INTRODUCTION
Cirripedes play an important role in the structure and function of intertidal ecosystems. Cirripedes are the most successful fouling animals because of their prolific settlement and wide distribution (Khandeparker & Anil, Reference Khandeparker and Anil2007; Maréchal & Hellio, Reference Maréchal and Hellio2011). Their life cycle includes six nauplius and one non-feeding cypris larval stages (Høeg & Møller, Reference Høeg and Møller2006). Cirripede cyprids are adapted to locate suitable substratum for attachment and metamorphosis to juvenile barnacles (Lagersson & Høeg, Reference Lagersson and Høeg2002; Aldred & Clare, Reference Aldred and Clare2008; Maruzzo et al., Reference Maruzzo, Conlan, Aldred, Clare and Høeg2011). Cypris attachment and metamorphosis is also referred to as ‘cypris settlement’ (Clare & Matsumura, Reference Clare and Matsumura2000). Metamorphosis is defined as the subsequent sequence of morphological, physiological and biochemical events that transform a permanently attached cyprid into a sessile filter feeding juvenile (Thiyagarajan, Reference Thiyagarajan2010).
Although the substratum selection, attachment and settlement mechanisms of cirripede cyprids have been extensively studied, little attention has been given to the intervening and critical series of metamorphic events from cyprid to juvenile barnacle that follow cypris settlement (Høeg & Møller, Reference Høeg and Møller2006; Anil et al., Reference Anil, Khandeparker, Desai, Baragi and Gaonkar2010; Thiyagarajan, Reference Thiyagarajan2010).
The few early observations on the morphological changes in balanomorphan metamorphosis either did not use laboratory-reared animals or failed to provide precise timing of metamorphic events (Doochin, Reference Doochin1951; Daniel, Reference Daniel1958). Walley (Reference Walley1969) described the detailed histological changes of Semibalanus balanoides metamorphosis by relying on field-sampled specimens without determining the precise sequence or age. Glenner & Høeg (Reference Glenner and Høeg1993, Reference Glenner and Høeg1998) and Takenaka et al. (Reference Takenaka, Suzuki, Yamamoto, Yamamoto and Yoshi da1993) focused on specific organs rather than the overall course of the metamorphosis of laboratory-reared cirripedes. Only a few studies provide timing of metamorphic events. Kühl (Reference Kugele and Yule1950) divided the metamorphosis of Amphibalanus improvisus into a series of characteristic phases. Maruzzo et al. (Reference Maruzzo, Aldred, Clare and Høeg2012) presented an accurate timeline and detailed description of morphological events of cyprid to juvenile barnacle in the model species of A. amphitrite (Darwin, 1854). Høeg et al. (Reference Høeg, Maruzzo, Okano, Glenner and Chan2012) observed the cypris metamorphosis and described the timing of the overall series of events in four cirripede species including the suspension-feeding pedunculate (Lepas sp. and L. anserifera), sessile Thoracica (Megabalanus rosa) and parasitic Rhizocephala (Sacculina carcini). At present, only two accurate descriptions of metamorphosis in pedunculated barnacles of Thoracica are available (Høeg et al., Reference Høeg, Maruzzo, Okano, Glenner and Chan2012).
Capitulum mitella (Linnaeus, 1758), formerly assigned to the genus Pollicipes (Leach, 1817), is a dominant intertidal cirripede (Lee et al., Reference Lee, Shim and Kim2000). The soft part of C. mitella has been a seafood source in China, thus its economic value has led to increased fishery pressure and declining populations. The pedunculate cirripede Pollicipes is of considerable economic importance in Europe, particularly in Spain and Portugal (Borja et al., Reference Borja, Muxika and Bald2006; Jacinto et al., Reference Jacinto, Cruz, Silva and Castro2010). Although numerous studies have focused on the biology and ecology of Pollicipes species, inducing the attachment and metamorphosis of Pollicipes cyprids in a laboratory setting remains a challenge (Kugele & Yule, Reference Kühl1996). These cyprids cannot settle to juvenile barnacles and complete their life cycle under laboratory conditions, thereby hindering further research.
Within the last two decades, our research group has dealt with the ecology, reproduction, gametogenesis, embryonic development, larval development and culture conditions of C. mitella. Recently, we described the cypris morphology of C. mitella in detail (Rao & Lin, Reference Rao and Lin2014) and successfully induced its metamorphosis into juvenile barnacle after artificial exposure to Juvenile hormone III. However, an in-depth understanding of this metamorphosis process has not been established.
