Introduction
Coral reefs are among the most biodiverse ecosystems on Earth (Veron, Reference Veron1995) and provide food, income, coastal protection and many other services for millions of people worldwide (Kennedy et al., Reference Kennedy, Perry, Halloran, Iglesias-Prieto, Schönberg, Form, Carricart-Ganivet, Fine, Eakin and Mumby2013; Pendleton et al., Reference Pendleton, Comte, Langdon, Ekstrom, Cooley, Suatoni, Beck, Brander, Burke, Cinner and Doherty2016). The rapid and unprecedented degradation of coral reefs over the last decades is of crucial concern (Hoegh-Guldberg, Reference Hoegh-Guldberg1999; Hughes et al., Reference Hughes, Baird, Bellwood, Card, Connolly, Folke, Grosberg, Hoegh-Guldberg, Jackson, Kleypas and Lough2003). The distribution and abundance of coral reefs has decreased by ~50% over the past 30 years (Hoegh-Guldberg et al., Reference Hoegh-Guldberg, Jacob, Taylor, Bindi, Brown, Camilloni, Diedhiou, Djalante, Ebi, Engelbrecht, Guiot, Hijioka, Mehrotra, Payne, Seneviratne, Thomas, Warren and Zhou2018) and without any regulation of human-induced pressure more than 90% will be in danger by 2030 (Burke et al., Reference Burke, Reytar and Spalding2011). Indeed, coral reefs are endangered by multiple local drivers (pollution, overfishing, unsustainable coastal development; Burke et al., Reference Burke, Reytar and Spalding2011; Halpern et al., Reference Halpern, Frazier, Potapenko, Casey, Koenig, Longo, Lowndes, Rockwood, Selig, Selkoe and Walbridge2015; Cheal et al., Reference Cheal, MacNeil, Emslie and Sweatman2017) and global drivers (ocean warming, deoxygenation, acidification, sea-level rise, intensifying storms; Gattuso et al., Reference Gattuso, Hoegh-Guldberg and Pörtner2014; IPCC, 2014) acting in concert. Although the most immediate threat to coral reefs is the rising seawater temperature (with a 99% loss of coral reefs expected under a warming of 2°C above the pre-industrial period; Frieler et al., Reference Frieler, Meinshausen, Golly, Mengel, Lebek, Donner and Hoegh-Guldberg2013; Hoegh-Guldberg et al., Reference Hoegh-Guldberg, Cai, Poloczanska, Brewer, Sundby, Hilmi, Fabry and Jung2014; Schleussner et al., Reference Schleussner, Lissner, Fischer, Wohland, Perrette, Golly, Rogelj, Childers, Schewe, Frieler and Mengel2016; Hughes et al., Reference Hughes, Barnes, Belwood, Cinner, Cumming, Jackson, Kleypas, Van De Leemput, Lough, Morrison and Palumbi2017), ocean acidification (OA) has also been proven to impact corals.
Ocean surface water average pH has decreased by 0.2 pH units since 1870–1899 (Bopp et al., Reference Bopp, Resplandy, Orr, Doney, Dunne, Gehlen, Halloran, Heinze, Ilyina, Seferian and Tjiputra2013; Gattuso et al., Reference Gattuso, Magnan, Billé, Cheung, Howes, Joos, Allemand, Bopp, Cooley, Eakin and Hoegh-Guldberg2015), a shift that is unprecedented in the last 65 Ma (Ridgewell & Schmidt, Reference Ridgewell and Schmidt2010). The atmospheric partial pressure of carbon dioxide (pCO2) was about 278 ppm during the pre-industrial period and has almost doubled to reach about 408 ppm in 2018 (Blunden & Arndt, Reference Blunden and Arndt2019). Without additional efforts to constrain emissions, it is expected to reach between 720 and >1000 ppm by the year 2100 (‘baseline scenarios’, ranging between RCP6.0 and RCP8.5; IPCC, 2014). This would in turn decrease the average pH of ocean surface waters by 0.2–0.32 pH units (58–109% increase in acidity, based on RCP6.0 and RCP 8.5; IPCC, 2014).
Ocean acidification impacts organisms producing calcium carbonate shells and skeletons, including scleractinian corals (Gattuso & Hansson, Reference Gattuso and Hansson2011) by increasing the number of protons in seawater and modifying the seawater carbonate chemistry. Research on the effects of OA on tropical scleractinian corals is relatively recent (last two decades; Gattuso et al., Reference Gattuso, Frankignoulle, Bourge, Romaine and Buddemeier1998) with a cumulative body of work emerging from naturally acidified sites and manipulation experiments in the laboratory and in the field. Multiple lines of evidence show that OA can negatively affect physiology with consequences for the ability to calcify (calcium carbonate precipitation) of several reef organisms (Raven et al., Reference Raven, Caldeira, Elderfield, Hoegh-Guldberg, Liss, Riebesell, Shepherd, Turley and Watson2005; Hendriks et al., Reference Hendriks, Duarte and Álvarez2010; Hofmann et al., Reference Hofmann, Barry, Edmunds, Gates, Hutchins, Klinger and Sewell2010; Kroeker et al., Reference Kroeker, Kordas, Crim and Singh2010), including corals (for reviews, see Erez et al., Reference Erez, Reynaud, Silverman, Schneider, Allemand, Erez, Silverman, Schneider, Reynaud, Allemand, Dubinsky and Stambler2011; Chan & Connolly, Reference Chan and Connolly2013). Furthermore, OA may also negatively alter calcification rates through increased skeletal porosity (Tambutté et al., Reference Tambutté, Venn, Holcomb, Segonds, Techer, Zoccola, Allemand and Tambutté2015; Foster et al., Reference Foster, Falter, McCulloch and Clode2016). Higher skeletal porosity, acting individually or in concert with decline in coral calcification, may weaken coral skeletons and in turn increase their susceptibility to storm damage and sea-level rise (e.g. increase coral breakages; Silbiger et al., Reference Silbiger, Guadayol, Thomas and Donahue2014).
