INTRODUCTION
The conventional view is that planktonic organisms comprise large panmictic populations due to the lack of physical barriers to dispersal and gene flow in the open ocean (Palumbi, Reference Palumbi1994; McQuinn, Reference McQuinn1997). However, nearly all species exhibit population structure to some degree (Ehrlich & Raven, Reference Ehrlich and Raven1969) and even the apparent high dispersal potential of the plankton does not always translate into spatial population homogeneity (Bucklin et al., Reference Bucklin, Lajeunesse, Curry, Wallinga and Garrison1996a, Reference Bucklin, Sundt and Dahleb, Reference Bucklin, Astthorsson, Gislason, Allen, Smolenack and Wiebe2000; Kirby et al., Reference Kirby, Lindley and Batten2007). For example, in the open-ocean, at macrogeographical scales, hundreds to thousands of kilometres (Haury et al., Reference Haury, McGowan, Wiebe and Steele1978), distance appears to restrict gene flow. Studies of the North Atlantic copepods Calanus finmarchicus and Nannocalanus minor have revealed genetic differentiation between samples of these species collected from the western and eastern North Atlantic seaboards (Bucklin et al., Reference Bucklin, Lajeunesse, Curry, Wallinga and Garrison1996a, Reference Bucklin, Sundt and Dahleb, Reference Bucklin, Smolenack, Bentley and Wiebe1997, Reference Bucklin, Astthorsson, Gislason, Allen, Smolenack and Wiebe2000). High levels of gene flow in the plankton also appear incompatible with the high diversity of planktonic copepod species (Mauchline Reference Mauchline1998; Goetze, Reference Goetze2005).
The copepod Centropages typicus is an important member of the zooplankton in the North Atlantic and Mediterranean. Previous reports indicate that C. typicus persists throughout the winter in the water column in four centres of distribution in the North Atlantic: the American continental shelf; the western English Channel; the eastern North Sea (German and Southern Bights); and north of Ireland (Lee, Reference Lee1971; Lindley & Reid, Reference Lindley and Reid2002). It also persists further south in the Bay of Biscay and Iberian Atlantic coastal waters (Beaugrand et al., Reference Beaugrand, Lindley, Helaouet and Bonnet2007) and throughout the Mediterranean (Razouls, Reference Razouls1973; Mazzocchi et al., Reference Mazzocchi, Christou, Capua, de Puelles, Fonda-Umani, Molinero, Nival and Siokou-Frangou2007; unpublished data from Continuous Plankton Recorder (CPR) samples in the Mediterranean). Although C. typicus is most abundant in the neritic-coastal zone and continental shelf waters it also occurs into more oceanic areas (Barnard et al., Reference Barnard, Batten, Beaugrand, Buckland, Conway, Edwards, Finlayson, Gregory, Halliday, John, Johns, Johnson, Jonas, Lindley and Nyman2004; Beaugrand et al., Reference Beaugrand, Lindley, Helaouet and Bonnet2007). Interestingly, the long-term mean annual distribution of C. typicus shows that during its peak of abundance at the end of summer, this species expands from its centres of distribution right across the southern part of the North Atlantic (Lee Reference Lee1971; Beaugrand et al., Reference Beaugrand, Lindley, Helaouet and Bonnet2007). The seasonal expansion of C. typicus into oceanic waters means that during part of the year individuals originating from different overwintering centres in the North Atlantic and the Mediterranean Sea can potentially mix at ‘the edge’ of their distribution and interbreed.
The chela of the male fifth pereiopod limb (P5) of C. typicus plays a key role in its reproduction. Therefore, morphological differences of this appendage may prevent breeding between individuals belonging to different populations and species (Goetze, Reference Goetze2008). Lee (Reference Lee1971) suggested that morphological variation among the chela of the male P5 of C. typicus could be used as an indicator to reflect differentiation among specimens from overwintering centres in the eastern North Atlantic and western North Atlantic and the Mediterranean Sea. Thus, the present study compares the morphology of the chela of the male P5 of C. typicus, from samples collected in 2005, with similar measurements made by Lee in 1961 (Lee, Reference Lee1971), to determine if population differentiation observed by Lee (Reference Lee1971) has been maintained. In addition, we complement our morphological analyses with genetic variation in C. typicus to determine whether the morphological variations might represent genetically differentiated populations.