In this study, the metamorphosis of laboratory-reared cyprids of the pedunculate cirripede, C. mitella was successfully induced in a laboratory setting. A timeline and a detailed description of the morphological events during the metamorphosis were derived from light microscopy and scanning electron microscopy(SEM) analyses. Results of this study provide the basis for understanding the process of cyprid metamorphosis and settlement ecology of C. mitella.
MATERIALS AND METHODS
Induction of metamorphosis
Adult specimens of Capitulum mitella were collected in August 2014 in the intertidal zone of Dinghai, Fuzhou, Fujian, China (26°16′N 119°48′E). Cyprids were reared according to the method established by Rao & Lin (Reference Rao and Lin2014). Juvenile hormone III (JH-III) (purity ≥ 65%) was purchased from Sigma Chemical Company. Assay was carried out in a 100 ml beaker containing 50 ml filtered seawater of 1 µg ml−1 JH-III. Two hundred cyprids (3–5 days old) were added to the beaker up to 4 days to complete metamorphosis. Assay was replicated three times. The experimental conditions were maintained in the dark at 25 ± 1°C with a salinity of 28. The metamorphosis process of cyprid to juvenile barnacle was continuously followed, photographed and timed using light microscope and dissection microscope. The process was observed for 4–5 days until cyprids fully metamorphosed into juveniles. Further development of the juveniles were observed until completion of the fourth moult.
Scanning electron microscopy (SEM)
Representative specimens of the newly metamorphosed, the first moulted, the second moulted, the third moulted and the fourth moulted juveniles were collected. The cypris carapaces of the specimens were separated using acupuncture needles under binocular dissecting microscope. The specimens were fixed in 2.5% glutaraldehyde (in seawater) for 2 h, rinsed with distilled water for 30 s for SEM analysis, post-fixed in 1% OsO4, dehydrated in a graded series of ethanol, and then critical-point dried with liquid CO2. Dried juveniles were mounted on carbon stubs with conducting carbon cement and then sputter-coated with gold. Images of the samples were obtained using a JEOL JSM-6380LV SEM operated at 15 kV.
RESULTS
After exposure to the reagent, a majority of the cyprids metamorphosed into juvenile barnacles without cementing to the substratum, while the minority of cyprids attached and metamorphosed into juvenile barnacles. The process of metamorphosis of unattached and attached cyprids did not differ in terms of time sequence, morphological changes and duration of metamorphosis. Hence the results presented here were for the unattached cyprids only. The morphological changes of the cyprids were evident through cypris carapaces. The process was observed by light microscopy to follow the metamorphic events and timing.
Analysis of cypris metamorphosis using a light microscopy
METAMORPHIC EVENTS AND THEIR TIMING
Phase 1–Triggering of metamorphosis
After 0–3.5 h of reagent exposure, a majority of cyprids moved up and down or slid on the surface of the water (Video S1), whereas the minority of cyprids explored the bottom of the container by walking bipedally on the antennules (Video S2). After 3.5 h of reagent exposure, the cyprids gradually weakened swimming ability and sank to the bottom of container; however, the thoracopods and antennules were repetitively extended and retracted (Video S3). After 5 h of reagent exposure, a majority of cyprids ceased swimming and sank to the bottom of container. The thoracopods were entirely contained in the carapace, or with their setae slightly protruded out of the carapaces (Figure 1A). The antennules retracted in front of the body and occasionally shook slightly.