Overall, there is a broad agreement that OA has deleterious impacts on fundamental processes of coral biology (Kleypas et al., Reference Kleypas, Feely, Fabry, Langdon, Sabine and Robbins2006; Doney et al., Reference Doney, Fabry, Feely and Kleypas2009; Hendriks et al., Reference Hendriks, Duarte and Álvarez2010; Kroeker et al., Reference Kroeker, Kordas, Crim and Singh2010; Chan & Connolly, Reference Chan and Connolly2013). However, contrasting results were documented (Ries et al., Reference Ries, Cohen and McCorkle2009; De Putron et al., Reference De Putron, McCorkle, Cohen and Dillon2011; Rodolpho-Metalpa et al., Reference Rodolpho-Metalpa, Houlbrèque, Tambutté and Hall-Spencer2011) and a call for more research has been made (Atkinson & Cuet, Reference Atkinson and Cuet2008). Comprehensive reviews have noted a high degree of variability in the rate of coral growth decline with decreasing pH levels (Erez et al., Reference Erez, Reynaud, Silverman, Schneider, Allemand, Erez, Silverman, Schneider, Reynaud, Allemand, Dubinsky and Stambler2011; Pandolfi et al., Reference Pandolfi, Connolly, Marshall and Cohen2011; Chan & Connolly, Reference Chan and Connolly2013). Variations between studies are partly explained by differences in experimental designs (e.g. duration of exposure, irradiance levels, abundance of food and nutrients, carbonate chemistry measurement methods used, etc.; Langdon & Atkinson, Reference Langdon and Atkinson2005; Kleypas et al., Reference Kleypas, Feely, Fabry, Langdon, Sabine and Robbins2006) but other explanations, based on coral's biological traits have also been proposed (and discussed, see Comeau et al., Reference Comeau, Edmunds, Spindel and Carpenter2014a). Based on the proposed ‘Two compartment proton flux model’ (Jokiel, Reference Jokiel2011), differences in corallum morphology (‘Branching’ vs ‘Mounding’) and skeletal porosity (‘Perforate’ vs ‘Imperforate’) imply differential spatial separation of the areas of rapid photosynthesis and the areas of rapid calcification in the corallum. When the spatial separation between these areas is higher (as is the case with ‘Mounding’ and/or ‘Perforate’ skeletal properties), it reduces the competition for HCO3− between photosynthesis and calcification and thus enhances the rapid recycling of materials between these processes (Jokiel, Reference Jokiel2011). In addition, variations in coral calcification rates (‘Fast’ vs ‘Slow’) may be responsible for interspecific differences as the requirement for carbonate ions of slow-growing corals is lower than for fast-growing corals (Rodolpho-Metalpa et al., Reference Rodolpho-Metalpa, Houlbrèque, Tambutté and Hall-Spencer2011). Local adaptation to the present natural variability in pH is an additional source of variation in the response of corals to decreasing pH (Sanford & Kelly, Reference Sanford and Kelly2011; Rivest & Gouhier, Reference Rivest and Gouhier2015; Vargas et al., Reference Vargas, Lagos, Lardies, Duarte, Manríquez, Aguilera, Broitman, Widdicombe and Dupont2017). Corals that have historically been exposed to high variability in pH may have physiologically acclimatized/adapted to these conditions and may therefore be more resilient to low pH conditions (for examples, see Rivest & Gouhier, Reference Rivest and Gouhier2015).
A reef is composed of a multitude of coral species. However, most studies have focused on one or two coral species within a reef and fail to integrate the role of other species composing a coral reef landscape. Evaluating differences in species responses to decreasing pH within a given reef landscape is an important first step to better understand the future consequences of OA on local to regional coral assemblages. Here, we exposed a coral assemblage composed of seven abundant coral species of the lagoon of Mo'orea (Acropora cytherea, Acropora hyacinthus, Acropora pulchra, Leptastrea pruinosa, Montipora grisea, Pavona cactus, Pocillopora verrucosa) to three pH treatments (ambient: pHT ~7.95, low pH as projected for near-future: pHT ~7.7, and extreme low pH: pHT ~7.3; i.e. pHT: pH on the total scale) for 48 days to examine whether the responses to OA differ among these coral species. The effect of pH on coral survival, growth and photo-physiological responses was measured. Our experiment will allow several alternative hypotheses to be tested: (i) species will have similar sensitivities to decreased pH, as a consequence of local adaptation; (ii) species will have different sensitivities to decreased pH, as expected based on biological traits. In such a case, resilience to decreased pH will be greater in (a) mounding corals (M. grisea and L. pruinosa) compared with branching corals (all five other species); (b) perforate skeletons (A. pulchra, M. grisea, A. cytherea, A. hyacinthus) compared with imperforate (other three species); (c) slow-growing (L. pruinosa and P. verrucosa as the two slowest calcifiers, based on our data) compared with fast-growing (A. pulchra and A. hyacinthus as the two fastest calcifiers, based on our data).
Materials and methods
Organism collection and experimental conditions
Seven coral species were considered in this study, namely: Acropora cytherea, Acropora hyacinthus, Acropora pulchra, Leptastrea pruinosa, Montipora grisea, Pavona cactus and Pocillopora verrucosa. For each species, fragments (N = 4 per colony, N = 5 colonies, 5–7 cm length) were collected with a hammer and a chisel in the lagoon of Mo'orea, French Polynesia (17°29′17″S 149°53′3″W), at ~1–2 m depth. Upon arrival at the laboratory, coral fragments were glued on a plastic support and placed in a 1000 litre aquarium for a 2-week acclimation period with flow-through seawater (2–3 complete renewals per day) under natural light conditions (temperature ~27°C; salinity ~35; pHT ~ 7.95). Fragments from each colony were randomly assigned (one fragment per colony in each aquarium, N = 4 aquaria, N = 35 coral fragments per aquarium) in four 200 litre aquaria, to minimize the effects caused by their spatial distribution. These four aquaria were used as the seawater acidification experiment (treatment and control) aquaria. They were left for another 1-week acclimation period to ambient conditions (aerated natural seawater, temperature ~27°C; salinity ~35; pHT ~7.95; artificial light provided by Aqua Illumination Hydra (32HD 90W) following a light/dark cycle of 12 h/12 h and a daily maximum irradiance of 650 μmol photons m−2 s−1). Temperature in these aquaria was controlled with heater chillers (TK500 TECO) and monitored using Hobo Pendant temperature loggers (±0.5°C). Approximately a third of the water in the aquaria was replaced each afternoon with seawater collected from surface waters of the Bay in front of the station (‘Opunohu Bay), allowing a complete renewal of the water every 2–3 days.