MATERIALS AND METHODS
Plankton samples
For the morphological analysis we extracted specimens of C. typicus males from CPR samples collected in 2005 in the western North Atlantic (WA), at two locations in the eastern North Atlantic (sites EA1 (English Channel) and EA2 (North Sea)) and by net haul from the Mediterranean (Table 1). For a description of the CPR sampling see Batten et al. (Reference Batten, Clarke, Flinkman, Hays, John, John, Jonas, Lindley, Stevens and Walne2003). The specimens in the Mediterranean were obtained from samples collected in 2005 from the Gulf of Naples, at Station MC, by the Stazione Zoologica di Napoli (Table 1). Our sample sites correspond to those analysed by Lee (Reference Lee1971) from CPR samples taken in 1961 in the standard CPR areas in the North Atlantic, and broadly to western Mediterranean samples, also obtained in 1961, from net haul material collected by the Castellon Marine Laboratory, Spain and the L'Institut Océanographique d'Algiers.
Table 1. Centropages typicus. Mean (± SE) prosome length (μm) measured from six (N) specimens collected in 2005 from the eastern Atlantic (i.e. English Channel, EA1; North Sea, EA2), western Atlantic (WA) and the Mediterranean (Med). The latitude and longitude of the areas sampled in the present study and the corresponding Continuous Plankton Recorder standard areas (D3, C1 and E10) and the Mediterranean area (MED) from Lee (Reference Lee1971) are also shown.

Material for the genetic analysis was collected by net hauls from the Canadian coast of the north-west Atlantic (WA, 44°28′N 63°2′W) and the English Channel (EA1) (site L4, Plymouth, UK, 50°15′N 04°13′W) between July and September 2005 and from the North Sea (EA2) (German Bight, 54°4′N 7°21′E) in July 2005. A Mediterranean sample (MED) of C. typicus was obtained from the coast of Naples at Station MC (40°48′N 14°15′E) in June 2005. The sampling locations, in the present study, lie within the CPR regions D3 and C1, E10 in the North Atlantic and the Mediterranean Sea sampling sites reported by the Lee (Reference Lee1971) morphological study (Table 1). Individual C. typicus were identified under a stereo microscope and stored in ethanol at –20°C prior to DNA extraction.
Lee (Reference Lee1971) showed that the populations from these overwintering areas were most likely to mix, hence interbreed, at ‘the edge’ of these regions, between August and October when this species reaches its maximum spatial distribution across the North Atlantic. Therefore, the C. typicus specimens analysed in the current paper originated from samples collected at the core of each distribution centre between August and October.
Morphological analysis
Lee (Reference Lee1971) identified morphological differentiation among samples of C. typicus by examination particularly of the third exopodite segment of the chela (the male right 5th pereiopod (P5)) (Figure 1). Lee (Reference Lee1971) also found that male C. typicus from the eastern and western North Atlantic had consistently differing morphologies and that males from the Mediterranean (Castellon) differed from both Atlantic forms (Figure 2A).

Fig. 1. Line drawing of male Centropages typicus, dorsal view, showing the position of the chela on the P5 (figure modified from Rose, 1933).

Fig. 2. The chelae of male P5 of Centropages typicus from (a) eastern North Atlantic, (b) western North Atlantic and (c) Mediterranean. Above, (A) drawings after Lee (Reference Lee1971) and below, (B) the digitized images from specimens sampled in the same areas in 2005.
In this study, the chela of 24 specimens of C. typicus from samples collected in 2005 (Table 1) were dissected under a stereo microscope and the prosome length and the chelae were measured from digital images (magnification × 670) obtained by inverted microscopy (Figure 2B). The shape of the distal part of the chela of specimens from each sample site was also measured from the digital images following the method described by Lee (Reference Lee1971) (Figure 3). Briefly, lines were drawn through the bases of the segmental spines and the corresponding basal joints. The angle where these lines intersected was bisected and a line was drawn through the distal angle of the joint of the inner segmental spine to meet this bisector at right angles; this line was then projected to meet a perpendicular line at the point ‘O’ from the tip of the segment and the distance ‘r’ from the point ‘O’ was measured at 5° intervals (Figure 3). Finally, the radius length measurements were standardized as a percentage of the prosome length. Differences in the shape of the curves derived from the radius plot of the P5 of each specimen from the different areas were investigated using the one-way analysis of similarity (ANOSIM) test, with pair-wise comparisons on Euclidean distance between radial measurements standardized to body length using the software package Primer-e V.6 (Clarke & Gorley, Reference Clarke and Gorley2006).

