INTRODUCTION
Marine dinoflagellates of the order Dinophysiales Kofoid are a group of thecate and motile forms that are laterally depressed with a sagittal serrate suture extended throughout the body, and show cingular and sulcal lists of variable development. In general the epitheca is reduced whereas the hypotheca is elongated (species of the genus Amphisolenia Stein are extremely elongated forms, reaching more than 1 mm). The thecae have two valves, left and right, and a number of plates, usually 18 with certain exceptions: 6–7 in the epitheca (2 apical and 4 epithecal), four cingular, four sulcal and four hypothecal (Sournia, Reference Sournia1986; Fensome et al., Reference Fensome, Taylor, Norris, Sarjeant, Wharton and Williams1993). The number of recognized species within the order varies from 240 to 382 (Sournia, Reference Sournia and Lassus1995), with the highest diversity found in tropical waters and certain forms are limited to oligotrophic waters (Sournia, Reference Sournia1986), and yet other forms have umbrophylic preferences (Sournia, Reference Sournia1982). Most forms are truly planktonic, but species of Synophysis Nie & Wang have benthic habits (Hernández-Becerril, Reference Hernández-Becerril1988a; Faust, Reference Faust1993; Hoppenrath, Reference Hoppenrath2000). One of the most diverse genera is Dinophysis Ehrenberg, with more than 200 species recognized (Sournia, Reference Sournia1986), due in part to the transfer of species of the genus Phalacroma Stein (Abé, Reference Abé1967a; Balech, Reference Balech1967). Several species of Dinophysis are considered to be toxic for they produce okadaic acid or dinophysistoxin, which cause diarrhoetic shellfish poisoning (DSP) (Lee et al., Reference Lee, Igarashi, Fraga, Dahl, Hovgaard and Yasumoto1989; Godhe et al., Reference Godhe, Svensson and Rehnstam-Holm2002; Taylor et al., Reference Taylor, Fukuyo, Larsen, Hallegraeff and Hallegraeff2003).
Many species of this order are not photosynthetic, but species that are photosynthetic, especially belonging to Dinophysis, contain pigments and chloroplasts of endosymbiotic origin, related to Cryptophyta (Schnepf & Elbrächter, Reference Schnepf and Elbrächter1988, Reference Schnepf and Elbrächter1999), Chrysophyceae or Haptophyceae (Hallegraeff & Lucas, Reference Hallegraeff and Lucas1988), and Prasinophyceae (Berland et al., Reference Berland, Maestrini, Grzebyk and Thomas1995a); furthermore, Schnepf & Elbrächter (Reference Schnepf and Elbrächter1999) have mentioned that chloroplasts in Dinophysiales are not typical of the dinoflagellates. In addition, kleptochloroplasts have been recently reported for the species Dinophysis mitra (Schütt) Abé (Koike et al., Reference Koike, Sekiguchi, Kobiyama, Takishita, Kawachi, Koike and Ogata2005). Various species of Dinophysis are mixotrophs or even heterotrophs, and may develop ‘peduncular’ structures or organic tubes as strategies to feed (Hansen, Reference Hansen1991; Jacobson & Andersen, Reference Jacobson and Andersen1994; Hansen & Calado, Reference Hansen and Calado1999; Jacobson, Reference Jacobson1999). Cyanobacteria symbionts are relatively commonly found in species of the genera Amphisolenia, Citharistes Stein, Histioneis Stein, Ornithocercus Stein and Triposolenia Kofoid; in Ornithocercus, Histioneis and Citharistes species these associations are called ‘phaeosomes’ and are located in special chamber-like structures or ‘phaeosomal chambers’ (Taylor, Reference Taylor1982; Hallegraeff & Jeffrey, Reference Hallegraeff and Jeffrey1984; Gaines & Elbrächter, Reference Gaines, Elbrächter and Taylor1987; Lucas, Reference Lucas1991; Janson et al., Reference Janson, Carpenter and Bergman1995; Schnepf & Elbrächter, Reference Schnepf and Elbrächter1999). More recently, picoplanktonic cells have been found attached to the cellular surface in species of Dinophysis, and this fact has been thought to provide food in this genus (Imai & Nishitani, Reference Imai and Nishitani2000; Nishitani et al., Reference Nishitani, Sugioka and Imai2002).
No species of the order has been successfully cultured (Nishitani et al., Reference Nishitani, Miyuamura and Imai2003), although incubation of cells up to 30 days have been achieved (Koike at al., 2006), and thus details on the biology of most species within the order are unknown, in general, for instance sexual reproduction. Some sexual processes in Dinophysis have been recently suggested from live and preserved field samples (MacKenzie, Reference MacKenzie1992; Berland et al., Reference Berland, Maestrini and Grzebyk1995b; Subba Rao, Reference Subba Rao1995; Giacobbe & Gangemi, Reference Giacobbe and Gangemi1997); presence of possible ‘gametes’ and cysts in Dinophysis may be part of complex life cycles, including smaller forms that have been considered as true species, but now they should be regarded as part of those cycles (Bardouil et al., Reference Bardouil, Berland, Grzebyk and Lassus1991; Reguera et al., Reference Reguera, Bravo and Fraga1995; Reguera & González-Gil, Reference Reguera and González-Gil2001; Dodge, Reference Dodge and Norton2003; Taylor et al., Reference Taylor, Fukuyo, Larsen, Hallegraeff and Hallegraeff2003). Some patterns of the cellular cycle have been approached in field samples, through the postmitotic index (Reguera et al., Reference Reguera, Garcés, Pazos, Bravo, Ramilo and González-Gil2003). Evidence for engulfment of ‘small cells’, as part of the sexual life cycle of Dinophysis fortii Pavillard, was recently found by Koike et al. (Reference Koike, Nishiyama, Sayito, Imai, Koike, Kobiyama and Ogata2006).
Most species of the genera Amphisolenia, Histioneis and Triposolenia show very low density populations, thus many forms of these genera are considered rare: many species have been described on the basis of one single specimen (Kofoid, Reference Kofoid1907; Wood, Reference Wood1954; Taylor, Reference Taylor1976; Hernández-Becerril & Meave, Reference Hernández-Becerril and Meave del Castillo1999), and they have not been reported since then.