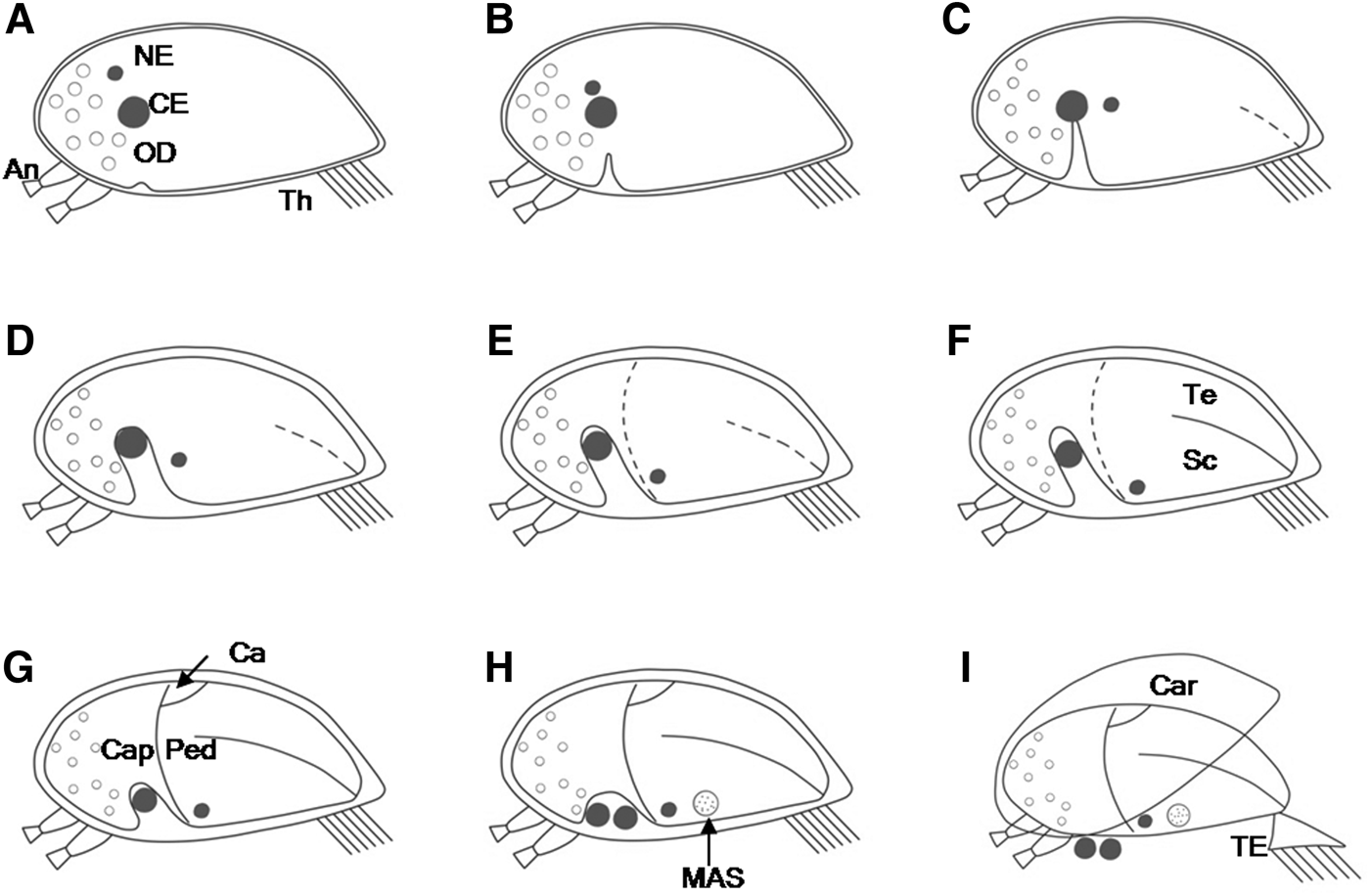
Fig. 1. Events of cypris metamorphosis in Capitulum mitella under light microscopy: (A) at 3.5–5 h of reagent exposure, the cyprid weakens swimming ability and finally ceases swimming; (B) at 6 h, the epidermis ventral to the compound eyes begins to invaginate slightly (black arrow); (C) at 12 h, the invagination of the epidermis continues (black arrow), the black nauplius eye begins to move ventrally; (D) at 24 h, the invagination extends to the compound eyes, the nauplius eye continues to move ventrally, the first sign of shell-plate formation is appearance of a brown boundary (white dotted line); (E) at 36 h, the invagination bypasses the compound eyes and inclines to the top of the head, the nauplius eye migrates to the ventral of the metamorphosing specimen (white dotted line showing the boundary of shell plates); (F) at 48 h, the invagination is at its highest degree, the larval shape slightly resembles that of a juvenile (black arrow showing border of capitulum and peduncle); (G) at 60 h, the shell-plates and border are more apparent (black arrow); (H) at 72 h, the invagination gradually becomes shallow, the juvenile rudiment has form. An, antennule; CE, compound eye; NE, nauplius eye; Sc, scutum; Te, tergum; Th, thoracopod. Scale bar = ~100 µm.
After 6 h of reagent exposure, the epidermis ventral to the compound eyes began to invaginate slightly, which was an indicator of triggering metamorphosis. Yellow oil droplets began to gather in the anterior part of cyprids at this time (Figure 1B, 3A).
Phase 2–Invagination and separation of epidermis
After 12 h of reagent exposure, the invagination continued and all cyprids were almost quiescent and immobile. The black nauplius eye began to move ventrally (Figure 1C, 3B).
