uriz2002; Van Soest et al., Reference Van Soest, Boury-Esnault, Hooper, Rützler, De Voogd, Alvarez de Glasby, Hajdu, Pisera, Manconi, Schönberg, Janussen, Tabachnick, Klautau, Picton, Kelly, Vacelet, Dohrmann, Díaz and Cárdenas2015), and in south-eastern Brazil in 10–12 m depth (present study; Figure 1).
MOLECULAR ANALYSIS
ML phylogenetic reconstruction using 28S sequences recovered the Brazilian specimens together with Mediterranean C. viridis sequences in a highly supported monophyletic clade (Figure 4). The phylogenetic relationship among sequences within this clade became apparent without any geographic structure. The C. viridis clade was clustered closely to C. aprica, followed by C. varians and C. schmidti in a basal polytomy. Cliona jullieni formed the most basal clade among the sequences of the C. viridis complex analysed.
REMARKS AND DISCUSSION
The Brazilian specimens examined in the present study were conspecific with Cliona viridis and thus recognized as belonging to the C. viridis species complex, as indicated by the presence of a series of characters, i.e. the brownish green colour, gamma or beta growth form, typical subtylostyles and slender spirasters, and the presence of photosymbionts (Carballo et al., Reference Carballo, Sánchez-Moyano and García-Gómez1994; Rosell & Uriz, Reference Rosell and Uriz2002; Schönberg, Reference Schönberg, Moosa, Soegiarto, Romimohtarto, Nontji, Soekarno and Suharsono2000b). The complex comprises an unknown number of species mostly distributed in warm waters and largely follows the distribution of coral reefs (Van Soest et al., Reference Van Soest, Boury-Esnault, Hooper, Rützler, De Voogd, Alvarez de Glasby, Hajdu, Pisera, Manconi, Schönberg, Janussen, Tabachnick, Klautau, Picton, Kelly, Vacelet, Dohrmann, Díaz and Cárdenas2015). Five or six species have previously been assigned to the C. viridis complex from the Tropical Western Atlantic: C. aprica, C. caribbaea Carter, Reference Carter1882, C. tenuis Zea & Weil, Reference Zea and Weil2003, C. tumula Friday et al., Reference Friday, Poppel and Hill2013 and C. varians (Duchassaing & Michelotti, Reference Duchassaing and Michelotti1864) (Zea & Weil, Reference Zea and Weil2003; Escobar et al., Reference Escobar, Zea and Sánchez2012; Friday et al., Reference Friday, Poppel and Hill2013). Cliona varians is well known from the north-east and south-east coast of Brazil (Muricy et al., Reference Muricy, Esteves, Moraes, Santos, Silva, Almeida, Klautau and Lanna2008; Hajdu et al., Reference Hajdu, Peixinho and Fernandez2011). However, this list is expected to be incomplete, as some massive species should be part of it (presently Spheciospongia spp.), and in addition less typical species were not formally included in the complex, e.g. C. schmidti and C. jullieni from the Mediterranean and Indo-Pacific region (see Barucca et al., Reference Barucca, Azzini, Bavestrello, Biscotti, Calcinai, Canapa, Cerrano and Olmo2007). These two species were included in our phylogenetic analysis, because they possess similar skeletal characters and molecular properties, and at least C. jullieni has photosymbionts (e.g. Barucca et al., Reference Barucca, Azzini, Bavestrello, Biscotti, Calcinai, Canapa, Cerrano and Olmo2007; Schönberg, unpublished data).