The classification into families is based on morphological characters: shape of thecae, position of flagellar and ventral pores, development of cingular and sulcal lists and the presence of spines (Fensome et al., Reference Fensome, Taylor, Norris, Sarjeant, Wharton and Williams1993). Sournia (Reference Sournia1986) proposed Dinophysiaceae Stein, Oxyphysaceae Sournia and Citharistaceae Kofoid & Skoksberg, whereas Fensome et al. (Reference Fensome, Taylor, Norris, Sarjeant, Wharton and Williams1993) and Steidinger & Tangen (Reference Steidinger, Tangen and Tomas1997) mentioned Dinophysiaceae Stein, Amphisoleniaceae Lindemann and Oxyphysaceae Sournia. Hernández-Becerril et al. (Reference Hernández-Becerril, Meave del Castillo, Flores-Granados and Barreiro2003) also included the family Citharistaceae, considering that the genus Citharistes has particular characteristics: the location of the ‘phaeosomal chamber’ in the hypotheca (not the cingulum) and the largest hypothecal plates which join two other smaller intercalary plates (six hypothecal) (Balech, Reference Balech1971, Reference Balech1988). Sournia (Reference Sournia1986) recognized only 11 genera within the order, whereas Fensome et al. (Reference Fensome, Taylor, Norris, Sarjeant, Wharton and Williams1993) considered 14 genera. More recently, Gómez (Reference Gómez2005a) included three families: Citharistaceae, Dinophysiaceae and Oxyphysaceae, with 12 genera. A revision of the order in Mexican waters was recently done by Hernández-Becerril et al. (Reference Hernández-Becerril, Meave del Castillo, Flores-Granados and Barreiro2003), including historical, taxonomic and distribution information of the group, with a list of 90 taxa (species and varieties) in 11 genera and no illustrations; various records need to be confirmed. Finally, a recent report of a species of the genus Dinofurcula Kofoid & Skogsberg, Dinofurcula cf. ultima (Kofoid) Kofoid & Skogsberg, was made from the Gulf of Tehuantepec (Hernández-Becerril & Bravo-Sierrra, Reference Hernández-Becerril and Bravo-Sierra2004). In this work we attempt to give contemporary information on species of the order Dinophysiales in the central and southern, tropical Mexican Pacific to produce a general guide to the group.
MATERIALS AND METHODS
This work is based on analysis of preserved net samples collected during the period 1980–2006 on coasts of the central and southern, tropical Mexican Pacific (states of Jalisco, Colima, Micoacán, Guerrero, Oaxaca and Chiapas). Figure 1 shows sampling points. Phytoplankton net (47, 54, 60, 64 and 66 µm mesh) hauls (either horizontal or vertical, up to 80 m) were done to collect samples, which were preserved in formalin 4%. Parts of the material are deposited in: (1) the Instituto de Ciencias del Mar y Limnología, UNAM; (2) the Phycological collection, Laboratorio de Biología Acuática, Facultad de Biología, UMSNH; (3) the Department of Ecology (CUCBA, U de G); and (4) the Section of Algae, Herbarium of the Faculty of Sciences (FCME, UNAM).

Fig. 1. Map showing the sampling locations in the tropical Mexican Pacific for this study.
Species were identified and measured in a light microscope (Carl Zeiss Axiolab and Olympus), in bright field, using fresh or rinsed material. Occasional observations were done by SEM (JEOL JMS) following conventional protocols (e.g. rinsing, drying and coating material). The terminology used was according to Fensome et al. (Reference Fensome, Taylor, Norris, Sarjeant, Wharton and Williams1993), Steidinger & Tangen (Reference Steidinger, Tangen and Tomas1997) and Hernández-Becerril & Meave (Reference Hernández-Becerril and Meave del Castillo1999). References, dimensions and distribution information are provided for each species.
RESULTS AND OBSERVATIONS
Forty-one species from five genera were identified. The systematic account, and references, measurements and distribution data are given for each species.
References: Tai & Skogsberg, Reference Tai and Skogsberg1934, p. 433, figure 4; Balech, Reference Balech1988, p. 39, pl. 5, figures 5–8; Hernández-Becerril, Reference Hernández-Becerril1992, p. 102, figures 1–6; Larsen & Moestrup, Reference Larsen, Moestrup and Lindley1992, p. 3, figure 1a–d; Steidinger & Tangen, Reference Steidinger, Tangen and Tomas1997, p. 429, pl. 11; Balech, Reference Balech and Sar2002, p. 130, figures 9–12.

Fig. 2–13. Light microscopy (LM) and scanning electron microscopy (SEM). Figure 2. Dinophysis acuminata, SEM. Figure 3. Dinophysis amandula, LM. Figure 4. Dinophysis apicata, LM. Figure 5. Dinophysis argus, LM. Figures 6 & 7. Dinophysis caudata, LM and SEM, respectively. Figure 8. Dinophysis cuneus, LM. Figures 9 & 10. Dinophysis diegensis, a pair of cells and a single cell, LM. Figure 11. Dinophysis doryphora, LM. Figures 12 &13. Dinophysis fortii, LM. Scale bars: Figures 4–8, 20 µm; Figures 2,3,9–13, 10 µm.
Conspicuous synonyms: Dinophysis borealis Paulsen, Dinophysis lachmanii Paulsen, Dinophysis boehmii Paulsen (others in Gómez, Reference Gómez2005a).
Schiller, Reference Schiller1933, p. 120, Gómez, Reference Gómez2005a, p. 141.
Remarks: this species shows a high morphological variability, hence the difficulty to positively identify it and the number of synonyms. Balech (Reference Balech and Sar2002) considered at least two varieties, Dinophysis acuminata var. acuminata, and D. acuminata var. lachmanii Paulsen.
Dimensions: 59–65 µm (62 µm) length (L), 38–41 µm (40 µm) width (W).
Local distribution: localities in Jalisco, Colima, Michoacán and Oaxaca.
General distribution: temperate to tropical waters.
References: Sournia, Reference Sournia1973, p. 18; Balech, Reference Balech1988, p. 50, pl. 10, figures 16 &17.
Conspicuous synonyms: Phalacroma ovum Schütt, Dinophysis amygdala Balech.
Schiller, Reference Schiller1933, p. 81, figures 73a–c.
Dimensions: 65–75 µm (71 µm) L, 54–58 µm (56 µm) W.
Local distribution: localities in Jalisco.
General distribution: warm waters (tropical to subtropical).
References: Abé, Reference Abé1967a, p. 73, figure 23c–g; Taylor, Reference Taylor1976, p. 33, figure 36.