Fig. 3. The construction used to analyse the shape of the third exopodite segment of the male P5 of Centropages typicus showing the radial length (r) and origin (O). Diagram modified from Lee (Reference Lee1971). See Materials and Methods for further explanation.
DNA extraction and amplification
Total genomic DNA was extracted from individual C. typicus using a standard proteinase-k, phenol-chloroform extraction protocol. Briefly, individual copepods were placed into 1.5 ml Eppendorf tubes each containing 200 µl cell lysis buffer (10 mM Tris–Cl pH 7.9, 100 mM EDTA and 0.5% SDS) with 4 µl proteinase-K solution (10 mg/ml) and digested for 3 hours at 55°C. Following phenol-chloroform purification the DNA was recovered by precipitation using NaCl and EtOH, and then resuspended in 50 µl nanopure H2O. A 1 µl aliquot of the extracted C. typicus DNA was then used as a template in a polymerase chain reaction (PCR). Two loci were amplified from each individual using Taq DNA polymerase. A 709 base pairs (bp) region of the mitochondrial cytochrome c oxidase subunit I (COI) was amplified using the primers LCO and HCO (Folmer et al., Reference Folmer, Black, Hoeh, Lutz and Vrijenhoek1994) and a 781 bp fragment of the putative C. typicus nuclear rDNA internal transcribed spacer (ITS) array was amplified using the primers ITS1 and ITS4 (White et al., Reference White, Bruns, Lee, Taylor, Innis, Gelfand, Sninsky and White1990); this nuclear region comprises a partial sequence of the 18S rRNA gene, complete sequences of the ITS1, 5.8S rRNA gene and ITS2 and a partial sequence of the 28S rRNA gene. The PCR annealing temperatures were 54°C and 50°C for the ITS1 and COI primers respectively, with extension times of 1 minute in both cases. All other PCR conditions were identical for both loci. Each 50 µl PCR reaction contained 10 pmol of each primer, 200 µM of each dNTP, 1.5 mM MgCl2, 1 × reaction buffer and 2.5 units recombinant Taq DNA polymerase (Invitrogen). The thermal profile began with a denaturation step at 94°C for 2 minutes followed by 35 cycles of 94°C for 1 minute, annealing at 50°C or 54°C for 1 minute, and extension at 72°C for 1 minute. The cycle was completed with a final extension step of 72°C for 10 minutes. Amplification products were purified and sequenced directly using the forward amplification primer. To confirm that sequence variations in COI PCR products represented genetic variations in C. typicus, rather than nucleotides misincorporated during the PCR, a total of 8 randomly chosen individuals were reamplified and sequenced on the opposite strand. To confirm the observed variation in ITS1, a single individual representing each ITS variant was cloned and a total of 8 clones from each individual were sequenced on both strands using vector specific sequencing primers.