We excluded most of the known brown-coloured, symbiotic Indo-Pacific species typical for the C. viridis species complex from the present comparison as they are spatially removed from our sample area and largely differ in morphology from our samples (e.g. C. orientalis Thiele, 1900, C. albimarginata Calcinai et al., 2005, C. caesia (Schönberg, Reference Schönberg2000a)). We considered it most likely that our material derived from the Western Atlantic and might have originated from the larger Caribbean Region. However, the Caribbean species C. caribbaea and C. tenuis can attain only alpha or beta growth forms (Rützler, Reference Rützler1974; Zea & Weil, Reference Zea and Weil2003), and C. aprica only occurs in alpha form (Zea & Weil, Reference Zea and Weil2003), while our material was sampled as beta and gamma sponges. Additionally, these three species have tiny (1–4 mm), circular or oval oscules and shorter tylostyles, up to 400 µm (Schönberg, Reference Schönberg, Moosa, Soegiarto, Romimohtarto, Nontji, Soekarno and Suharsono2002; Zea & Weil, Reference Zea and Weil2003), whereas our specimens have larger papillae and longer tylostyles (Table 1). According to its original description, C. paucispina Rützler, Reference Rützler1974 from Bermuda also belongs into the C. viridis species complex and was thus considered. It develops small colonies in beta form and has zooxanthellae, but has tylostyles of a mean length of 340 µm and spirasters with reduced spination and thus differs from our material (Rützler, Reference Rützler1974). Cliona varians can develop a similar habit compared with our specimens, forming thick crusts to gamma form specimens (Hill, Reference Hill1999). Schönberg (Reference Schönberg, Moosa, Soegiarto, Romimohtarto, Nontji, Soekarno and Suharsono2000b) included this species in the C. viridis complex. Nevertheless, it was clearly different from our material for possessing typically C-shaped spirasters (anthosigmas; confirmed after examination of a schizolectotype of this species in the present study). Indeed, C. varians never develops ostial papillae rising above the surface (e.g. Muricy et al., Reference Muricy, Esteves, Moraes, Santos, Silva, Almeida, Klautau and Lanna2008; Hajdu et al., Reference Hajdu, Peixinho and Fernandez2011).
The next best connectivity was assumed to Mediterranean and Central East Atlantic species, which yielded a good match. The specimens described in the present study are very similar to those belonging to C. viridis in gamma or beta growth from the Mediterranean by their large and irregular papillae over 2 cm in diameter (Carballo et al., Reference Carballo, Sánchez-Moyano and García-Gómez1994, Figure 8B; Rosell & Uriz, Reference Rosell and Uriz2002, Figure 17D–E), moss green to brownish colour in life and long tylostyles up to 500 µm long, and slender spirasters with up to five twists and 34 µm long (Table 1). Spirasters were relatively common in the specimen MNRJ 14017 in beta growth form, and were apparently absent in one specimen in gamma growth form (MNRJ 14019). However, spirasters were probably overlooked in the latter specimen, since they are usually very scarce in specimens of this species in gamma growth form (Carballo et al., Reference Carballo, Sánchez-Moyano and García-Gómez1994). An additional specimen in gamma form from the Mediterranean Sea examined in the present study for comparison (MNRJ 13458) exhibited the same convoluted surface due to the contraction of conspicuous papillae, a pale brown colour after fixation, and similarly, did not show spirasters. Hence, we assume that the Brazilian C. viridis specimens in beta and gamma form represent different stages of growth or ecophenotypes of the same species. Our morphological identification was confirmed by molecular analysis in this study (Figure 4).
The size and shape of erosion chambers and pits or scars caused by the Brazilian specimens were very similar to those described for Mediterranean specimens of this species (Rosell & Uriz, Reference Rosell and Uriz2002). Nevertheless, they were also similar to the traces created by other unrelated excavating species, i.e. Siphonodictyon labyrinthicum (Hancock, 1849) and Spiroxya levispira (Topsent, 1898) (see Rosell & Uriz, Reference Rosell and Uriz2002) and hence were not useful in species recognition in the present study. Chamber morphology can vary and is not usually distinctive for a single sponge species (e.g. Bromley & D'Alessandro, Reference Bromley and D'Alessandro1984, Reference Bromley and D'Alessandro1990). Notwithstanding, the microanatomy of the wall pitting in galleries created by Cliona spp. was described for only a few species and should be better investigated (e.g. Bromley, Reference Bromley1978; Calcinai et al., Reference Calcinai, Arillo, Cerrano and Bavestrello2003, Reference Calcinai, Bavestrello and Cerrano2004; Schönberg & Shields, Reference Schönberg, Shields, Wisshak and Tapanila2008).
Overall, the Brazilian specimens are morphologically very similar to beta and gamma forms of the Mediterranean C. viridis complex. Indeed, our specimens were phylogenetically closer to the Mediterranean members of this species complex than to the Caribbean C. aprica and Brazilian C. varians. Furthermore, we showed that in our selection of samples C. jullieni and C. schmidti were only distantly related to the clade formed by C. aprica and C. viridis. Following White's (Reference White1977) proposal on the species complex concept – ‘A species complex consists of sibling species which, by definition, are species with obscure morphological differences. What, then, is the possible role of morphology in understanding species complexes? It is to find out if the seemingly undifferentiated members of a supposed complex are, in reality, a group of species differentiated sufficiently well for some or all of them to be identified morphologically’ – we have tentatively identified our samples as C. viridis. Nevertheless, only a detailed revision of the C. viridis complex at least in its type-locality (the Mediterranean) and connective areas (i.e. Eastern Atlantic and Tropical Western Atlantic) would possibly allow a better explanation for the morphological affinities between the Brazilian and Mediterranean populations of this species and the taxonomic implications of our findings for unravelling this highly productive, reef shaper complex of species.