Basynonym: Phalacroma apicatum Kofoid & Skogsberg
Kofoid & Skogsberg, Reference Kofoid and Skogsberg1928, p. 111, figure 10; Schiller, Reference Schiller1933, p. 76, figure 68a–c.
Dimensions: 92–100 µm (95 µm) L, 76–80 µm (78 µm) W.
Local distribution: one locality in Jalisco.
General distribution: tropical to subtropical.
Remarks: this species is closely related to Dinophysis argus (Stein) Abé, from which it can be distinguished for its ‘more sharply conical epitheca’, and its more developed left sulcal list (according to Abé, Reference Abé1967a; Taylor, Reference Taylor1976).
References: Abé, Reference Abé1967a, p. 71, figure 23a,b; Taylor, Reference Taylor1976, p. 33, pl. 4, figure 35; Balech, Reference Balech1988, p. 51, pl. 11, figures 7–10; Hernández-Becerril, Reference Hernández-Becerril1988b, p. 426, figure 6.
Basynonym: Phalacroma argus Stein
Schiller, Reference Schiller1933, p. 74, figure 67a; Steidinger & Tangen, Reference Steidinger, Tangen and Tomas1997, p. 437, pl. 14.
Dimensions: 90 µm L, 80 µm W.
Local distribution: localities in Jalisco, Michoacán and Guerrero.
General distribution: temperate, subtropical and tropical.
References: Taylor, Reference Taylor1976, p. 34, pl. 6, figure 59; Balech, Reference Balech1988, p. 45, pl. 8, figures 2 & 3; Hernández-Becerril, Reference Hernández-Becerril1992, p. 106, figure 21; Larsen & Moestrup, Reference Larsen, Moestrup and Lindley1992, p. 6, figure 3a,b; Steidinger & Tangen, Reference Steidinger, Tangen and Tomas1997, p. 431, pl. 12; Balech, Reference Balech and Sar2002, p. 132, figure 22.
Synonym: Dinophysis homunculus Stein
Stein, Reference Stein1883, pl. 21, figures 1, 2 & 5–7.
Dimensions: 68–90 µm (75 µm) L, 62–72 µm (68 µm) W.
Local distribution: widely distributed from Jalisco to Chiapas.
General distribution: cosmopolitan in temperate to tropical waters.
References: Abé, Reference Abé1967a, p. 68, figure 21a–h; Taylor, Reference Taylor1976, p. 35, pl. 5, figures 46 & 47; Dodge, Reference Dodge1985, p. 19; Balech, Reference Balech1988, p. 51, pl. 11, figures 4–6; Rivera Tenenbaum et al., Reference Rivera Tenenbaum, Menezes, Castro Viana, Queiroz Mendes, Eduardo, Ferreira Hatherly and Rivera Tenenbaum2006, p. 118.
Phalacroma cuneus Schütt
Jörgensen, Reference Jörgensen1923, p. 11, figure 11; Steidinger & Tangen, Reference Steidinger, Tangen and Tomas1997, p. 439, pl. 14.
Dimensions: 87–92 µm (90 µm) L, 92–95 µm (93 µm) W.
Local distribution: localities in Jalisco and Michoacán.
General distribution: warm waters (tropical to subtropical).
References: Kofoid, Reference Kofoid1907, p. 313, pl. 33, figures 57, 59–61; Balech, Reference Balech1988, p. 185, pl. 7, figure 9; Hernández-Becerril, Reference Hernández-Becerril1992, p. 106, figures 7–12.
Dimensions: 59–65 µm (62 µm) L, 34–38 µm (36 µm) W.
Local distribution: localities in Jalisco and Michoacán.
General distribution: temperate to tropical waters.
Remarks: This species is also considered more recently as a ‘small cell’ of D. caudata (Reguera & González-Gil, Reference Reguera and González-Gil2001; Dodge, Reference Dodge and Norton2003). Our Figure 10 shows a ‘mature’ cell, with own morphological characteristics, different from D. caudata.
References: Norris & Berner, Reference Norris and Berner1970, p. 161, figures 23–45; Taylor Reference Taylor1976, p. 35, pl. 4, figures 41 & 42; Balech, Reference Balech1988, p. 55, pl. 13, figures 11–13; Hernández-Becerril, Reference Hernández-Becerril1988b, p. 426, figure 7; Licea et al., Reference Licea, Moreno, Santoyo and Figueroa1995, p. 19, pl. 6, figure 5.
Basynonym: Phalacroma doryphorum Stein
Jörgensen, Reference Jörgensen1923, p. 16, figure 17.
Dimensions: 69–79 µm (75 µm) L, 67–69 µm (68 µm) W.
Local distribution: from Jalisco to Chiapas.
General distribution: tropical to subtropical form.

Fig. 14–25. Light microscopy (LM) and scanning electron microscopy (SEM). Figure 14. Dinophysis hindmarchii, LM. Figure 15. Dinophysis hastata, LM. Figures 16 & 17. Dinophysis favus, lateral and ventral views, respectively, LM. Figures 18 & 19. Dinophysis mitra, LM and SEM. Figure 20. Dinophysis ovum, LM. Figures 21 & 22. Dinophysis porodictyum, SEM and LM. Figures 23 & 24. Dinophysis rapa, LM. Figure 25. Dinophysis rotundata, LM. Scale bars: 10 µm.
References: Taylor, Reference Taylor1976, p. 36, pl. 5, figures 50 & 51.
Basynonym: Phalacroma favus Kofoid & Michener
Jörgensen, Reference Jörgensen1923, p. 15, figure 16; Steidinger & Tangen, Reference Steidinger, Tangen and Tomas1997, p. 439, pl. 14.
Dimensions: 64–71 µm (67 µm) L, 53–55 µm (54 µm) W.
Local distribution: Jalisco, Colima and Michoacán.
General distribution: distributed from temperate to tropical waters.
Remarks: this species resembles Dinophysis hindmarchii (Murray & Whitting) Balech, but whereas D. favus has its dorsal margin straight and is less rounded than D. hindmarchii, the posterior process of the latter is more rounded and short, and has its left sulcal list shorter, with a robust and large R3.