Genetic data analysis
A consensus ITS and COI sequence obtained from C. typicus was putatively identified to similar sequences deposited on GenBank using the BLAST search algorithm. All the COI and ITS sequences were aligned manually. Genetic data analysis focused on the mtDNA COI locus using the software packages Arlequin 3.0 (Excoffier et al., Reference Excoffier, Laval and Schneider2005) and GenAlEx 6 (Peakall & Smouse, Reference Peakall and Smouse2005). Mean nucleotide diversity (π) (calculated as the average pairwise divergence between all haplotype combinations) was estimated according to Nei (Reference Nei1987). Haplotype diversity was also calculated following Nei (Reference Nei1987, equation 8.4). Genetic differentiation among samples was examined using an analysis of molecular variance (AMOVA) (Excoffier et al., Reference Excoffier, Smouse and Quattro1992) to determine the distribution of molecular variance among the 4 samples, WA, EA1, EA2 and Med. The AMOVA calculates φST, which is an analogous statistic to FST (Wright, Reference Wright1931) and considers both the evolutionary distance among haplotypes and the haplotype frequency in the population. In the analysis of C. typicus, in this study, the total molecular variance is the sum of the covariance component due to differences among haplotypes within a sample WA, EA1, EA2 and Med, and the covariance component due to differences among haplotypes in different samples within the group of samples. Because of the low nucleotide diversity and therefore small distances among haplotypes, uncorrected pairwise distances were used in all calculations. The level of significance was tested using a permutation procedure with 16,000 permutations (Excoffier et al., Reference Excoffier, Laval and Schneider2005). In addition to AMOVA, standard FST values were also calculated between pairs of samples of C. typicus and their significance was tested using a permutation procedure. We also conducted a principal coordinate analysis (PCA), using Genalex 6 (Peakall & Smouse, Reference Peakall and Smouse2005), on the standardized covariance matrix of genetic distances using Nei's (Reference Nei1972) estimate of genetic distance; this was to determine major patterns in the haplotype dataset. Analysis of variation in the ITS1 region was more simply reduced to the presence or absence of a single PCR variant in the sample due to the nature of the genetic variation observed.
RESULTS
Morphological variations
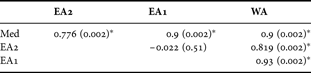
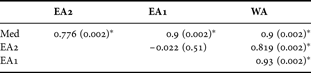
The plot of the mean radius of the chela of C. typicus calculated from the raw data in Appendix 3 of Lee (Reference Lee1971) shows a clear morphological differentiation between the Mediterranean (Med) and western Atlantic (E10) samples and between these and the two eastern Atlantic samples (D3 and C1) (Figure 4A). We found similar differences among the chelae of specimens of C. typicus from samples collected in 2005 for the corresponding areas in the Mediterranean (Med) and western Atlantic (WA) samples and between these and the two eastern Atlantic (EA1 & EA2) samples (Figure 4B). Therefore, differences in curvature of the third exopodite segment in these recent specimens are consistent with Lee's earlier work. Table 2 provides the results of the one-way ANOSIM test confirming that samples of C. typicus differed significantly (Global R-statistics = 0.638, P < 0.001, permutations = 999) based upon chela morphology. The ANOSIM pair-wise comparison also showed that there was no significant difference between the morphology of the chelae of C. typicus from EA1 and EA2 sites in the eastern Atlantic (Table 2).

Fig. 4. Plot of radial length ‘r’ at 5° intervals (see Figure 3) of the male P5 of Centropages typicus: (A) redrawn from raw data (cm) in Appendix 3 of Lee (Reference Lee1971); and (B) radial length data standardized to the body length (%) of specimens collected in 2005 in the same areas as summarized in Table 1. Bars indicate standard errors (SE).
Table 2. Results of the one-way analysis of similarity test (Global R-statistics = 0.638, P < 0.001, permutations = 999) with the pair-wise comparison between the standardized radial lengths (i.e. % body length) of the 5th modified pereiopod of Centropages typicus collected in 2005 from the eastern Atlantic (i.e. English Channel, EA1; North Sea, EA2), western Atlantic (WA) and Mediterranean (Med). The values in parentheses are the P values and the asterisk (*) indicates a significant difference between pairs.
Variation in COI sequences
A total of 560 bp of the COI gene was obtained from 116 individuals (Table 3). A comparative search in GenBank yielded a 98% similarity to a previously identified mtDNA COI sequence from C. typicus (accession number EU016221). All the putative C. typicus COI DNA sequences translated into amino acid sequences, which, together with the absence of any double peaks in the chromatograms, suggested they were unlikely to be nuclear pseudogenes. The C. typicus COI sequences obtained in this study can be found under the GenBank accession numbers GU132316–GU132431.
Table 3. Variation in mitochondrial cytochrome c oxidase subunit I (COI) and the internal transcribed spacer (ITS) rDNA of Centropages typicus from the eastern Atlantic (i.e. English Channel, EA1 and North Sea, EA2), western Atlantic (WA) and Mediterranean (Med).