After 24 h of reagent exposure, the invagination extended to the compound eyes and the epidermis of whole cyprid began to separate slightly from the carapace while oil droplets became small (Figure 1D, 3C). The nauplius eye continued to move ventrally. The first sign of shell-plate formation was appearance of a brown boundary at the posterior end of cyprid.
After 36 h of reagent exposure, the invagination bypassed the compound eyes and inclined to the top of the head (Figure 1E, 3D). The boundary of shell plates got longer and more obvious. Scutum and tergum developed beneath the cypris carapace. The nauplius eye migrated to the ventral of the metamorphosing specimen. The separation of the epidermis from the carapace was evident; the shape of whole specimen was different from that of a cyprid.
Phase 3–Formation of juvenile rudiment
After 48 h of reagent exposure, the invagination was at its highest degree at this time, and the compound eyes were completely separated from the metamorphosing specimen. The specimen emerged vaguely at the incipient border of capitulum and peduncle, situated at approximately a third of the body length from the anterior end. The scutum and tergum rudiment appeared beneath the carapace. The specimen shape slightly resembled that of a juvenile (Figure 1F, 3E).
After 60 h of reagent exposure, the metamorphosis further progressed as the border and shell-plates became more apparent (Figure 1G, 3F). The peduncle region was yellow and the capitulum region was brown. The peduncle was hook-shaped for the invagination existence, and the oil droplets became more vague and smaller.
After 72 h of reagent exposure, the peduncle and capitulum were clear. The scutum and tergum gradually became thicker, while the carina appeared. The internal region of the capitulum was opaque and the invagination of the peduncle gradually became shallow (Figure 1H, 3G).
Phase 4–Shedding of thoracopodal exuvium
After 84 h of reagent exposure, the metamorphosing cyprid began to assume a perfect juvenile barnacle outline (Figures 2A, 3H). The tergum, scutum and carina of the capitulum were clear, whereas the rostrum and upper latus were vaguely visible. The musculus adductor scutorum and nauplius eye were visible through the shell-plates. The hook-shaped peduncle gradually became a short column, while the compound eyes gradually were expelled to the side of the peduncle.

Fig. 2. Events of cypris metamorphosis in Capitulum mitella under light microscopy: (A) at 84 h, the forthcoming metamorphic cyprid assumes a perfect juvenile barnacle outline; (B) at 96 h, the cyprid sheds the thoracopodal exuvium and metamorphoses into a juvenile barnacle; (C) the cirri of a newly formed juvenile extend out of the shell-plates; (D) the shed thoracopodal exuvium; (E) the shed cirrum cuticle for the first time; (F) the second moulted juvenile and shed cirrum cuticle (black arrow); (G) the third moulted juvenile and shed cirrum cuticle (black arrow); (H) the fourth moulted juvenile and shed cirrum cuticle (black arrow). An, antennule; CE, compound eye; Cap, capitulum; Car, carapace; Ci, cirri; MAS, musculus adductor scutorum; NE, nauplius eye; Th, thoracopod. Scale bar = ~100 µm.

Fig. 3. Schematic drawing of major events in metamorphosis of Capitulum mitella, detailed explanations refer to the captions of Figures 1 & 2: (A) at 6 h; (B) at 12 h; (C) at 24 h; (D) at 36 h; (E) at 48 h; (F) at 60 h; (G) at 72 h; (H) at 84 h; (I) at 96 h. An, antennule; CE, compound eye; Cap, capitulum; Car, carapace; Ca, carina; MAS, musculus adductor scutorum; NE, nauplius eye; OD, oil droplet; Ped, peduncle; Sc, scutum; Te, tergum; Th, thoracopod; TE, thoracopod exuvium.
After 96 h of reagent exposure, the majority of cyprids had moulted and metamorphosed into juveniles (Figure 2B, C, 3I). The anterior end of the ventral carapace margin was splayed by the metamorphosing specimen, and the juvenile cirri, still encased in the cuticle of cypris thoracopods, stretched out of the shell-plates and pushed against the cuticle of cypris thoracopods. The thoracopodal exuvium was separated from the metamorphosing specimen (Figure 2D), and the juvenile stretched out of the cypris carapace. Finally the cyprid metamorphosed into a juvenile barnacle. The cypris carapace and the compound eyes were shed but remained loosely around the basal part of the juvenile. The cyprid shedding of the thoracopodal exuvium marked transition from the cyprid to the first instar juvenile.