References: Tai & Skogsberg, Reference Tai and Skogsberg1934, p. 439, figure 5, pls. 11 & 12; Balech Reference Balech1988, p. 43, pl. 6, figures 18 & 19; Hernández-Becerril, Reference Hernández-Becerril1988b, p. 426, figures 4 & 35; Larsen & Moestrup, Reference Larsen, Moestrup and Lindley1992, p. 6, figure 4a–c; Steidinger & Tangen, Reference Steidinger, Tangen and Tomas1997, p. 431, pl. 11; Balech, Reference Balech and Sar2002, p. 131, figure 15.
Dimensions: 59–62 µm (61 µm) L, 44–48 µm (46 µm) W.
Local distribution: from Jalisco to Guerrero.
General distribution: widely distributed in warm and temperate waters.
References: Norris & Berner, Reference Norris and Berner1970, p. 165, figures 45–59; Taylor, Reference Taylor1976, p. 37, pl. 5, figures 52–55; Dodge, Reference Dodge1985, p. 21; Balech, Reference Balech1988, p. 54, pl. 13, figures 1–3; Larsen & Moestrup, Reference Larsen, Moestrup and Lindley1992, p. 7, figure 5a,b; Steidinger & Tangen, Reference Steidinger, Tangen and Tomas1997, p. 433, pl. 12.
Dimensions: 56–78 µm (67 µm) L, 53–64 µm (58 µm) W.
Local distribution: from Jalisco to Chiapas.
General distribution: tropical to subtropical.
References: Balech Reference Balech1988, p. 52, pl. 12, figure 4; Rivera Tenenbaum et al., Reference Rivera Tenenbaum, Menezes, Castro Viana, Queiroz Mendes, Eduardo, Ferreira Hatherly and Rivera Tenenbaum2006, p. 148.
Basynonym: Phalacroma hindmarchii Murray & Whitting
Murray & Whitting, Reference Murray and Whitting1899, p. 330, pl. 5, figure 15.
Dimensions: 70 µm L, 65 µm W.
Local distribution: localities in Jalisco and Michoacán.
General distribution: distributed in tropical to subtropical waters.
References: Taylor, Reference Taylor1976, p. 39, pl. 5, figure 49; Balech, Reference Balech1988, p. 45, pl. 8, figures 9–11; Balech, Reference Balech and Sar2002, p. 132, figures 20 & 21.
Basynonym: Phalacroma mitra Schütt
Steidinger & Tangen, Reference Steidinger, Tangen and Tomas1997, p. 439, pl. 14.
Dimensions: 72–82 µm (77 µm) L, 63–65 µm (64.55 µm) W.
Local distribution: from Jalisco to Oaxaca.
General distribution: distributed from temperate to tropical waters.
References: Jörgensen, Reference Jörgensen1923, p. 22, figure 26; Schiller, Reference Schiller1933, p. 116, figure 109; Dodge, Reference Dodge1982, p. 53, figure 3J; Konovalova, Reference Konovalova1998, p. 68, pl. 4, figure 13.
Dimensions: 42–51 µm (47 µm) L, 36–40 µm (38 µm) W.
Local distribution: localities in Jalisco, Colima and Michoacán.
General distribution: temperate to tropical waters.
References: Abé, Reference Abé1967a, p. 61, figure 17a–f; Taylor, Reference Taylor1976, p. 40, pl. 4, figure 45; Dodge, Reference Dodge1985, p. 25; Balech, Reference Balech1988, p. 50, pl. 10, figures 18–20.
Basynonym: Phalacroma porodictyum Stein
Jörgensen, Reference Jörgensen1923, p. 9, figure 6.
Dimensions: 70–78 µm (74 µm) L, 65–69 µm (67 µm) W.
Local distribution: from Jalisco to Oaxaca.
General distribution: temperate to tropical.
References: Taylor, Reference Taylor1976, p. 40, pl. 5, figure 48a,b, pl. 41, figure 48; Balech, Reference Balech1988, p. 44, pl. 8, figures 6–8; Licea et al., Reference Licea, Moreno, Santoyo and Figueroa1995, p. 21, pl. 6, figure 6.
Basynonym: Phalacroma rapa Stein
Jörgensen, Reference Jörgensen1923, p. 14, figure 14; Steidinger & Tangen, Reference Steidinger, Tangen and Tomas1997, p. 439, pl. 14.
Dimensions: 87 µm L, 85 µm W.
Local distribution: localities in Jalisco, Colima and Michoacán.
General distribution: distributed from temperate to tropical waters.
References: Tai & Skogsberg, Reference Tai and Skogsberg1934, p. 426, figure 2A–L; Balech, Reference Balech1976, p. 91, figure 4O–T; Larsen & Moestrup, Reference Larsen, Moestrup and Lindley1992, p. 9, figure 8a–d; Balech, Reference Balech and Sar2002, p. 131, figures 16–19.
Basynonym: Phalacroma rotundatum (Claparède & Lachmann) Kofoid & Michener
Jörgensen, Reference Jörgensen1923, p. 5, figure 2; Steidinger & Tangen, Reference Steidinger, Tangen and Tomas1997, p. 439, pl. 14.
Synonym: Dinophysis whittingae Balech
Balech, Reference Balech and Sar2002, p. 131.
Dimensions: 42–50 µm (46 µm) L, 42–51 µm (47 µm) W.
Local distribution: from Jalisco to Chiapas.
General distribution: widely distributed in temperate and warm waters.

Fig. 26–37. Light microscopy (LM) and scanning electron microscopy (SEM). Figure 26. Dinophysis schuettii, LM. Figure 27. Ornithocercus formosus, LM. Figure 28. Ornithocercus magnificus, LM. Figures 29 & 30. Ornithocercus heteroporoides, SEM and LM. Figure 31. Ornithocercus splendidus, LM. Figure 32. Ornithocercus heteroporus, LM. Figure 33. Ornithocercus cristatus, LM. Figures 34 & 35. Ornithocercus orbiculatus, LM. Figures 36 & 37. Ornithocercus steinii, LM and SEM. Scale bars: Figures 27,28,31,34–37, 20 µm; Figures 26,29,30,32,33, 10 µm.
References: Norris & Berner, Reference Norris and Berner1970, p. 179, figures 92–112; Taylor, Reference Taylor1976, p. 41, pl. 6, figures 65 & 66; Balech, Reference Balech1988, p. 53, pl. 12, figures 7–9; Hernández-Becerril, Reference Hernández-Becerril1992, p. 107, figures 13–18; Steidinger & Tangen, Reference Steidinger, Tangen and Tomas1997, p. 433, pl. 12.