aHaplotype diversity h was calculated following Nei (Reference Nei1987, equation 8.4); bmean nucleotide diversity, π, was calculated as the average pairwise divergence between all haplotype combinations and was estimated according to Nei (Reference Nei1987).
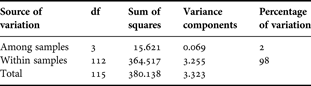
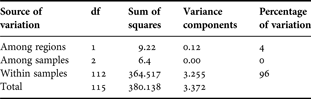
There were 131 variable nucleotide positions (in 560 bp) among the 116 COI sequences obtained and there were no insertions or deletions. Translation of the nucleotide sequences indicated that all nucleotide substitutions were silent except for a single substitution in one individual within the EA2 sample. Although haplotype diversity was high with 100 unique haplotypes, nucleotide diversity was low (Table 3). Standard deviations for these two parameters overlapped for all populations. The mean nucleotide diversities, π (± SE), for the entire sample and for within samples were 0.0118 ± 0.0014 and 0.0117 ± 0.0015, respectively. A total of eight individuals were re-amplified and on each occasion the new sequence confirmed the haplotype obtained originally. The most common COI haplotype was represented 11 times among the 116 individuals occurring in 3, 4 and 4 C. typicus individuals from the Med, EA1 and WA, respectively. The greatest difference among haplotypes was seen between two individuals from the Med and EA1 samples that differed from each other by 18 nucleotides. The AMOVA on the COI sequences (Table 4A) indicated that 2% of the total variance was due to variation among the four sampling sites, φ st: 0.0207, probability random value > observed value = 0.006. An AMOVA comparing the Mediterranean sample to the Atlantic samples revealed that a significant proportion (4%) of the total variance among samples was due to variation between these two regions (Table 4B), φ ct: 0.036, probability random value > observed value = 0.002. No significant differentiation was observed when the WA sample was compared to EA1 and EA2 samples. Population pairwise FST values also indicated that C. typicus in the Mediterranean were significantly different from those from the EA1 and WA (P < 0.05 after sequential Bonferroni correction) (Table 5). A similar result was obtained when the smaller sized EA1 and EA2 samples were pooled into a single eastern Atlantic group. Figure 5 shows the results of the PCA that also suggests a small group of Mediterranean haplotypes differ from those found in the Atlantic.

Fig. 5. Principal coordinate analysis on the standardized covariance matrix of genetic distances for the mtDNA COI of Centropages typicus. The first and second principal components explained 48.69% and 13.65% of the variation, respectively. The plot shows a small cluster of unique Mediterranean haplotypes.
Table 4A. Analysis of molecular variance on the cytochrome c oxidase subunit I sequences of Centropages typicus.

Table 4B. Analysis of molecular variance on the cytochrome c oxidase subunit I sequences comparing Mediterranean to Atlantic samples of Centropages typicus.

Table 5. FST and corresponding P values in parentheses (above diagonal) and the results of exact permutation tests for population differentiation (Raymond & Rousset, 1995) (below diagonal) using variation in mtDNA cytochrome c oxidase subunit I of Centropages typicus. An asterisk denotes significance difference (P < 0.05) after sequential Bonferroni correction.

Variation in ITS1 sequences
A total of 462 bp of the nuclear rDNA ITS array was obtained from 102 individuals (Table 3). Comparative searches in GenBank also yielded close matches to Calanoida ITS sequences including a partial sequence of Centropages bradyi (GenBank accession number AY335898.1). Only a single nucleotide site in ITS1 varied among the sequenced PCR products, which appeared as a polymorphic nucleotide transition (C-T). DNA sequences of 8 separate cloned PCR products confirmed the presence of both ‘C’ and ‘T’ ITS1 variants within a single individual. However, among the 102 individuals examined only two PCR variants were observed; these were monomorphic ‘C’ and polymorphic ‘C/T’ PCR products, the monomorphic ‘T’ PCR product was never found. The number of monomorphic ‘C’ PCR products varied considerably among the 4 samples (Table 3). For example, the WA sample comprised 80% monomorphic ‘C’ PCR products, whereas the eastern Atlantic samples were 100% polymorphic ‘C/T’ and the Mediterranean sample was 11% monomorphic for the ‘C’ PCR product. A consensus C. typicus rDNA ITS array sequence can be found under the GenBank accession number GU125729.