After this acclimation period, coral fragments were exposed to experimental pH conditions: ambient pHT ~7.95 (2 control aquaria), pHT ~7.7 (hereafter referred to as ‘low pH’) and pHT ~7.3 (‘extreme low pH’, as those experienced by corals through the Cretaceous and in the early Eocene; Pearson & Palmer, Reference Pearson and Palmer2000; Pelejero et al., Reference Pelejero, Calvo and Hoegh-Guldberg2010). The low pH scenario is here representative of ‘near-future’ conditions as the average environmental pH variability for a fringing reef on the north shore of Mo'orea was 7.989 ± 0.038 (mean ± SD), with minimum pH of 7.84 observed over a 3-month period (Rivest & Gouhier, Reference Rivest and Gouhier2015). The use of an extreme low pH treatment provides the opportunity to identify clear trends in coral physiology breakdown (Krief et al., Reference Krief, Hendy, Fine, Yam, Meibom, Foster and Shemesh2010; Rodolpho-Metalpa et al., Reference Rodolpho-Metalpa, Houlbrèque, Tambutté and Hall-Spencer2011; McCulloch et al., Reference McCulloch, Falter, Trotter and Montagna2012; Vidal-Dupiol et al., Reference Vidal-Dupiol, Zoccola, Tambutté, Grunau, Cosseau, Smith, Freitag, Dheilly, Allemand and Tambutté2013; Tambutté et al., Reference Tambutté, Venn, Holcomb, Segonds, Techer, Zoccola, Allemand and Tambutté2015). The manipulation of pH was achieved by bubbling pure CO2 into the seawater. pH was controlled using a pH-stat system (IKS, Aquastar, Karlsbad, Germany, sensitivity of ± 0.05 pH units). It was gradually reduced by 0.15 pH units day−1, starting with the extreme low pH and then the low pH to ensure that all aquaria reach their targeted pH value concomitantly. Once reached (Day 0), pH levels were maintained for a 48-day experimental period. Temperature and salinity were monitored daily using a certified thermometer (VWR traceable digital thermometer with probe, VWR International, LLC) and a conductivity meter equipped with a conductivity electrode (Mettler-Toledo, Switzerland), respectively.
All pH values in the text are expressed in total scale (pHT). The pH electrodes of the pH-stat system were inter-calibrated every week using a portable pH logger (Metrohm, Switzerland) and a glass combination electrode (Metrohm, Primatrode with NTC) calibrated on the total scale using a TRIS buffer provided by the Marine Physical Laboratory from Scripps Institution of Oceanography (Dickson et al., Reference Dickson, Sabine and Christian2007). Furthermore, to ensure that the regulation was optimal, pH values were recorded daily with the same equipment as described above. Measurements of total alkalinity (A T) in each aquarium (triplicate 50 ml samples per aquarium) were performed every 2 days to ensure a stable A T level. Samples were collected in the morning (before the renewal of the water) and analysed within 1 day by Gran titration using open-cell potentiometric titration with an automated titrator (888 Titrando, Methrom). Titrations of certified reference materials provided by A. G. Dickson (batch 171) were performed every 2–3 titrations and the deviation from the nominal value (2217.40 ± 0.63 μmol kg−1) was always below 5%. Parameters of the carbonate chemistry system (pCO2 and ΩAr) were determined from pHT, A T, salinity and temperature using the free-access package CO2Sys (Lewis et al., Reference Lewis, Wallace and Allison1998).
Biological parameter measurements
All coral fragments (N = 140), individually tag-identified, were weighed using the Buoyant Weight (BW) technique (Davies, Reference Davies1989) before the start of the experiment (Day 0) and at the end (Day 48). Daily relative growth rate (RGR, expressed in % per day) of each live coral nubbin was then calculated following the equation:
 $${\rm \;RGR} = {\rm \;}\displaystyle{{\left({\displaystyle{{BW{\rm \;}( {{\rm Day}\;48} ) -BW{\rm \;}( {{\rm Day}\;0} ) } \over {BW{\rm \;}( {{\rm Day}\;0} ) }} \times {\rm \;}100} \right)} \over {48}}$$
$${\rm \;RGR} = {\rm \;}\displaystyle{{\left({\displaystyle{{BW{\rm \;}( {{\rm Day}\;48} ) -BW{\rm \;}( {{\rm Day}\;0} ) } \over {BW{\rm \;}( {{\rm Day}\;0} ) }} \times {\rm \;}100} \right)} \over {48}}$$In this equation, BW (Day 48) and BW (Day 0) refers to the buoyant weight of the nubbin at day 48 and day 0, respectively. It is divided by 48 to get the RGR value (%) per day. A positive value reflects skeletal growth over the time course of the experiment while a negative value indicates a net dissolution of the coral skeleton.
Photosynthetic efficiency (Fv/Fm) of each individually tag-identified coral fragment was measured in triplicate using a fluorimeter Diving-PAM (Walz, Germany) every ~2 days, one hour after dark (~7:30 p.m. local time). The mortality (viz. complete loss of living tissues and algal overgrowth), tissue necrosis (viz. loss of living tissues in some portions of the skeleton) and colour of each coral fragment were evaluated every 2–3 days. The colour was documented using the reference colour chart developed by Siebeck et al. (Reference Siebeck, Marshall, Kluter and Hoegh-Guldberg2006), which provides a proxy for the number of Symbiodinium within the organism (referred to hereafter as the colour index). Colour of the coral fragments was visually observed and compared with the reference colour chart to attribute an index ranging from 1 (extremely pale colour) to 7 (darkest colour) for each nubbin. The index was then transformed in per cent value according to the original colour of each nubbin (knowing that the colour index value that was attributed at day 1 equals 100%). This allows documenting the temporal changes of colour of each nubbin over the course of the experiment. At the end of the experiment (Day 48), all coral fragments were bleached to remove living tissues. Coral skeletons were observed under an optical microscope (Leica M80, Leica Microsystems, Switzerland) to examine the impact of low pH levels on the structure of the skeleton.