Dimensions: 41–47 µm (44 µm) L, 36–39 µm (37 µm) W.
Local distribution: from Jalisco to Chiapas.
General distribution: tropical to subtropical.
References: Stein, Reference Stein1883, pl. 22, figure 13; Schiller, Reference Schiller1933, p. 254, figure 250; Taylor, Reference Taylor1976, p. 44, pl. 9, figure 89.
Dimensions: 84 µm L, 50 µm W.
Local distribution: localities in Michoacán.
General distribution: tropical to subtropical.
References: Stein, Reference Stein1883, pl. 22, figures 5 & 6; Balech, Reference Balech1971, p. 15, pl. 3, figures 40–46; Balech, Reference Balech1988, p. 63, pl. 15, figure 8; Rivera Tenenbaum et al., Reference Rivera Tenenbaum, Menezes, Castro Viana, Queiroz Mendes, Eduardo, Ferreira Hatherly and Rivera Tenenbaum2006, p. 126.
Synonym: Parahistioneis crateriformis (Stein) Kofoid & Skogsberg
Schiller, Reference Schiller1933, p. 211, figure 200a,b.
Dimensions: 84 µm L, 66 µm W.
Local distribution: localities in Colima.
General distribution: tropical to subtropical.
References: Murray & Whitting, Reference Murray and Whitting1899, pl. 32, figure 4a,b; Schiller, Reference Schiller1933, p. 215, figure 205a,b; Balech, Reference Balech1988, p. 65, pl. 15, figure 4; Gómez, Reference Gómez2005b, figure 30; Rivera Tenenbaum et al., Reference Rivera Tenenbaum, Menezes, Castro Viana, Queiroz Mendes, Eduardo, Ferreira Hatherly and Rivera Tenenbaum2006, p. 134.
Synonym: Parahistioneis para (Murray & Whitting) Kofoid & Skogsberg
Kofoid & Skogsberg, Reference Kofoid and Skogsberg1928, p. 601, figure 85, 6; Taylor, Reference Taylor1976, p. 53, pl. 9, figures 87 & 88, pl. 41, figure 490.
Dimensions: 85 µm L, 35 µm W.
Local distribution: localities in Jalisco and Michoacán.
General distribution: tropical to subtropical.
References: Kofoid, Reference Kofoid1907, p. 205, pl. 16, figure 99; Taylor, Reference Taylor1976, p. 47, pl. 10, figure 95.
Dimensions: 130 µm L, 48 µm W.
Local distribution: localities in Jalisco.
General distribution: tropical to subtropical.
References: Böhm, Reference Böhm1931, p. 499, figures 5 & 6; Schiller, Reference Schiller1933, p. 244, figure 238a,b; Hernández-Becerril, Reference Hernández-Becerril1988b, p. 427, figure 13; Gómez, Reference Gómez2005b, figure 16.
Dimensions: 102 µm L, 59 µm W.
Local distribution: localities in Michoacán.
General distribution: tropical to subtropical.
Remarks: this species is considered as a synonym of H. mitchellana Murray & Whitting by Taylor (Reference Taylor1976).
References: Tai & Skogsberg, Reference Tai and Skogsberg1934, p. 458, figure 11; Balech, Reference Balech1988, p. 38, pl. 5, figures 3 & 4.
Dimensions: 58 µm L, 53 µm W.
Local distribution: localities in Michoacán.
General distribution: temperate to subtropical.
New record for the Mexican Pacific.
References: Matzenauer, Reference Matzenauer1933, p. 447, figure 11; Balech, Reference Balech1967, p. 93, pl. 2, figures 38–46.
Synonyms: Dinophysis moresbyensis Wood
Wood, Reference Wood1963a, p. 7, figure 18.
? Ornithocercus carpentariae Wood
Wood, Reference Wood1963b, p. 5, figure 11.
Dimensions: 65 µm L, 38 µm W.
Local distribution: localities in Jalisco, Michoacán, Oaxaca and Chiapas.
General distribution: tropical to subtropical.
References: Kofoid & Skogsberg, Reference Kofoid and Skogsberg1928, p. 577, figure 91, pl. 17, figures 4 & 5; Schiller, Reference Schiller1933, p. 207, figure 197a–d; Taylor, Reference Taylor1976, p. 48, pl. 7, figure 75.
(as Ornithocercus quadratus in Hernández-Becerril, Reference Hernández-Becerril1988b, p. 427, figures 11 & 41).
Dimensions: 98 µm L, 70 µm W.
Local distribution: one locality in Jalisco.
General distribution: tropical to subtropical.
Reference: Abé, Reference Abé1967b, p. 83, figure 29a–c.
Dimensions: 66–69 µm L, 61–63 µm W.
Local distribution: localities in Michoacán.
General distribution: temperate to subtropical.
Remarks: this species and Ornithocercus heteroporus Kofoid are closely related, however, O. heteroporoides appears with a body larger and its cingular lists more developed than the former. Ornithocercus heteroporus has its left cingular list triangular, with no lobes.
References: Kofoid, Reference Kofoid1907, p. 207, pl. 12, figure 70; Abé, Reference Abé1967b, p. 81, figure 28a,b; Taylor, Reference Taylor1976, p. 48, pl. 8, figure 83; Balech, Reference Balech1988, p. 59, pl. 14, figure 4; Steidinger & Tangen, Reference Steidinger, Tangen and Tomas1997, p. 436, pl. 13.
Synonym: Ornithocercus biclavatus Wood
Wood, Reference Wood1954, p. 211, figure 66.
Dimensions: 66 µm L, 55 µm W.
Local distribution: from Jalisco to Chiapas.
General distribution: tropical to subtropical.
References: Abé, Reference Abé1967b, p. 88, figure 32a–d; Norris, Reference Norris1969, p. 178, figures 1–15; Taylor Reference Taylor1976, p. 49, pl. 7, figures 67–69, pl. 42, figure 505a,b; Balech, Reference Balech1988, p. 61, pl. 14, figures 7 & 8; Steidinger & Tangen, p. 436, pl. 13.
Dimensions: 85–96 µm (90 µm) L, 43–50 µm (48 µm) W.
Local distribution: from Jalisco to Chiapas.
General distribution: tropical to subtropical.
References: Kofoid & Skogsberg, Reference Kofoid and Skogsberg1928, p. 559, pl. 17, figure 7; Schiller, Reference Schiller1933, p. 203, figure 193; Balech, Reference Balech1988, p. 61, pl. 15, figure 2.
