INTRODUCTION
The species-rich genus Euplotes has been revealed to be much more divergent than previously believed and is of extremely wide distribution and high adaptive potentialities (Curds, Reference Curds1975; Lobban et al., Reference Lobban, Modeo, Verni and Rosati2005; Schwarz et al., Reference Schwarz and Stoeck2007; Wilbert & Song, Reference Wilbert and Song2008). Species identification and separation is still uneasy mainly because: (1) many of the features used for distinguishing species are known to vary considerably even within clonal cultures; (2) there is a large amount of confusion in the literature concerning the identity of certain common species; and (3) there is the added difficulty of having to search through a considerable body of literature before an identification can be attempted (Curds, Reference Curds1975; Yonezawa, Reference Yonezawa1985; Song et al., Reference Song, Warren and Hill1998; Lobban et al., Reference Lobban, Modeo, Verni and Rosati2005; Schwarz & Stoeck, Reference Schwarz and Stoeck2007).
In recent decades, from various habitats, many new Euplotes species have been studied, and added to this genus (Song et al., Reference Song, Warren and Hill1998; Lobban et al., Reference Lobban, Modeo, Verni and Rosati2005; Schwarz & Stoeck, Reference Schwarz and Stoeck2007; Wilbert & Song, Reference Wilbert and Song2008; Jiang et al., in press). Recent studies have demonstrated that a complete description of a ciliate species might include details of living morphology, infraciliature and/or silverline system as well as molecular data in order to solve taxonomic problems (Shao et al., Reference Shao, Song, Li, Warren, Al-Rasheid, Al-Quraishy, Al-Farraj and Lin2008; Song et al., Reference Song, Shao, Yi, Li, Warren, Al-Rasheid and Yang2009; Chen et al., in press). As regards SSr RNA sequence information, about 1/5 of Euplotes morphospecies have been involved so far (Yi et al., Reference Yi, Song, Clamp, Chen, Gao and Zhang2009; Jiang et al., in press).
Euplotes encysticus was originally reported by Yonezawa (Reference Yonezawa1985) as a fresh water form. Although Matsusaka et al. (Reference Matsusaka, Noguchi and Yonezawa1989) described its cortical morphogenesis during encystment, the circumscription of this ‘new’ species has not been clearly provided in the authors' opinion.
As a new contribution, a new definition of E. encysticus is provided based on a population adaptable to both brackish (salinity 6‰) and fresh water. Furthermore, the characters were raised to distinguish E. encysticus between the most similar congener E. muscorum based on both morphology and phylogenetic analyses.
MATERIALS AND METHODS
Sample collection, observation and identification
Samples were collected from a coastal sandy beach (22°43′N 114°32′E) of the Daya Bay, South China Sea in December, 2007, by digging a hole about 10 cm deep in the sand, waiting for seawater to seep into the hole, then taking the seawater with some sand back to the laboratory. Water temperature was about 12°C, and salinity 6.0‰. Uniprotistan cultures can be kept at room temperature (about 20°C) using distilled water, to which rice grains were added to enrich bacterial food (Hu, Reference Hu2008).
Live observations were carried out using an oil immersion objective with bright field and Nomarski differential interference contrast optics (Shao et al., Reference Shao, Song, Li, Warren, Al-Rasheid, Al-Quraishy, Al-Farraj and Lin2008). The infraciliature was revealed using the protargol method (Wilbert, Reference Wilbert1975). In order to demonstrate aspects of the silverline system, the Chatton–Lwoff method was used (Wilbert & Song, Reference Wilbert and Song2008). Measurements and counts were performed at a magnification of 1250. Drawings were made with the aid of a camera lucida. Terminology and systematics are mainly according to Lynn (Reference Lynn2008) and Song et al. (Reference Song, Shao, Yi, Li, Warren, Al-Rasheid and Yang2009).