Phase 5 – Early development of juvenile barnacle
The juvenile cirri shed their cuticle for the first time at 1.5–2 days after juvenile formation (Figure 2E). At 3–4 days after juvenile formation, the cirri shed their cuticle for the second time (Figure 2F). At 7–9 days, the cirri shed their cuticle for the third time and the juvenile immediately commenced suspension feeding with its cirri (Figure 2G, Video S4). At this stage, the thoracopods began to extend as a basket of cirri. At 12–15 days, the cirri shed their cuticle for the fourth time (Figure 2H). The carapace and the paired compound eyes of cyprid fell away after a period of time in water current; however, a pair of spent antennules remained for a longer period of time.
Morphology of a juvenile barnacle
The newly formed juvenile completely assumed the shape and armature of all shell-plates found in adult specimens (Figure 4). Tergum, scutum and upper latus were paired, and carina and rostrum were unpaired. Rostro-carnal length of the juvenile was 240–260 µm. However, some traces of the cypris structure still remained. The cypris carapace was shed but remained loosely attached at the peduncle sides, the compound eyes and the pair of spent antennules still attached to the ventral (rostral) side of the peduncle base. The musculus adductor scutorum and nauplius eye were clearly seen through the scutum. The cirri could extend out of the shell-plates to move (Video S5). The column-shaped peduncle still contained a few oil droplets. The peduncle was very flexible (Figure 2B, C) and could contract, bend and extend its length (Video S6).
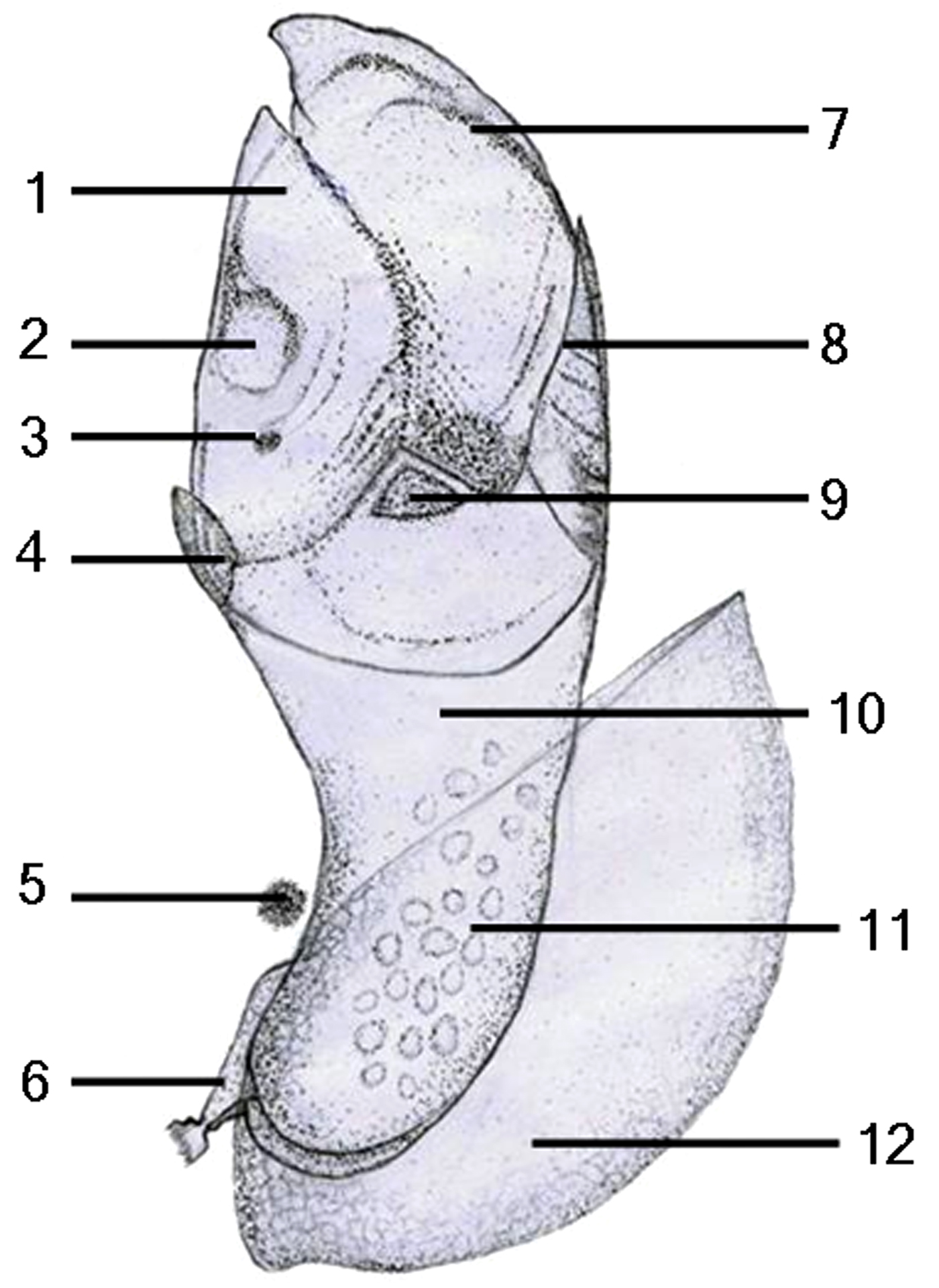
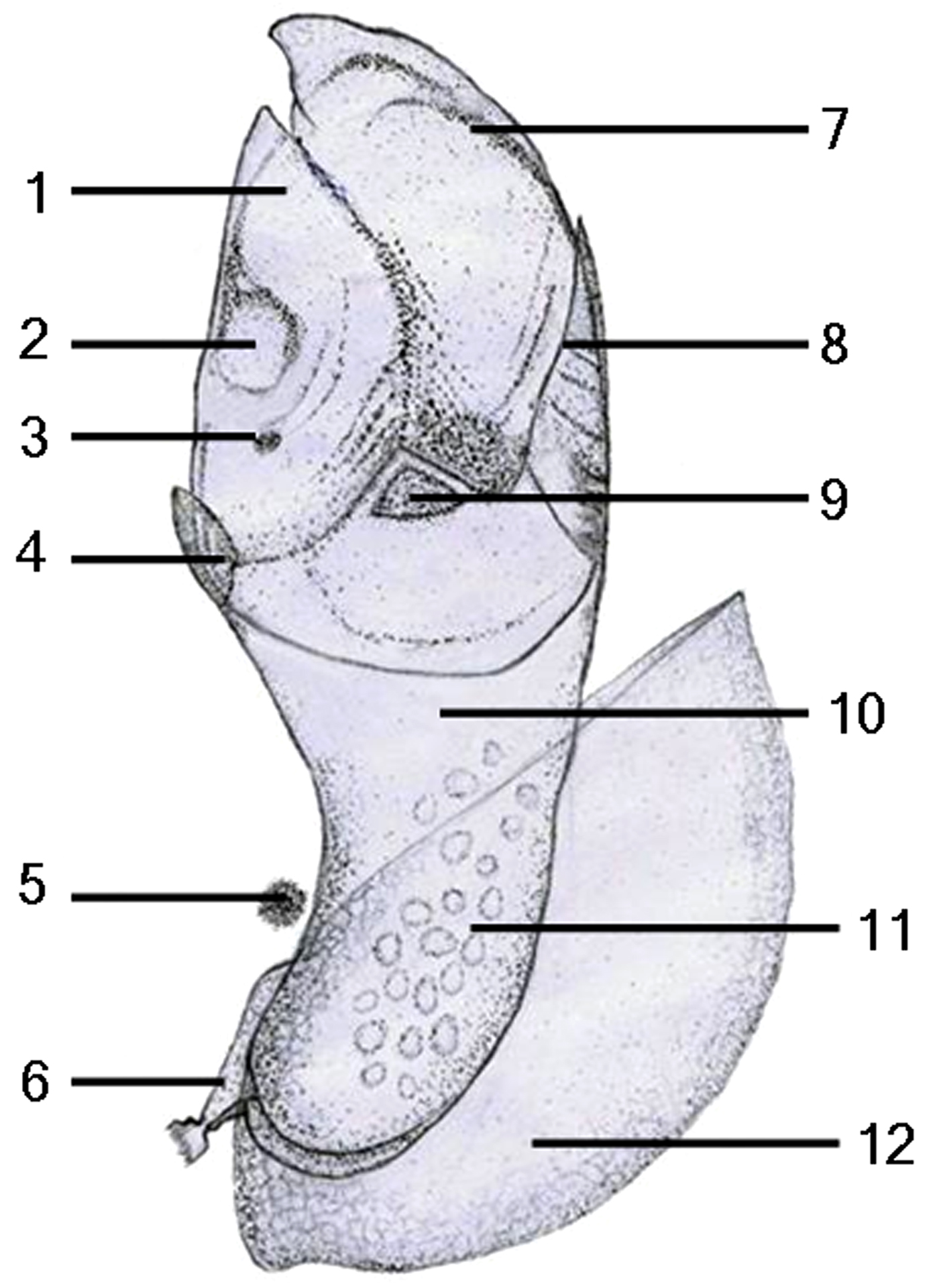
Fig. 4. Schematic drawing of the newly formed juvenile in Capitulum mitella: 1, scutum; 2, musculus adductor scutorum; 3, nauplius eye; 4, rostrum; 5, compound eye; 6, antennule; 7, tergum; 8, carina; 9, upper latus; 10, peduncle; 11, oil droplet; 12, carapace.