Data analysis
All statistical tests and graphs were performed using R software (R Core Team, 2017). While our measurements from individual organisms in each tank are pseudoreplicated, the potential implications of pseudoreplication were reduced by the large volume of the tanks (200 l) and the replacement of one third of the water in the aquaria ensuring high water quality. Based on this rationale, individual organisms were treated as statistical replicates (Comeau et al., Reference Comeau, Carpenter, Lantz and Edmunds2016). Moreover, the comparison of the effect size for the RGR between the four aquaria reveals a similar variability between the two high pH aquaria (1 and 2) and the expected decreasing trend with decreasing pH as well as variability deviating from the control (Supplementary Figure S1). These support the robustness of our experimental system and the validity of the observed pH effects.
Following Shapiro–Wilk's tests and Levene's tests to evaluate the assumptions of normality and equality of variance necessary for parametric analysis, tissue necrosis, relative growth rate, photosynthetic efficiency and colour index were analysed using one-way ANOVA on each species independently (Supplementary Table S1). When data distribution did not follow normality, data were analysed via univariate non-parametric Kruskal–Wallis tests for each factor. Statistical difference between the two control aquaria was tested and results were pooled when no significant difference was reported (Supplementary Figure S2 and Table S2). Post-hoc tests were performed for pairwise comparisons (Tukey test following ANOVA/Dunn test following Kruskal–Wallis; Supplementary Table S1).
Results
Carbonate chemistry
Targeted pH levels were successfully reached in all treatments, with seawater pHT maintained at 7.69 ± 0.12 (low pH) and 7.27 ± 0.17 (extreme low pH) compared with 7.95 ± 0.11 (mean ± SE) in the ambient aquaria, corresponding to pCO2 of 999.6 μatm, 3218 μatm and 480.8 μatm, respectively (Table 1). pH levels in the two control aquaria were not statistically different (Tukey test, P = 0.81) and were thus pooled together. All treatments were statistically different from the others (Tukey test, P < 0.005). A T in the ambient pH was 2133.3 ± 90.4 and increased in the other treatments reaching 2199.0 ± 27.9 under low pH and 2487.5 ± 128.8 under extreme low pH, reflecting skeletal dissolution. Seawater was supersaturated with respect to aragonite in the ambient (ΩAr = 2.74) and low pH (ΩAr = 1.7) but was undersaturated with respect to aragonite in the extreme low pH (ΩAr = 0.79).
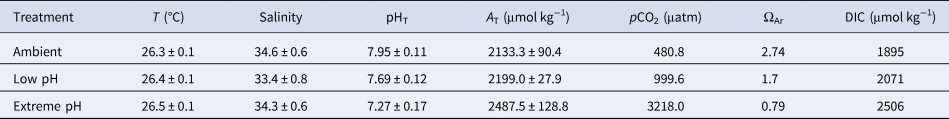
Table 1. Mean physical parameters and carbonate chemistry in the three experimental treatments
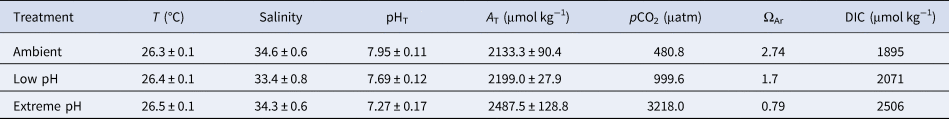
Data are means ± standard error. Parameters of the carbonate chemistry were calculated from pH in the total scale (pHT), total alkalinity (A T), temperature (T) and salinity, with the free-access package CO2Sys (Lewis et al., Reference Lewis, Wallace and Allison1998). Constants used were from Mehrbach et al. (Reference Mehrbach, Culberson, Hawley and Pytkowicx1973), as refitted by Dickson & Millero (Reference Dickson and Millero1987).