DISCUSSION
Observation of the long-term mean annual distribution of the abundance of C. typicus would suggest that populations originating from the overwintering distribution centres identified by Lee (Reference Lee1971) in the North Atlantic mix and interbreed when this species expands across the southern part of the North Atlantic during summer–autumn (Lee, Reference Lee1971; Beaugrand et al., Reference Beaugrand, Lindley, Helaouet and Bonnet2007). Moreover, North Atlantic populations of C. typicus could also mix with Mediterranean conspecific populations transported by hydrographical circulation through the Gibraltar Strait (Hardy, Reference Hardy1958; Patarnello et al., Reference Patarnello, Volckaert and Castillo2007). In contrast, the morphological differences we found between the chela of C. typicus from the American continental shelf, European Atlantic shelf and Mediterranean would suggest that specimens from the different overwintering centres would not interbreed and instead they represent three different populations. Hence, our results agree with those reported by Lee (Reference Lee1971). It is also noteworthy that the shape of the chela of specimens collected in 2005, in the present study, is identical to the shape of those reported by Lee for the same overwintering centres (Lee, Reference Lee1971; Figures 2 & 4). These findings clearly show that both the male chela morphology and distribution of the C. typicus found in the core areas that we investigated has not changed over the past 40 years.
Genetic analysis also provided some evidence for differentiation among samples, although neither nuclear nor mtDNA variation completely resolved population genetic structure in these samples. The AMOVA and the PCA on mtDNA COI variation suggested slight differentiation between samples from the Mediterranean and from the Atlantic; genetic analyses reveal that this difference can be explained by differentiation between the most distantly related samples from the western Atlantic and the Mediterranean and so perhaps reflects isolation by distance alone. Variation in the nuclear rDNA ITS1 also revealed tentative genetic differences between C. typicus from the western Atlantic and those from the eastern Atlantic and Mediterranean.
The high numbers of both unique mtDNA COI haplotypes and variable nucleotide sites we observed in C. typicus are greater than seen in some other North Atlantic copepod taxa previously (Bucklin & Kocher, Reference Bucklin and Kocher1996; Bucklin et al., Reference Bucklin, Lajeunesse, Curry, Wallinga and Garrison1996a, Reference Bucklin, Sundt and Dahleb; Reference Bucklin, Smolenack, Bentley and Wiebe1997; Bucklin & Wiebe, Reference Bucklin and Wiebe1998; Zane et al., Reference Zane, Ostellari, Maccatrozzo, Bargelloni, Cuzin-Roudy, Buchholz and Patarnello2000; Papadopoulos et al., Reference Papadopoulus, Peijnenburg and Luttikhuizen2005) and may reflect the differing population biology of these species. Centropages typicus has a shorter generation time and more generations in a season than other species, such as Calanus helgolandicus. Although our results may be explained by isolation by distance, and while the amount of the total variance explained by variation among the four samples of C. typicus (2%) was much lower than reported among samples of C. helgolandicus and C. euxinus from the eastern North Atlantic, the Mediterranean and the Black Sea (46%) (Papadopoulos et al., 2005), the existence of population differentiation between Atlantic and Mediterranean C. typicus would agree with similar observations in the copepod C. helgolandicus and the euphausid Meganyctiphanes norvegica (Zane et al., Reference Zane, Ostellari, Maccatrozzo, Bargelloni, Cuzin-Roudy, Buchholz and Patarnello2000; Papadopoulos et al., 2005), which may arise from a combination of the oceanography and evolutionary history of the Mediterranean basin (Patarnello et al., Reference Patarnello, Volckaert and Castillo2007). The absence of population genetic variation in mtDNA among the WA, EA1 and EA2 samples of C. typicus in the North Atlantic while different from early studies on C. finmarchicus and N. minor, based on the analysis of mtDNA (Bucklin et al., Reference Bucklin, Lajeunesse, Curry, Wallinga and Garrison1996a, Reference Bucklin, Sundt and Dahleb), would agree with a more recent analysis of C. finmarchicus based upon both mitochondrial and nuclear DNA markers (Provan et al., Reference Provan, Beatty, Keating, Maggs and Savidge2009).