Sequence availability and phylogenetic analyses
The SSU rRNA gene sequences of E. encysticus and reference sequences from GenBank databases were aligned using the Clustal W implemented in Bioedit 7.0 (Hall, Reference Hall1999) and refined by removing ambiguous gaps at both termini of the alignment. The Kimura two-parameter distance method (Kimura, Reference Kimura1980) in program MEGA-4.0 (Tamura et al., Reference Tamura, Dudley, Nei and Kumar2007) was used for distance analyses. Protocruzia contra was used as an out-group.
Phylogenetic trees were constructed according to the methods reported in Yi et al. (Reference Yi, Song, Shao, Warren, Al-Rasheid, Roberts, Miao, Al-Quraishy and Chen2008). A maximum parsimony (MP) tree was obtained via random addition and swapped using the tree–bisection–reconnection (TBR) algorithm. Gaps were treated as missing data. MP analysis was performed with the software package PAUP* 4.0b10 (Swofford, Reference Swofford2002), and the support for the internal branches was estimated using the bootstrap method with 1000 replicates.
The program MrModeltest v.2.0 (Nylander, Reference Nylander2004) selected the GTR + I + G as the best model with Akaike information criterion, which was then used for both BI and ML analyses. BI analysis was performed with MrBayes 3.1.2 (Ronquist & Huelsenbeck, Reference Ronquist and Huelsenbeck2003) using the Markov chain Monte Carlo algorithm. The program was run for 1,000,000 generations with a sample frequency of 100 and a burn-in of 2500. The ML tree was constructed with the PhyML V2.4.4 program (Guindon & Gascuel, Reference Guindon and Gascuel2003).
RESULTS
Euplotes encysticus Yonezawa, Reference Yonezawa1985 (Table 1; Figures 1 & 3)

Fig. 1. Euplotes encystycus from life (A, E, G), after protargol staining (B, C, D, F) and silver nitrate impregnation (H, I). (A) Ventral view of a typical individual; (B) to show different shapes of macronucleus; (C, D) ventral (C) and dorsal views (D), showing the infraciliature; (E) the lateral view, arrows mark the ridges; (F) arrows showing the variability of AZM; (G) dorsal side, showing the granules around the dorsal cilia; (H, I) ventral and dorsal views of the silverline system. AZM, adoral zone of membranelles; CC, contractile vacuole; CVP, contractile vacuole pore; DK, dorsal kineties; FVC, frontoventral cirri; LMC, marginal cirri; Ma, macronucleus; Mi, micronucleus; PM, paroral membrane; TC, transverse cirri. Scale bars = 50 µm.
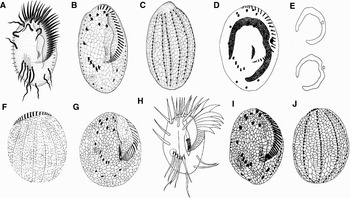
Fig. 2. Euplotes muscorum from Dregesco, Reference Dragesco1970 (A–C), from Jo & Shin, Reference Jo and Shin2003 (D–G); Euplotes encystycus from Yonezawa, Reference Yonezawa1985 (H) and Matsusaka et al. (Reference Matsusaka, Noguchi and Yonezawa1989) (I, J).

Fig. 3. Euplotes encystycus from life (A–G), after protargol (H, K, L) and silver nitrate impregnation (I, J). (A, B) Ventral (A) and dorsal (B) views, arrows mark the ridges; (C) showing the protrusion at anterior portion of cell (arrow); (D) ventral side, arrow refers to the contractile vacuole (CV); (E) arrows indicate the granules around the dorsal cilia; (F) to show the detail of dorsal side, arrows mark the ridges; (G) the cyst, arrow marks the contractile vacuole; (H) to show the transverse cirri; (I, J) ventral (I) and dorsal (J) sides of silverline system; (K, L) to show the AZM curved in different extent (arrow). AZM, adoral zone of membranelles. Scale bars (A–D) = 50 µm; (G) = 20 µm.
Table 1. Morphometric data of Euplotes encysticus. All data based on protargol-impregnated specimens and measurements in µm.