Coral responses to OA
Mortality and tissue necrosis
After 48 days, no coral mortality was recorded for all species and all pH treatments, except for A. pulchra for which 40% of the coral fragments died in the extreme low pH treatment. Despite this, tissue necrosis was frequently observed, with a significantly higher tissue loss at extreme low pH compared with the ambient in all species (Figure 1A; Supplementary Table S1). The higher proportions of tissue loss under extreme low pH were noted for A. pulchra (mean tissue loss of 37.5%; Dunn test, P = 0.01) and M. grisea (mean tissue loss of 22%; Dunn test, P = 0.001). Under low pH, no differences were observed in comparison to ambient conditions, except for L. pruinosa (Figure 1A; Supplementary Table S1; mean tissue loss of 4.4%; Tukey test, P = 0.03).
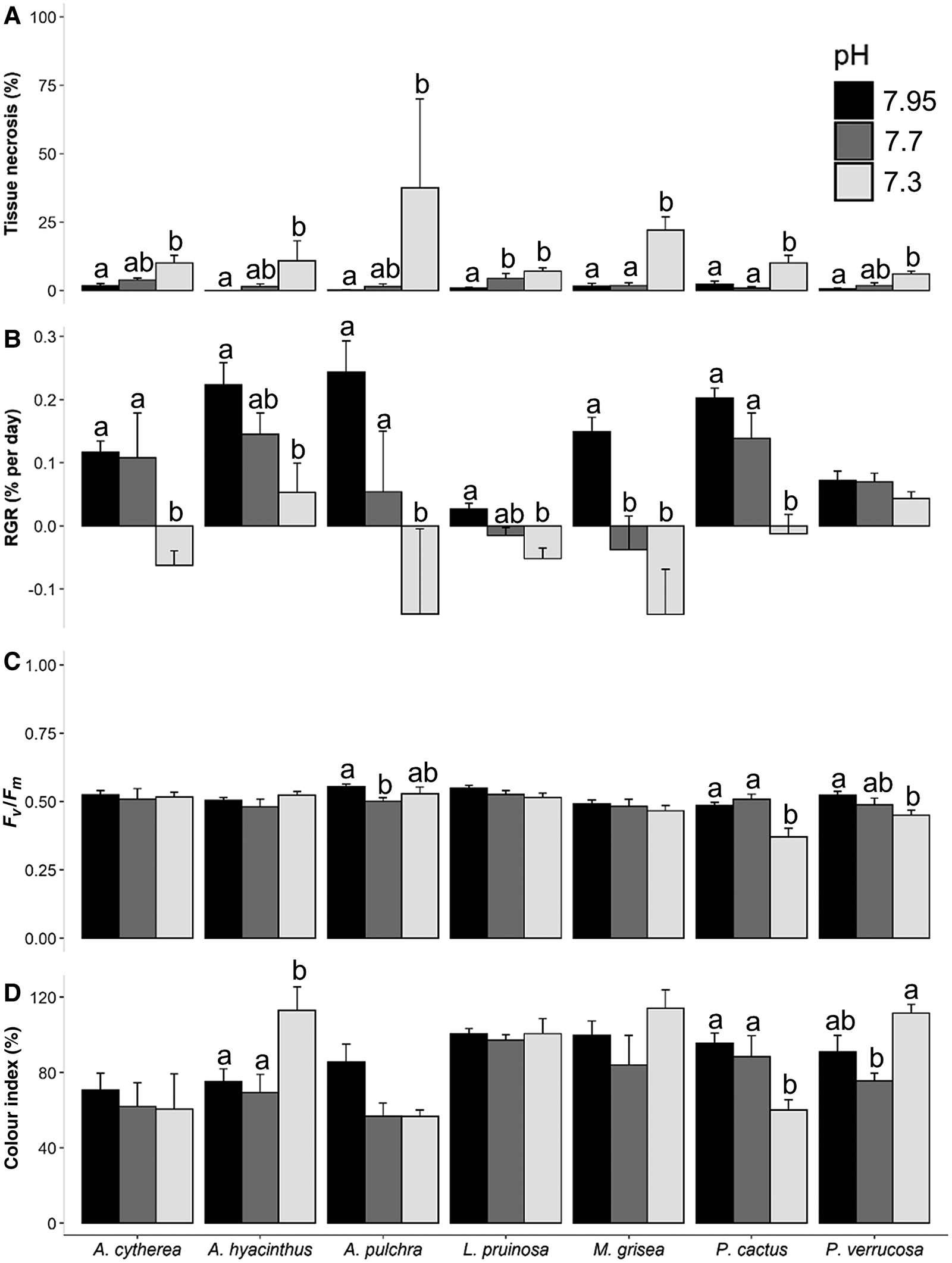
Fig. 1. Effects of experimental ocean acidification (pH levels) on the different parameters after 48 days for each studied species: (A) Tissue necrosis (proportion of dead tissue; %); (B) Relative growth rate (RGR, % weight increase relative to day 0; % per day); (C) Photosynthetic efficiency (Fv/Fm); (D) Relative change in colour (change in colour index relative to day 0; %). Light grey, dark grey and black bars represent extreme low (7.3), low (7.7) and ambient pH (7.95) treatment, respectively. Data are means ± standard error. Letters indicate statistical differences for each species (see Supplementary Table S1 for results of statistical analyses).
Relative growth rate
Under near-future conditions of pH (low pH), significant negative effect on the relative growth rate (RGR in % per day) was only observed for M. grisea (Tukey test, P = 0.001, Supplementary Table S1; Figure 1B). Despite this, exposure to extreme low pH significantly decreased the RGR for most species leading to a 13, 7, 16, 4, 10 and 31% decrease in skeletal weight at extreme low pH relative to the ambient, for A. cytherea, A. hyacinthus, A. pulchra, L. pruinosa, M. grisea and P. cactus, respectively. Only P. verrucosa remained unaffected under the extreme low pH (one-way ANOVA, P = 0.4; Supplementary Table S1; Figure 1B).
Microscopy analysis of coral skeleton
Microscopy analyses revealed skeletal dissolution only under extreme low pH conditions compared with the ambient (Figure 2A, B; see Supplementary Figure S3 for images of all species). This was particularly marked for Acropora sp. and M. grisea with reduction of the radial symmetry of the calyx, the number and size of the septa and irregularities in the thickness and microstructure of the calyx wall.
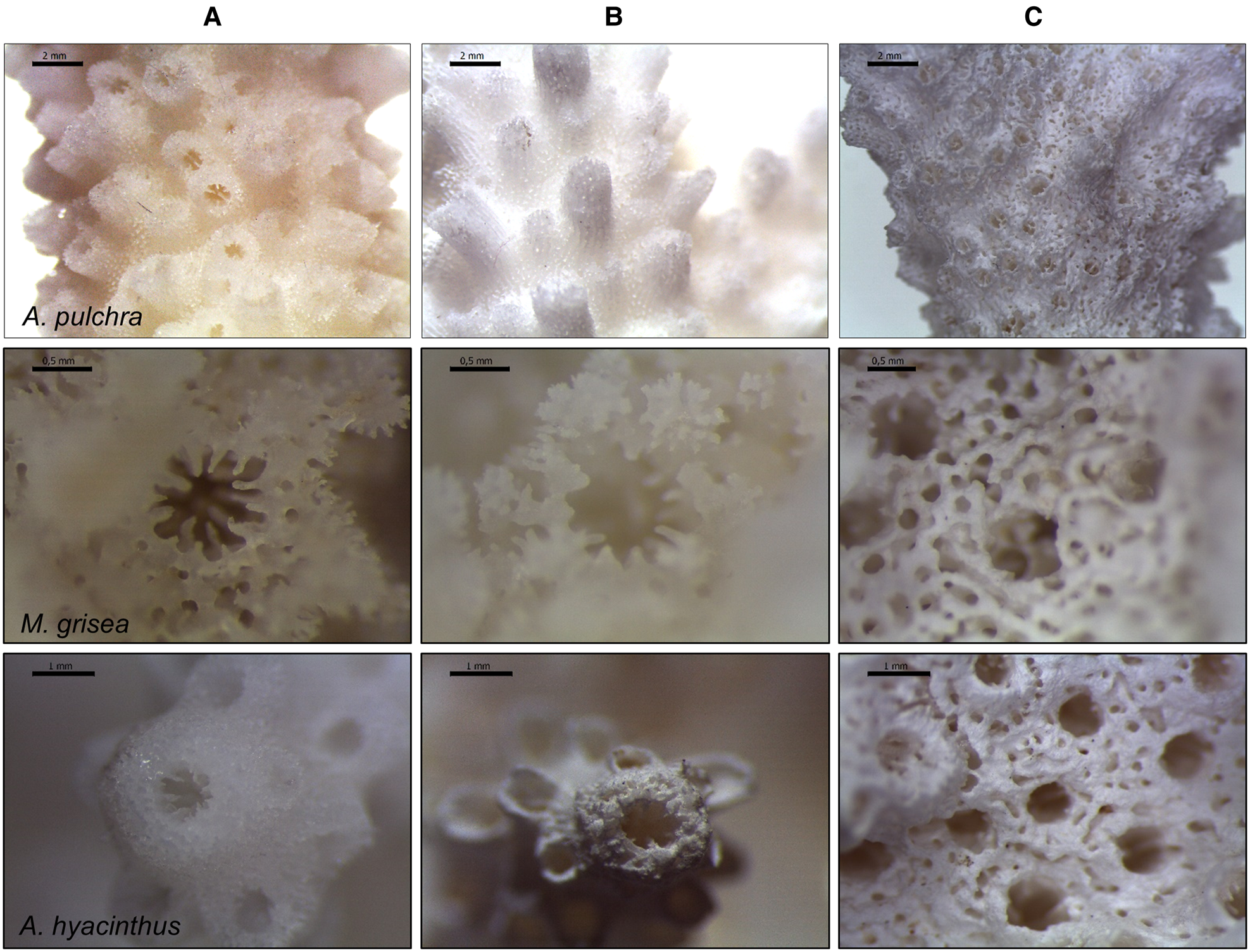
Fig. 2. Microscopy images of the calcium carbonate (CaCO3) skeleton of Acropora pulchra (scale bar: 2 mm), Montipora grisea (0.5 mm) and Acropora hyacinthus (2 mm). Comparison between one individual in the ambient pH (A) and one individual showing tissue necrosis in the extreme low pH, with focus on live portions (covered by living tissues, B) and on dead portions of the skeleton (free from living tissues, C).