While we cannot exclude the possibility that the absence of the ‘T’ rDNA ITS1 sequence from our sample of 102 individuals does not simply reflect the failure of this variant to amplify, for example due to a linked mutation at the priming site, one explanation for the presence of the heterozygous C/T and homozygous ‘C’ rDNA ITS1 PCR sequences we observed (and the complete absence of the homozygous ‘T’ PCR product) is one of intragenomic variation in the rDNA tandem repeat. Although nucleotide diversity among tandem rDNA repeat elements is generally low in most species due to concerted evolution (Brown et al., Reference Brown, Wensink and Jordan1972), intragenomic variation does occur (Harris & Crandall, Reference Harris and Crandall2000). Under this scenario, the two variants would then comprise two different rDNA array haplotypes, a homologous ‘C’ array and a heterologous ‘C/T’ array. In this respect C. typicus appears to differ from other members of the order Calanoida where, so far, no intragenomic or within species variation in the rDNA array has been observed (Goetze, Reference Goetze2003). Since we only sequenced PCR products directly for the majority of individuals it is impossible to determine the frequency of each array within the samples. However, the different proportions of the ‘C/T’ PCR product among the samples of C. typicus tentatively suggest that there may be restricted gene flow between western and eastern Atlantic samples. This observation, in contrast to the results obtained with COI for C. typicus, would agree with population genetic studies of C. finmarchicus and N. minor (Bucklin et al., Reference Bucklin, Lajeunesse, Curry, Wallinga and Garrison1996a, Reference Bucklin, Sundt and Dahleb).
Cryptic species are common in marine habitats (Knowlton, Reference Knowlton1993). Morphological variations in other copepod taxa treated as cosmopolitan species have sometimes proved to represent diagnostic species differences upon genetic analysis (Staton et al., Reference Staton, Wickliffe, Garlitska, Villanueva and Coull2005). Genetic analyses have also indicated that there are high levels of cryptic diversity at the species level among copepods (Goetze, Reference Goetze2005). Although copepods may show eco-phenotypic variations (Fleminger & Hulsemann, Reference Fleminger and Hulsemann1987), the morphological differences we have described in C. typicus are in the male sexual appendage, which is used to hold the female during transfer of the spermatophore. Chela morphology in Centropages spp. may be central to reproductive isolation between species since pheromonal cues do not appear sufficient to prevent heterospecific encounters between males and females (Goetze, Reference Goetze2008). It is therefore tempting to speculate whether this morphological variation may reflect incipient speciation in C. typicus. In the North Atlantic this may also be represented in the variation in ITS1 among samples of C. typicus that are both geographically most separate and show the greatest morphological differences. The lack of genetic differentiation in mtDNA in the North Atlantic among samples of C. typicus may simply reflect the large population sizes of planktonic organisms, the high dispersal potential in the ocean (Provan et al., Reference Provan, Beatty, Keating, Maggs and Savidge2009), and the high haplotype diversity and small sample sizes in our study. Experimental crosses between C. typicus from the eastern and western North Atlantic and Mediterranean might resolve the extent of genetic isolation between these different population centres.
ACKNOWLEDGEMENTS
We thank Erica Head (Bedford Institute of Oceanography, Canada), Adrianna Ianora, Grazia Mazzocchi and Iole di Capua (Stazione Zoologica ‘A. Dohrn', SZN), Maarten Boesma (Alfred Wegener Institut für Polar-und Meeresforschung; Biologische Anstalt Helgoland) and John Fraser (FRS) for sending specimens for the genetic analysis. Rowena Stern (SAHFOS) and Andrew Griffiths (Marine Biological Association of the United Kingdom) are gratefully acknowledged for their constructive criticism. We thank the owners, masters and crews of the ships that tow the Continuous Plankton Recorder on a voluntary basis. We also thank the Associate Editor and two anonymous referees for improving the paper with their comments and recommendations.