Dimensions: 90–95 µm (93 µm) L, 88–94 µm (92 µm) W.
Local distribution: localities in Jalisco and Michoacán.
General distribution: tropical to subtropical.
References: Kofoid & Skogsberg, Reference Kofoid and Skogsberg1928, p. 521, figures 77 & 85, pl. 16, figures 2 & 4, pl. 17, figure 3; Abé, Reference Abé1967b, p. 81, figure 27a–c; Taylor, Reference Taylor1971, figures 13–17; Taylor, Reference Taylor1976, p. 52, pl. 8, figures 85 & 86, pl. 40, figure 486, pl. 42, figure 504; Balech, Reference Balech1988, p. 59, pl. 14, figures 5 & 6; Steidinger & Tangen, Reference Steidinger, Tangen and Tomas1997, p. 437, pl. 13.
Dimensions: 105 µm L, 68 µm W.
Local distribution: from Jalisco to Chiapas.
General distribution: tropical to subtropical.
References: Schiller, Reference Schiller1933, p. 202, figure 192a–f; Abé, Reference Abé1967b, p. 94, figure 35a–c; Taylor, Reference Taylor1976, p. 52, pl. 7, figures 72 & 73; Balech, Reference Balech1977, p. 26, figures 1–14; Balech, Reference Balech1988, p. 61, pl. 15, figure 1; Steidinger & Tangen, p. 437, pl. 13.
Synonym: Ornithocercus serratus Kofoid
Kofoid, Reference Kofoid1907, p. 207, figure 95; Jörgensen, Reference Jörgensen1923, p. 38, figure 52.
Dimensions: 98–108 µm (105 µm) L, 88–94 (92 µm) W.
Local distribution: from Jalisco to Chiapas.
General distribution: tropical to subtropical.
References: Schiller, Reference Schiller1933, p. 178, figure 169a–e; Abé, Reference Abé1967b, p. 111, figure 42a–k; Taylor, Reference Taylor1976, p. 28, pl. 2, figures 21 & 22, pl. 3, figures 21b & 22b; Balech, Reference Balech1988, p. 69, pl. 17, figures 2, 3 & 13; Hernández-Becerril, Reference Hernández-Becerril1988a, p. 521, figures 3–5; Steidinger & Tangen, Reference Steidinger, Tangen and Tomas1997, p. 426, pl. 10.

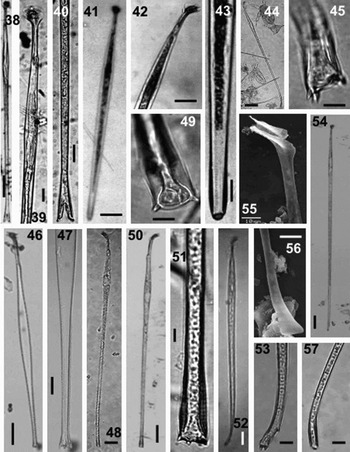
Fig. 38–57. Light microscopy (LM) and scanning electron microscopy (SEM). Figures 38–40. Amphisolenia michoacana, complete cell, anterior and posterior parts, respectively, LM. Figures 41–43. Amphisolenia fusiformis, complete cell, anterior and posterior parts, respectively, LM. Figures 44 & 45. Amphisolenia palmata, complete cell and terminal process, LM. Figures 46 & 47. Amphisolenia palaeotheroides, complete cell and terminal process, LM. Figures 48 & 49. Amphisolenia lemmermanni, complete cell and terminal process, LM. Figures 50 & 51. Amphisolenia rectangulata, complete cell and terminal process, LM. Figures 52 & 53. Amphisolenia bidentata, complete cell and terminal process, LM. Figures 54–57. Amphisolenia truncata, complete cell, anterior and posterios parts, respectively, LM. Scale bars: Figure 44, 100 µm; Figures 38,41,46,48,50,52,54, 50 µm; Figures 39,40,42,47, 20 µm; Figures 43,45,49,51,53, 55–57, 10 µm.

Fig. 58–65. Light microscopy. Figures 58 & 59. Amphisolenia thrinax. Figure 60. Metaphalacroma skogsbergi. Figure 61. Histioneis biremis. Figure 62. Histioneis schilleri. Figure 63. Histioneis crateriformis. Figure 64. Histioneis pulchra. Figure 65. Histioneis para. Scale bars: Figure 58, 100 µm; Figure 59, 50 µm; Figures 60–65, 20 µm.
Dimensions: 667–754 µm (726 µm) L, 18–25 µm (21 µm) W.
Local distribution: from Jalisco to Chiapas.
General distribution: tropical to subtropical.
Diagnosis: cellula solitaria, crassa. Cellula fusiforme, elongata. Testa conspicua, episoma plana. Alae cingulares anteriores et posteriores parvae. Alae sulcales parvae. Collum rectus. Corpo medio leniter inflato, leniter plus angustus ex extremitas posterior. Margines rectae et expolitae. Extremitas posterior rotunda, habens ora conspicua, spinae vel processus terminalis carentes. Chloroplasti carentes?

Fig. 66A–C. Amphisolenia fusiformis, complete cell, posterior and anterior parts, respectively.
Description: cell found solitary, large. Cell fusiform, elongate and uniformly wider at the third part of the body (mid-body), then tapering slightly toward caudal part. Head conspicuous, reduced epitheca, cingular lists approximately equal, little developed. Neck short and sulcal lists also reduced. Margins of the body are straight and smooth. The terminal portion ends somewhat rounded, with a perceptible rim and no process, spines or spinules. No chloroplasts are apparent. One single cell found.
Dimensions: 516 µm L, 26 µm W.
Iconotype: Figure 66A–C has been chosen as Iconotype of the species.
Type locality: Bahia de Maruata, Michoacán, Mexico (18°13′36″N 103°11′21″ W).
Etymology: the species has been named for its fusiform shape.
Diagnosis: cellula solitaria, crassa. Cellula elongata. Testa conspicua, episoma plana. Alae cingulares anteriores et posteriores parvae. Alae sulcales parvae. Collum rectus et brevis, umerus conspicuus. Corpo medio inflato, leniter plus angustus ex extremitas posterior. Extremitas posterior curvata tenera et bifurcata. Duo processus posteriores, breves, aequales, curvatus. Spinae parvae terminalis praesentes ex processus posteriores. Chloroplasti carentes?