Nonetheless, the most striking differences at these pH levels were observed between dead (here, referred to as tissue necrosis) and live skeletal portions (Figure 2B, C; Supplementary Figure S3). Indeed, for a given coral fragment affected by tissue necrosis, comparison of the skeletal structure between coral portions covered by living tissues and uncovered portions (free from living tissues; directly exposed to the acidified medium) showed that dead skeletal surface appeared smoother than the skeleton covered by living tissues. They suffered from severe deformities including the absence of sections of the skeleton, the almost complete disappearance of the calyx septa and the loss of 3D complexity of the corallites (e.g. A. pulchra; Figure 2C), illustrating the corrosive effects of extreme low pH on the calcareous skeletons.
Photo-physiological performance
Overall, there were limited effects of seawater pH on the photosynthetic efficiency of the seven studied species (Figure 1C; Supplementary Table S1). At low pH, only photo-physiological performances of A. pulchra were impacted (6% decrease in Fv/Fm related to ambient; Tukey test, P = 0.006) while, at extreme low pH, photo-physiological performances of P. cactus and P. verrucosa were affected with an average reduction by 25.6% and 14.2% respectively, compared with the ambient (Tukey test, P = 0.0001 and P = 0.01, respectively).
After 48 days, distinct trends of colour index were observed between species when corals were incubated at extreme low pH compared with ambient conditions (Figure 1D): an increased value for A. hyacinthus (Tukey test, P = 0.02), a decrease for P. cactus (P = 0.002) and no changes for all other species (P > 0.05; Supplementary Table S1). For P. verrucosa, an increase of colour index was noted in the extreme low pH compared with the low pH (P = 0.049).
Observation of the kinetics of the Fv/Fm over time did not reveal marked differences in trend between treatments for any species, except for P. cactus for which a reduction was observed after 20 days in the extreme low pH treatment (Supplementary Figure S4). Species showed more important variations of response for the colour index, with kinetics revealing three main trends (Supplementary Figure S5): (i) an increase over time when corals were exposed to extreme low pH levels (A. hyacinthus, P. verrucosa); (ii) a similar trend over time in all pH treatments (A. cytherea, L. pruinosa) or (iii) a decrease over time under low (A. pulchra) and extreme low (A. pulchra, P. cactus) pH levels. For M. grisea, the trend is less clear as it is similar in all treatments until about day 30 when the variability of response between nubbins starts to increase under low and extreme low pH treatments (Supplementary Figure S5).
Discussion
A clear understanding of coral species-specific responses to low pH is an important first step to assess how assemblage of species from a given environment will perform under future ocean acidification (Vargas et al., Reference Vargas, Lagos, Lardies, Duarte, Manríquez, Aguilera, Broitman, Widdicombe and Dupont2017). Here, we reported that all coral species (except Montipora grisea) from the Mo'orea lagoon were resilient to a reduced pH of 7.7, as expected for the near-future. The only significant effects at this pH level were an increased necrosis in L. pruinosa, a reduced RGR in M. grisea and a decreased Fv/Fm in A. pulchra. Thus, our data suggest that the coral landscape of Mo'orea may not be as sensitive to near-future ocean acidification alone.
Nonetheless, the incubation of corals under the extreme low pH scenario revealed species-specific responses with the observed sensitivity of certain species that contrasts with the tolerance of others. All tested species showed significant necrosis while exposed to pH 7.3. However, species-specific responses were observed for all other parameters. Acropora pulchra appeared to be the most sensitive and was the only species experiencing 40% mortality after a 48-day exposure to extreme low pH. On the other end of the spectra, P. verrucosa had a significantly reduced Fv/Fm but was the only species with no significant negative effect on its RGR. Negative relationships between coral growth/calcification and OA are widespread in coral studies (e.g. P. damicornis, Bahr et al., Reference Bahr, Jokiel and Rodgers2016; Putnam et al., Reference Putnam, Davidson and Gates2016; Comeau et al., Reference Comeau, Cornwall and McCulloch2017a; DeCarlo et al., Reference DeCarlo, Comeau, Cornwall and McCulloch2018; M. capitata, Jokiel et al., Reference Jokiel, Rodgers, Kuffner, Andersson, Cox and Mackenzie2008; Anderson et al., Reference Anderson, Kuffner, Mackenzie, Jokiel, Rodgers and Tan2009; Putnam et al., Reference Putnam, Davidson and Gates2016). However, there is also increasing evidence that this pattern is less ubiquitous than previously thought, with a variety of coral species that appear insensitive to OA (e.g. for the genus Porites, Comeau et al., Reference Comeau, Carpenter, Nojiri, Putnam, Sakai and Edmunds2014b; Barkley et al., Reference Barkley, Cohen, McCorkle and Golbuu2017; Sekizawa et al., Reference Sekizawa, Uechi, Iguchi, Nakamura, Kumagai, Suzuki, Sakai and Nojiri2017; Yuan et al., Reference Yuan, Guo, Cai, Huang, Zhou and Liu2019). The resilience of the genus Pocilloporidae to OA in French Polynesia (i.e. calcification unaffected by decreasing pH) has been previously highlighted by several experimental studies (Comeau et al., Reference Comeau, Carpenter, Nojiri, Putnam, Sakai and Edmunds2014b, Reference Comeau, Cornwall and McCulloch2017a, Reference Comeau, Cornwall, DeCarlo, Doo, Carpenter and McCulloch2019; Edmunds et al., Reference Edmunds, Doo and Carpenter2019). Comeau et al. (Reference Comeau, Carpenter, Nojiri, Putnam, Sakai and Edmunds2014b) reported the high resistance for P. damicornis in response to short-term OA exposure (pH up to 7.71) across a large spatial scale (Mo'orea, Hawai'i and Okinawa). Comeau et al. (Reference Comeau, Cornwall, DeCarlo, Doo, Carpenter and McCulloch2019) also noted that P. verrucosa, after a year-long exposure to increased pCO2 conditions (1500 μatm), was able to maintain its calcification rate despite a decrease in the pH of the calcifying fluid (pHCF) and they also reported an increase of calcium concentrations in this compartment. The latter observation has been suggested as a mechanism used by the genus Pocilloporidae to maintain constant precipitation of calcium carbonate despite decreasing pHCF (DeCarlo et al., Reference DeCarlo, Comeau, Cornwall and McCulloch2018).