SEM analysis of cypris metamorphosis
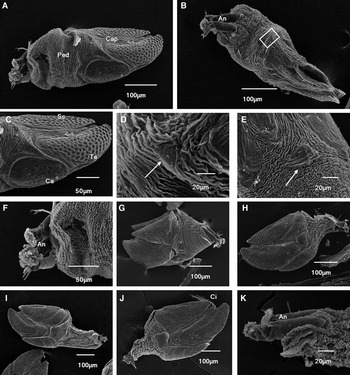
The morphology of the newly formed juvenile was observed by SEM. The juvenile consisted of capitulum and peduncle (Figure 5A, B). The shell-plates of the capitulum had developed, and included unpaired carina and rostrum and paired tergum, scutum and upper latus. Uniform circular depressions were distributed on the surface of the tergum, scutum and carina (Figure 5C). A few small socketed setae were also scattered on the depressions. The rostrum (Figure 5D) and upper latus (Figure 5E), which had the shape of small triangle, were not clear. The entire surface of the peduncle was covered with convoluted cuticular ridges (Figure 5F). Intact cyprid antennules were retained on the ventral (rostral) side of the base of the juvenile peduncle (Figure 5F).

Fig. 5. SEM analysis of the newly formed juvenile and early development of the juvenile barnacle in Capitulum mitella: (A) dorsal-lateral view of the newly formed juvenile; (B) ventral view of the newly formed juvenile; (C) the capitulum showing the scutum, tergum and carina; (D) magnified view of the rostrum (white arrow) from inset box in B; (E) the upper latus (white arrow); (F) the entire surface of the peduncle is covered with convoluted cuticular ridges. A pair of antennules remain attached to the base of the peduncle; (G) the first moulted juvenile; (H) the second moulted juvenile; (I) the third moulted juvenile; (J) the fourth moulted juvenile; (K) the cypris antennules remain attached to ventral view of the peduncle base in the fourth moulted juvenile. An, antennule; Ca, carina; Cap, capitulum; Ci, cirri; Ped, peduncle; Sc, scutum; Te, tergum.
The rostrum and upper latus of the first moulted juvenile were clear (Figure 5G). By comparison, the morphology structure of the second (Figure 5H), third (Figure 5I) and fourth moulted juveniles (Figure 5J, K) showed no significant difference.
DISCUSSION
In this study, the metamorphic events and timing of Capitulum mitella cyprids were observed in detail. The results showed that the metamorphosis of C. mitella was very similar to that of Lepas (Høeg et al., Reference Høeg, Maruzzo, Okano, Glenner and Chan2012). However, our observations were more detailed in some events than those of Høeg et al. (Reference Høeg, Maruzzo, Okano, Glenner and Chan2012). Høeg (Reference Høeg1987) suggested that a slight separation of the epidermis from the cypris cuticle when observed under TEM indicates the initiation of the metamorphic moult. In this observation, the slight invagination of the epidermis ventral to cypris compound eyes was a trigger for the metamorphosis. We believe that in lepadomorph barnacles, shedding of the thoracopodal exuvium rather than the carapace indicates completion of the metamorphosis. However, this process was not clearly described by Høeg et al. (Reference Høeg, Maruzzo, Okano, Glenner and Chan2012). The main difference between both Lepas species is the method of elimination of the cypris carapace (Høeg et al., Reference Høeg, Maruzzo, Okano, Glenner and Chan2012). The cypris carapace is shed from the top in Lepas sp., but from the bottom in L. anserifera. The carapace of L. anserifera remains at the basal position of the peduncle for several days. Lepas anserifera can extend their cirri for feeding while the carapace remains around the base of the peduncle. Capitulum mitella was consistent with L. anserifera in this respect. Lepadomorph Scalpellum scalpellum observation by Glenner & Høeg (Reference Glenner and Høeg1993) showed that the cypris carapace remains loosely attached to the basal part of the peduncle. They also indicated that the juvenile growth is not obstructed by the cypris carapace in lepadomorph barnacles despite being attached for a long time.