AM, adoral membranelles; AZM, adoral zone of membranelles; BBP, basal body pairs; BL, length of body; CV, coefficient of variation in %; DK, dorsal kinety; FVC, frontoventral cirri; Max, maximum; Mean, arithmetic mean; Min, minimum; N, number of specimens investigated; SD, standard deviation.
Morphology of live and impregnated specimens
Cell size about 80–90 × 50–65 µm in vivo. Body asymmetrically oval with posterior portion slightly wider than anterior; dorsoventrally flattened about 4:1 (Figure 1A, E). Peristome narrow and extremely long, extending approximately 75% of cell length, with a visible protrude at the anterior (Figure 3C, arrow). Adoral zone of membranelles (AZM) curved in varying degrees in posterior portion (Figures 1F, 3K, M). Cytoplasm colourless, often filled with many light-reflecting granules and few food vacuoles (Figure 3A, B). Single contractile vacuole located to right of transverse cirri (Figure 3D, arrow), about 10 µm in diameter, often pulsating quickly (<1 second) with a ~60 seconds interval. Six obvious dorso-lateral ridges (Figure 1E, arrows; Figure 3B, arrows). Dorsal kineties (DK) inserted in concaves down the ridges (Figure 3F, arrows). DK1, on left margin of ventral side, noticeably shortened (Figure 1C, arrow); other DKs extending over entire length of cell with about 24 cilia in each. The dorsal cilia are surrounded by ~6 elongated ellipsoidal granules, which are generally 1.5 × 0.2 µm in size (Figure 1G, arrows; Figure 3E, arrows).
Cirri usually strong. Frontoventral cirri 25–30 µm long, transverse cirri 30–40 µm, caudal and left marginal cirri about 20 µm (Figure 1A; Figure 3A). Paroral membrane (PM) about 10 µm long.
Macronucleus (Ma) usually C-shaped, with the anterior and posterior ends sometimes curving (Figure 1B). Micronucleus (Mi) small, nearly spherical and located in the upper right half of the macronucleus (Figure 1D).
After long time cultivation (about 1 month), cells can encyst because of food exhaustion. Cysts usually irregular spherical with scraggly border, about 40 µm in diameter; contractile vacuole detectable (Figure 3G, arrow).
Locomotion by crawling on crumb or substrate (Figure 1E).
Silverline system
Dorsal silverline system complex type, consisting of 4–6 polygenes between each kinety (Figures 1H, I, 3I, J). Contractile vacuole pore (CVP) located at level of transverse cirri (Figure 1H).
SSr RNA sequence analyses
The SSr RNA sequence (Accession number: FJ346569) for E. encysticus described in this study is 1865 bp long with 43.91% GC content. The alignment of SSr RNA sequences displays 38 bp differences between E. encysticus and E. muscorum and two populations of E. encysticus are almost identical (excluding the termini of the alignment). Treating gaps as missing data, the Kimura two-parameter distance between two E. encysticus was estimated to be 0.0006, which ranges from 0.0076 to 0.0082 between E. encysticus and E. muscorum.
Phylogenetic position of Euplotes encysticus based on SSr RNA sequences (Figure 4)
Trees constructed using different algorithms show similar topology and thus are combined. The analyses provide full posterior probability and bootstrap support for the monophyly of Euplotidae (100ML/100MP/1.00BI). Two populations of E. encysticus cluster together with high support value (93ML/73MP/0.85BI), then form a clear sister group to E. muscorum, which is strongly supported (98ML/97MP/1.00BI).
DISCUSSION
Though the previous form of E. encysticus was found in fresh water habitat (Yonezawa, Reference Yonezawa1985; Matsusaka et al., Reference Matsusaka, Noguchi and Yonezawa1989), it is rather certain that our isolation should be conspecific with the original because of the extreme similarities (Figure 2D–F), i.e. the general pattern of ciliature, the silverline-system, the dorso-lateral ridges, the capability of encystment and the quite adjacent number of all cirri and the adoral membranelles (AZM). The ‘minor difference’ in morphology is that our organism has a bigger body size in vivo (80–90 × 50–65 µm versus 62–74 × 41–53 µm in Yonezawa, Reference Yonezawa1985). In addition, by 0‰ to 6‰ in salinity, the discrepancy of the original niches of the two populations is rather inconspicuous, and it would even be neglected, because our isolation can reproduce well when it was implanted into fresh water directly.