Despite a significantly lower growth rate under extreme low pH compared with ambient conditions, A. hyacinthus was the only other species able to maintain a positive growth at pH 7.3. This moderate sensitivity was associated with a significant increase in colour index (i.e. zooxanthella density). This could provide additional energy to partly compensate for the costs associated with exposure to lowered pH conditions, although not enough to counteract the cost of maintaining a constant coral growth under extreme low pH as observed for P. verrucosa. The faster growth rate of A. hyacinthus under ambient conditions compared with P. verrucosa (by a factor of 4.9) is potentially a disadvantage under OA scenarios. Indeed, it is expected that fast-growing species will have higher energetic requirements relative to slow-growing species, given that they will need to export larger quantities of protons from the site of calcification (Rodolfo-Metalpa et al., Reference Rodolfo-Metalpa, Martin, Ferrier-Pagès and Gattuso2010; Comeau et al., Reference Comeau, Edmunds, Spindel and Carpenter2014a). This may explain the higher sensitivity of A. hyacinthus to decreasing pH compared with P. verrucosa.
All other five species showed a significant skeletal dissolution (negative RGR) when exposed to extreme low pH. Among them, A. pulchra and M. grisea harboured a striking decline in RGR (−86 and −120% compared with control, respectively). In comparison, Comeau et al. (Reference Comeau, Edmunds, Spindel and Carpenter2013) showed a 9% decline in area-normalized calcification on A. pulchra at pH 7.8 vs 8.05. These dissolutions of the calcareous skeletons of the nubbins might also explain the increase of alkalinity in the low and extreme pH compared with ambient conditions in our experiment. Indeed, the dissolution of calcium carbonate (CaCO3) in seawater increases the concentration of bicarbonate and carbonate ions, which in turn increases the alkalinity.
While the skeletal dissolution, observed for some species in our study, has been previously documented (Fine & Tchernov, Reference Fine and Tchernov2007; Kvitt et al., Reference Kvitt, Kramarsky-Winter, Maor-Landaw, Zandbank, Kushmaro, Rosenfeld, Fine and Tchernov2015), the dissolution can be, to some extent, attributable to the partial loss of living tissues covering coral skeletons. Microscopy observations of coral skeletons revealed that the direct contact of the non-covered skeleton with extreme pH seawater profoundly damaged its structure and morphology (e.g. calice, septum, corallite being smoother than those of the control treatment). In contrast, the presence of living tissues on the skeleton of the same individual limited this impairment. This highlights the protective role of coral tissues against adverse pH conditions, by creating a barrier between seawater and the calcified structure, which limits skeletal dissolution. It also supports a limited number of studies showing the beneficial and protective role of living tissues under reduced pH in other anthozoans: the temperate coral Cladocora caespitosa (Rodolpho-Metalpa et al., Reference Rodolpho-Metalpa, Houlbrèque, Tambutté and Hall-Spencer2011) and the octocoral Ovabunda macrospiculata (Gabay et al., Reference Gabay, Fine, Barkay and Benayahu2014).
The photosystem II (PSII) of the zooxanthellae inside coral tissues (measured by the photosynthetic efficiency, Fv/Fm) was only altered at low pH for A. pulchra. This suggests that, for most coral species, the productivity of the symbionts and the photosynthates available remained unchanged over the time course of the experiment, which is in line with a wide range of studies (e.g. for A. cervicornis, Enochs et al., Reference Enochs, Manzello, Carlton, Schopmeyer, Van Hooidonk and Lirman2014; Bedwell-Ivers et al., Reference Bedwell-Ivers, Koch, Peach, Joles, Dutra and Manfrino2017; Bielmyer-Fraser et al., Reference Bielmyer-Fraser, Patel, Capo and Grosell2018; for M. digitata, Biscéré et al., Reference Biscéré, Rodolfo-Metalpa, Lorrain, Chauvaud, Thébault, Clavier and Houlbrèque2015; Tambutté et al., Reference Tambutté, Venn, Holcomb, Segonds, Techer, Zoccola, Allemand and Tambutté2015; Nakamura et al., Reference Nakamura, Iguchi, Suzuki, Sakai and Nojiri2017) and corroborates previous works on similar lagoon species from Mo'orea (A. pulchra and P. cactus, Comeau et al., Reference Comeau, Carpenter and Edmunds2017b; P. verrucosa, Edmunds & Burgess, Reference Edmunds and Burgess2016; Comeau et al., Reference Comeau, Carpenter and Edmunds2017b; Evensen & Edmunds, Reference Evensen and Edmunds2017). Negative effects of extreme low pH treatment on coral photophysiology have been reported previously (Crawley et al., Reference Crawley, Kline, Dunn, Anthony and Dove2010; Krief et al., Reference Krief, Hendy, Fine, Yam, Meibom, Foster and Shemesh2010). Coral host cells can actively modulate the physiology of their algal symbionts as they contribute to the low pH (~4) in the symbiosome enveloping the algae, through host H+-ATPase (VHA) activity (Barott et al., Reference Barott, Venn, Perez, Tambutté and Tresguerres2015). The reduction of the VHA activity by the host under stressful conditions may significantly decrease H+ activity in the symbiosome, which may ultimately alter the exchange of compounds between each compartment (symbiosome lumen, coral cytoplasm and algal cytoplasm) and therefore affect symbiont photosynthesis, through reduced DIC supply (Barott et al., Reference Barott, Venn, Perez, Tambutté and Tresguerres2015).