Limited observations on the pedunculate barnacles demonstrate that the metamorphosis in lepadomorphs differs from balanomorphans (Glenner & Høeg, Reference Glenner and Høeg1993; Høeg et al., Reference Høeg, Maruzzo, Okano, Glenner and Chan2012; Maruzzo et al., Reference Maruzzo, Aldred, Clare and Høeg2012). A significant difference between lepadomorphs and balanomorphans is that a newly formed juvenile in lepadomorphs (Lepas sp., L. anserifera, S. scalpellum and C. mitella) is equipped with all shell-plates (Glenner & Høeg, Reference Glenner and Høeg1993; Høeg et al., Reference Høeg, Maruzzo, Okano, Glenner and Chan2012). However, shell-plates do not appear until some time after the cypris cuticle has been shed in balanomorphans such as Amphibalanus amphitrite, A. improvisus (Darwin, 1854), Semibalanus balanoides, Austrominius modestus (Darwin, 1854) and Megabalanus rosa (Glenner & Høeg, Reference Glenner and Høeg1993; Høeg et al., Reference Høeg, Maruzzo, Okano, Glenner and Chan2012; Maruzzo et al., Reference Maruzzo, Aldred, Clare and Høeg2012).
Høeg et al. (Reference Høeg, Maruzzo, Okano, Glenner and Chan2012) and Maruzzo et al. (Reference Maruzzo, Aldred, Clare and Høeg2012) concluded that the metamorphic events are related to the ecology of the species for various environmental conditions such as desiccation and predation, and environmental pressures in the intertidal zone might have influenced the metamorphic speed. For intertidal barnacles such as M. rosa, the whole metamorphosis is completed in ~ 6–10 h. By contrast, Lepas adapts to pelagic life and has a slow metamorphosis time lasting up to 3–4 days (Høeg et al., Reference Høeg, Maruzzo, Okano, Glenner and Chan2012). For A. amphitrite, ecdysis is observed at 8–16 h after initial attachment (Glenner & Høeg, Reference Glenner and Høeg1993), with metamorphosing specimens reaching the juvenile stage at 32 h after irreversible attachment (Maruzzo et al., Reference Maruzzo, Aldred, Clare and Høeg2012). In the present study, C. mitella cyprids completed metamorphosis at 3.5–4 days. The metamorphic duration of C. mitella was similar to that of Lepas and was much longer than that of M. rosa and A. amphitrite. Although C. mitella, M. rosa and A. amphitrite are intertidal barnacles, the former settles extensively on rock cracks in lower and upper midlittoral zones. Being as well protected from heat or desiccation as Lepas, C. mitella are able to withstand a long metamorphic duration (up to 3.5–4 days). Our results further supported this conclusion. Besides the ecology of the species, the metamorphic speed may also be related to the type of metamorphosis. In lepadomorphs (Lepas and C. mitella) all shell-plates become visible already beneath the carapace of the settled cyprid, while in balanomorphans the shell-plates do not appear until some time after the cypris cuticle has been shed. Therefore, the metamorphic speed in lepadomorphs is slower than that in balanomorphans. Nevertheless metamorphic observations on more species are necessary to support this viewpoint.
Despite intensive research on the mechanism of attachment and metamorphosis, the physiological mechanisms that regulate the metamorphic process remain unclear. Under normal circumstances, cyprids of C. mitella cannot commence metamorphosis. Thus, observations on metamorphic events and timing of C. mitella presented in this study were chemically induced metamorphosis. However, unattached juvenile or attached juvenile could normally moult and grow, cyprids’ failure to attach did not interfere with the process of metamorphosis. In this study, induced cyprids did not produce cement glue and cyprids were not attached to the substratum, making them far more suitable for sample preparation and analysis. Ecdysis of NA-induced cyprids in A. amphitrite is also considerably longer than in the natural system at 24–48 h post-treatment (Gohad et al., Reference Gohad, Aldred, Orihuela, Clare, Rittschof and Mount2012). Under natural conditions, ecdysis occurs at 8–16 h after initial attachment in A. amphitrite cyprids (Glenner & Høeg, Reference Glenner and Høeg1993). However, the metamorphosis of C. mitella under natural conditions remains unclear. Thus, the cypris metamorphosis of C. mitella under natural and laboratory conditions should be compared.
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at http://dx.doi.org/10.1017/S0025315416001089.
ACKNOWLEDGEMENTS
We are very grateful to Professor Jens Thorvald Høeg of the University of Copenhagen for very helpful comments on the manuscript.
FINANCIAL SUPPORT
This work was funded by the key project of science and technology department of Fujian province, China (2014N0011).