Fig. 67A–C. Amphisolenia michoacana, complete cell, posterior and anterior parts, respectively.
Description: solitary cell, very elongate and inflated about the third anterior part (mid-body). Conspicuous head, reduced epitheca, anterior and posterior cingular lists similar, poorly developed. Neck relatively straight and short, with sulcal lists reduced, shoulder conspicuous. Middle part of the body (mid-body) uniformly wider, becoming smoothly narrower toward the caudal part. The caudal portion is delicately curved at its base and bifurcates into two short, symmetric processes, each of them carries a single spine at the tip. Some inclusions in the cell were apparent. A single cell was found.
Dimensions: 625 µm L, 21 µm W.
Iconotype: Figure 67A–C has been chosen as iconotype of the species.
Type locality: Bahia de Maruata, Michoacán, Mexico (18°13′36″N 103°11′21″ W).
Etymology: the species has been named because it was originally found in the Mexican State of Michoacán.
References: Schiller, Reference Schiller1933, p. 179, figure 170a,b; Balech, Reference Balech1988, p. 70, pl. 17, figures 8 & 12; Hernández-Becerril, Reference Hernández-Becerril1988b, p. 427, figure 14.
Dimensions: 671–790 µm L, 20–22 µm W.
Local distribution: localities in Jalisco, Colima and Michoacán.
General distribution: tropical to subtropical.
References: Kofoid, Reference Kofoid1907, p. 199, pl. 14, figure 84; Kofoid & Skogsberg, Reference Kofoid and Skogsberg1928, p. 472, figure 56, pl. 11, figures 2–4; Schiller, Reference Schiller1933, p. 181, figure 172; Rampi, Reference Rampi1952, figure 5; Taylor, Reference Taylor1976, p. 29, pl. 2, figure 31, pl. 3, figure 31b.
Dimensions: 480 µm L, 25 µm W.
Local distribution: localities in Michoacán.
General distribution: tropical to subtropical.
References: Stein, Reference Stein1883, pl. 21, figures 11–15; Schiller, Reference Schiller1933, p. 180, figure 171a,b; Abé, Reference Abé1967b, p. 113, figure 43a–k; Balech Reference Balech1988, p. 69, pl. 17, figures 4–7.
Dimensions: 780 µm L, 25 µm W.
Local distribution: localities in Jalisco.
General distribution: warm waters (tropical to subtropical).
References: Kofoid, Reference Kofoid1907, p. 200, pl. 14, figure 83; Abé, Reference Abé1967b, p. 109, figure 41a–d; Balech Reference Balech1988, p. 186, pl. 83, figures 3–6.
Dimensions: 650–780 µm L, 20 µm W.
Local distribution: localities in Jalisco.
General distribution: tropical to subtropical.
References: Schiller, Reference Schiller1933, p. 183, figure 176; Abé, Reference Abé1967b, p. 114, figure 44a–f; Taylor, Reference Taylor1976, p. 30, pl. 2, figure 20; Balech, Reference Balech1988, p. 187, pl. 18, figures 5, 6 & 9.
Dimensions: 876 µm L, 48 µm W.
Local distribution: localities in Jalisco and Colima.
General distribution: tropical to subtropical.
New record for the Mexican Pacific.
References: Jörgensen, Reference Jörgensen1923, p. 40, figure 58; Schiller, Reference Schiller1933, p. 178, figure 168.
Dimensions: 800 µm L, 25 µm W.
Local distribution: localities in Jalisco and Colima.
General distribution: tropical to subtropical.
DISCUSSION
Diversity and distribution
A total of 41 species included in five genera of the order Dinophysiales was reported. The largest diversity was due to the genus Dinophysis, with 18 species, followed by Amphisolenia (9 species) and Ornithocercus (8 species). Two new records are annotated and illustrated: Metaphalacroma skogsbergii, and Amphisolenia thrinax. And additionally, the species Amphisolenia rectangulata, A. palmata, A. truncata, Dinophysis apicata, D. hindmarchii, Histioneis biremis, H. crateriformis, H. pulchra, Ornithocercus cristatus, O. heteroporoides and O. orbiculatus are illustrated for the first time in the waters of the Mexican Pacific. In our study, two species of the genus Amphisolenia were newly described: Amphisolenia fusiformis and A. michoacana.
Species found in our study that are associated to the production of okadaic acid or dinophysistoxin (which causes DSP) (Taylor et al., Reference Taylor, Fukuyo, Larsen, Hallegraeff and Hallegraeff2003) were basically Dinophysis species, such as D. acuminata, D. caudata, D. fortii, D. mitra and D. rotundata, however, no case of high cell densities of these species or poisoning (DSP) was reported in the study area or in the study period.
The diversity of the order Dinophysiales appears to be relatively low in our study, especially for samples studied that were obtained from a large area in the Mexican Pacific, and with respect to the more than 200 species recognized in Dinophysis (Sournia, Reference Sournia1986), or the list of 145 species of Dinophysis + Phalacroma (104 + 41) and 65 species of Histioneis (Gómez, Reference Gómez2005a). The revision of the order in Mexican waters (including both Mexican littorals, the Atlantic—Gulf of Mexico and the Mexican Caribbean—and the Pacific) listed 90 taxa (species and varieties) in 11 genera (Hernández-Becerril et al., Reference Hernández-Becerril, Meave del Castillo, Flores-Granados and Barreiro2003), and more recently, Okolodkov & Gárate-Lizárraga (Reference Okolodkov and Gárate-Lizárraga2006) provided a list of dinoflagellates from the Mexican Pacific (with no illustrations) and included 41 species of Dinophysis, 12 Ornithocercus, ten Histioneis and nine Amphisolenia species.
From the former two papers, it is clear that more studies need to be carried out to confirm occurrences of certain species and which records seem doubtful, for instance Dinophysis norvegica and D. sacculus, with limited distribution to the Mediterranean Sea and the North Atlantic, respectively. And although introduction of species (particularly dinoflagellates) may be possible, these two species show preferences for colder waters. We note the lack of a complete, contemporary account of dinoflagellates (with descriptions, measurements and illustrations) from Mexican waters.