Under extreme low pH exposure, photosynthetic efficiency was negatively impacted in P. cactus and P. verrucosa and remained unaltered in all five other species. The decrease in photosynthetic efficiency in P. cactus was associated with a decrease in colour index (i.e. loss of zooxanthellae). However, it was accompanied by an increased colour index compared with low pH condition in P. verrucosa, suggesting that other mechanisms/strategies are at play. Similarly, A. hyacinthus also showed an increased colour index under extreme low pH compared with ambient conditions, but its strategy here may benefit the organism under extreme conditions, taken that no differences in photosynthetic efficiencies were reported.
Several hypotheses have been proposed to explain species-specific responses based on coral functional traits (Comeau et al., Reference Comeau, Edmunds, Spindel and Carpenter2014a; Barner et al., Reference Barner, Chan, Hettinger, Hacker, Marshall and Menge2018), such as corallum morphology, skeletal porosity (Jokiel, Reference Jokiel2011) or calcification rate (Rodolpho-Metalpa et al., Reference Rodolpho-Metalpa, Houlbrèque, Tambutté and Hall-Spencer2011). It was proposed that mounding corals will be more resilient to reduced pH than branching corals because they interact differently with the environment (Jokiel, Reference Jokiel2011). This was challenged by our results as the two mounding species here (M. grisea and L. pruinosa) were more sensitive than the branched P. verrucosa. Comeau et al. (Reference Comeau, Carpenter, Nojiri, Putnam, Sakai and Edmunds2014b) also rejected this hypothesis with substantial effects observed in Psammocora profundacella. Similarly, if we look at the two types of skeleton encountered in corals (perforate, with tissues deeply into the skeleton and imperforate, with superficial tissues), it was initially assumed that corals with perforate skeletons may be less sensitive to decreased pH as they export protons more efficiently from the calcification site than imperforate ones (Jokiel, Reference Jokiel2011). Here, P. verrucosa and P. cactus, two species with imperforate skeletons, showed highly contrasted responses to reduced pH with P. cactus being less tolerant than P. verrucosa. Moreover, all perforate species (Acropora sp. and M. grisea) were highly impacted (notable declines in RGR) under the extreme low pH level, while this was not the case in the imperforate P. verrucosa. Finally, the suggestion that slow calcifiers will be less affected by exposure to low pH than fast calcifiers was partly discussed earlier. While the two species with the fastest calcification rate in our data, A. hyacinthus and A. pulchra, follow this trend, it is not as clear for the two slowest with dissolution observed for L. pruinosa and no effects for P. verrucosa under the extreme low pH. From these results, it is difficult to select one biological trait as a sole factor able to explain the interspecific variability in response to low pH conditions.
Local adaptation to present natural variability is another factor explaining species-specific response to OA (Vargas et al., Reference Vargas, Lagos, Lardies, Duarte, Manríquez, Aguilera, Broitman, Widdicombe and Dupont2017). Organisms that have evolved under highly variable environmental conditions are likely to be adapted to these local conditions and may have the physiological capacity to tolerate changing conditions. Based on Jensen's inequality, temporal variation in pH conditions can have predictable biological consequences that cannot be inferred from mean environmental conditions (Ruel & Ayres, Reference Ruel and Ayres1999). To determine the response of organisms to future pH levels, it is thus important to consider both exposure time and the magnitude of CO2 levels (Shaw et al., Reference Shaw, Munday and McNeil2013).
Environmental variability of pH was not characterized at our study site but Rivest & Gouhier (Reference Rivest and Gouhier2015) showed strong and consistent daily fluctuations on another site of the fringing reef of the north shore of French Polynesia. They recorded mean pH ± SD of 7.989 ± 0.038 with a minimum pH value of 7.84 and a maximum of 8.07, over about 3 months. In our study, the weak effects observed after 48 days under low pH (7.7) may be in part attributed to local adaptation to these pH fluctuations that are already going as low as 7.84. Nonetheless, the diversity in responses observed between species collected within the same coral assemblage (and thus exposed to identical levels of pH variability), highlights the importance of other factors (than all aforementioned hypotheses).
Conclusion
Our study revealed that seven common and abundant coral species from the lagoon of Mo'orea are resilient to 48 days of exposure to near-future pH conditions (pH 7.7). Despite this, understanding the impact on the reef landscape would require a more realistic design (including ecological interactions, long exposure time, fluctuating conditions, etc.), thus also considering other drivers and stressors that can modulate the effects of OA on corals or coral assemblages (Ban et al., Reference Ban, Graham and Connolly2014). Exposure to extreme low pH (7.3) revealed a diversity of responses in the seven tested species. Acropora pulchra was the only species experiencing increased mortality and all species experienced other sub-lethal negative effects. The host metabolism (tissue necrosis, relative growth rate) was affected to a larger extent while the photosynthetic activity of the algal symbionts (photosynthetic efficiency, colour index) remained rather unaffected by seawater acidification. Pocillopora verrucosa, the most abundant species in both the lagoon and the fore reef of Mo'orea (the genus Pocillopora represented 20.7% of the coral abundance in 2017; Edmunds, Reference Edmunds2018) appeared to be the most tolerant and was able to maintain its RGR, which is encouraging for the survival of this genus in the future. Altogether, the high interspecific variability of responses observed in seven species from the same assemblage supports that it would be overly simplistic to assume that biological traits or local adaptation are sufficient to allow determining the response of a coral species to decreased pH conditions.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0025315421000618
Financial support
This work was funded by the Foundation de France for a project called ‘ACID REEFS’, by Ministère de la Transition Écologique et Solidaire and the Foundation for Research on Biodiversity (FRB) for a project entitled ‘ACID REEFS’.