This is not the first time an eastern Atlantic/Mediterranean lineage of Cliona was found in south-eastern Brazil, since De Paula et al. (Reference De Paula, Zilberberg, Hajdu and Lôbo-Hajdu2012) reported an Irish lineage of C. celata in south-eastern Brazil. However, this is the first record of a Mediterranean lineage of C. viridis in the south-western Atlantic. The south-eastern coast of Brazil and the western Mediterranean are warm temperate and temperate ecoregions, respectively (Spalding et al., Reference Spalding, Fox, Gerald, Davidson, Ferdana, Finlayson, Halpern, Jorge, Lombana, Lourie, Martin, McManus, Molnar, Recchia and Robertson2007), which would possibly allow the development of sponge populations of a same species from both geographic regions (e.g. Klautau et al., Reference Klautau, Monteiro and Borojevic2004; Longo et al., Reference Longo, Mastrototaro and Corriero2007). Given the intense harbour activities near the sampling site (e.g. Ignacio et al., Reference Ignacio, Julio, Junqueira and Ferreira-Silva2010), we believe that our C. viridis specimens may have their origin in the Mediterranean Sea, and they were possibly brought to Brazil by ballast water, associated with traffic around oil platforms or other man-made structures. Nevertheless, as we have no confirmed evidence of introduction of Mediterranean lineages of this species in south-eastern Brazil we assumed that C. viridis is a cryptogenic species with a distribution extending from the Mediterranean to the eastern Atlantic and down to south-eastern Brazil. Recent expeditions conducted in February 2013, 2015 to the same locality failed to find additional specimens of this species, suggesting that C. viridis reached the south-eastern coast of Brazil, but apparently disappeared before firmly established (E. Esteves, personal observation) and re-colonized the continental islands off Rio after February 2015 (if specimens recently collected prove to be C. viridis). Otherwise, the Brazilian population of this zooxanthellate and thus temperature-dependent species (e.g. Schönberg & Suwa, Reference Schönberg, Suwa, Custódio, Lôbo-Hajdu, Hajdu and Muricy2007) might have reduced at south-east Brazil, caused by low temperatures stress occasioned by upwelling events in this area (Campos et al., Reference Campos, Velhote and da Silveira2000). Nevertheless, there is no evidence linking the absence or scarcity of the sponge in this area to thermal stress. A long-term study monitoring C. viridis at south-east Brazil is strongly recommended given its possible negative impacts on reef health and wide potential distribution. Additionally, phylogeographic, ecological and reproductive studies including other species belonging to this complex are necessary to elucidate the evolutionary history of C. viridis in the Tropical Western Atlantic.
ACKNOWLEDGEMENTS
We thank Cecília Pascelli, Fabiana Fernandes, Fener Abdalla, Filipp Soares, Kady Coelho and Wellington Vieira for help during collections. Kady Coelho is also thanked for underwater photographs. Eduardo Hajdu is acknowledged for providing facilities, including the use of laboratory space and scanning electron microscopy at Museu Nacional (UFRJ). Thanks to Fernando Moraes, Guilherme Muricy and the team of ‘Projeto Ilhas do Rio’, sponsored by Petrobras through the Program Petrobras Ambiental, for providing additional information and access to newer samples collected on continental islands off Rio de Janeiro. Sula S. Mota and Phillip Willenz gave us access to a schizolectotype of C. varians. Three anonymous referees gave us valuable comments on this manuscript.
FINANCIAL SUPPORT
Coordenação de Aperfeiçoamento de Pessoal de Nível Superior supported CVL with a PhD fellowship and ELE with a fellowship to attend the 9th International Porifera Conference, held in Fremantle (Western Australia). ELE and GLH received grants from the Fundação Carlos Chagas Filho de Amparo à Pesquisa do Rio de Janeiro and the Conselho Nacional de Desenvolvimento Científico e Tecnológico.