The species assemblages found in our study have an important tropical and subtropical component (28 species), whereas the species with temperate to tropical distribution are 13. Species of definite cold-water affinity were not represented. This is consistent with other works in other close or adjacent areas such as the Gulf of California and the Gulf of Tehuantepec (Hernández-Becerril, Reference Hernández-Becerril1988b, Reference Hernández-Becerrilc; Licea et al., Reference Licea, Moreno, Santoyo and Figueroa1995; Meave & Hernández-Becerril, Reference Meave del C., Hernández-Becerril and Tapia1998).
Taxonomy
There is an ongoing debate on the distinction and reliable characters with taxonomic value of the two closely related genera Dinophysis and Phalacroma. As pointed out above, the simultaneous and independent transfer of species of Phalacroma to Dinophysis (Abé, Reference Abé1967a; Balech, Reference Balech1967), merged them in one single genus (see list of basynonyms in many Dinophysis species cited here). According to traditional morphological criteria, the presence of a dome-shaped epitheca, which is conspicuous in Phalacroma, was one of the reasons to separate both genera (Stein, Reference Stein1883; Kofoid & Skogsberg, Reference Kofoid and Skogsberg1928), although later detailed morphological studies, especially at the sulcus level, provided evidence that no significant difference existed between them (Abé, Reference Abé1967a; Balech, Reference Balech1967).
Hallegraeff & Lucas (Reference Hallegraeff and Lucas1988) proposed additional criteria to separate both genera: morphology of the cells (areolation of the thecae, megacytic forms), type of nutrition (essentially photosynthetic or heterotrophic forms) and toxicity and ecology (coastal and oceanic forms); Steindinger & Tangen (1997) also considered the two genera apart. However, it is not easy to recognize the reproductive and nutritious biology, or the ecological characteristics of the species, moreover if they cannot be cultured, and the traditional morphological criteria are not practical or convincing.
Molecular phylogenies are based in protocols using single cells for PCR and have been carried out for a very limited number of species of members of the Dinophysiales, basically Dinophysis (Rehnstam-Holm et al., Reference Rehnstam-Holm, Godhe and Anderson2002; Edvarsen et al., Reference Edvardsen, Shalchian-Tabrizi, Jakobsen, Medlin, Dahl, Brubak and Paasche2003). In the phylogenetic trees produced, the separation of one heterotroph species, D. rotundata is significantly supported, but no conclusions on the possible reinstatement of Phalacroma were drawn (Edvarsen et al., Reference Edvardsen, Shalchian-Tabrizi, Jakobsen, Medlin, Dahl, Brubak and Paasche2003). Until new insights (morphological, ultrastructural, ecological, molecular and evolutive) are available on this conflict, we will consider that there are no significant differences between both genera and will use the name Dinophysis in priority.
On the other hand, we also consider that species of the genus Parahistioneis Kofoid & Skogsberg, established to accommodate certain species of Histioneis with ‘distinct’ morphological characters (lack of ‘rib’ in the posterior cingular list and anterior cingular list with no peduncular shape) (Kofoid & Skogsberg, Reference Kofoid and Skogsberg1928) are undistinguishable from species of Histioneis; Balech (Reference Balech1971, Reference Balech1988) mentioned that these characters are variable and show overlap, therefore he proposed Parahistioneis as a synonym of Histioneis. We followed these former criteria.
Smaller and slightly different forms of Dinophysis, which were described as new species in the past, are now currently considered as synonyms of other species, among them: Dinophysis dens Pavillard, D. skagii Paulsen, D. parvula (Schütt) Balech, and even D. diegensis are related to Dinophysis acuta Ehrenberg, D. acuminata, D. rotundata and D. caudata, respectively, as part of complex life cycles (Hernández-Becerril, Reference Hernández-Becerril1992; MacKenzie, Reference MacKenzie1992; Reguera & González-Gil, Reference Reguera and González-Gil2001; Dodge, Reference Dodge and Norton2003; Gómez, Reference Gómez2005a).
This may also be the case for many other forms of the Dinophysiales, in particular Ornithocercus. Species such as O. cristatus might be considered as ‘gametes’, cysts or life stages (schizonts or immature cells) of other species, although current evidence is still lacking (e.g. small cells inside larger ones, intergrades). Hernández-Becerril & Bravo-Sierra (2004) recently showed smaller cells of a member of the Dinophysiales, Dinofurcula cf. ultima and speculated on this subject.
The two species newly described here, on the basis of one specimen, Amphisolenia fusiformis and Amphisolenia michoacana, are absolutely conspicuous and unmistakably recognized, due to their size and general outline together. Amphisolenia fusiformis has a shape completely different from any other species of the genus: no other species is so uniform in width, which only close to the caudal part becomes slightly narrower, with a rounded end and a rim at the very end, and no projections or spines; most species of the genus show a wider mid-body. A species superficially similar is Amphisolenia laticincta Kofoid, which is much smaller than A. fusiformis and its body becomes considerably narrower toward its end; additionally its cingular lists are more separated from each other than in A. fusiformis.
Regarding Amphisolenia michoacana, the anterior part of the body is similar to many other species of the genus (head, neck and wider mid-body), but in this species the caudal portion bifurcates into two short symmetric processes, which do not spread widely or become branch-like as for example in A. bifurcata Murray & Whitting; the processes in A. michoacana show a single spine at the end. No species is like Amphisolenia michoacana regarding these characters. No closely related species are apparent in the extant species of Amphisolenia. Cell inclusions are present in Amphisolenia michoacana, although they are not apparent in Amphisolenia fusiformis. In other Amphisolenia species various endosymbionts (Cyanobacteria, Bacteria and Eukariots) have been found (Lucas, Reference Lucas1991).
These two species, as with most species of the genus, are extremely rare (only one specimen of each detected); many species have been described on the basis of one single specimen (Kofoid, Reference Kofoid1907; Wood, Reference Wood1954; Hernández-Becerril & Meave, Reference Hernández-Becerril and Meave del Castillo1999). But until detailed field studies are done on some genera of the order (Histioneis, Amphisolenia, etc.) (Gómez, Reference Gómez2005a), we will not be able to understand about life cycles and consequently the number of ‘recognized’ species will remain the same.
ACKNOWLEDGEMENTS
Partial support for this work was provided by PAPIIT (DGAPA, UNAM) (Project no. IN223206-2). Dr J.A. Aké-Castillo kindly helped with elaboration of drawings (Figures 66 and 67). Some observations presented here were part of the Bachelor degree thesis of K. E.-L. and M.A. T.-S.