In comparison with the congener E. encysticus shows great similarity with E. muscorum Dragesco, Reference Dragesco1970 (Figure 2A–J) in morphology. Thus, referring to all the data available we suggest the following points to discern the two species: (1) no cyst has been observed so far in E. muscorum (Dragesco, Reference Dragesco1970; Dragesco & Dragesco-Kernéis, Reference Dragesco and Dragesco-Kernéis1986; Jo & Shin, Reference Jo and Shin2003); (2) the obvious dorsal-lateral ridges and the granules surrounding the dorsal cirri have never been detected in E. muscorum; (3) the AZM of E. encysticus outspreads more broadly than that of E. muscorum both in living and protargol-impragenated specimens; and (4) the AZM covers 3/4 body length in E. encysticus, and 2/3 in E. muscorum. The dissimilarity between these two forms is clearly supported by the differences in their SSU rRNA gene sequences, of which E. encysticus differs from E. muscorum by 2.04% (in comparison to other morphological closely related Euplotes: Euplotes focardii versus Euplotes parkei 1.63% differences; Euplotes plicatum versus Euplotes bisulcatus 2.47%). These data, along with the phylogenetic position as revealed in molecular trees (Figure 4), support both the validity of E. encysticus as a well-outlined and distinctive member of the genus Euplotes and the morphological identification of E. encysticus.

Fig. 4. ML tree inferred from SSU rRNA gene sequences showing the position of E. encysticus. Numbers near branches represent the bootstrap values in the following order: ML/MP/BI. “-” reflects minor differences in topology that could not be represented on the consensus tree. The scale bar corresponds to 5 substitutions per 100 nucleotide positions. The highest supported (100ML/100MP/1.00BI) branches are marked with solid circles. Species newly described in this study are shown in bold text.
Yonezawa (Reference Yonezawa1985) raise the freshwater isolation as a new species mainly on: (1) the shape of right side of peristome in E. encysticus was straight, but in E. muscorum was wing shaped; (2) the position of micronucleus relative to macronucleus: in shallow depression in the centre of macronucleus in E. encysticus, while near the anterior corner of macronucleus in E. muscorum; and (3) the capability of cysting.
In our opinion, the shape of the lip may vary to a certain extent even in a same population. And as the figures in Jo & Shin (Reference Jo and Shin2003) indicated, the micronucleus of E. muscorum may be located also in shallow depression in the centre of macronucleus (Figure 2D, E), so these two points are not adopted here as distinguishing characters.
Since no clear diagnosis of E. encysticus based on modern methods is available, an improved definition is suggested according to previous and present studies (Yonezawa, Reference Yonezawa1985; Matsusaka et al., Reference Matsusaka, Noguchi and Yonezawa1989).
SYSTEMATICS
IMPROVED DIAGNOSIS
Medium-sized Euplotes, in vivo about 60–90 × 40–65 µm; body oval in outline; buccal field covering 3/4 of body, AZM broad; 9 frontoventral, 5 transversal, 2 caudal and 2 left marginal cirri; 30–43 adoral membranelles; 7–8 dorsal kineties within obvious ridges, the central one of which contains about 21–30 pairs of basal bodies, each dorsal cirri surrounded by several granules; dorsal silverline system complex type; capable of encystment.
ACKNOWLEDGEMENTS
This work was supported by ‘the Natural Science Foundation of China’ (Project Nos U0633006 and 30700069), the Center of Excellence in Biodiversity, King Saud University, and the 111 Project (No. B08049). Many thanks are due to Professor Weibo Song (OUC) and two anonymous referees for their constructive criticism and improvements to the English.







