INTRODUCTION
Bopyrina abbreviata Richardson, 1904 is a bopyrid isopod that infests Hippolyte zostericola (Smith, 1873), an important caridean in the transferral of energy from primary producers to higher trophic levels in the lagoon systems of the south-west Gulf of Mexico (Sánchez et al., Reference Sánchez, Raz-Guzmán, Barba, Kuo, Phillips, Walker and Kirkman1996; Barba et al., Reference Barba, Sánchez, Raz-Guzmán and Gallegos2000, Reference Barba, Raz-Guzmán and Sánchez2005). Information about the biology of B. abbreviata is limited to some reports about their population structure, reproduction and prevalence (Román-Contreras & Romero-Rodríguez, Reference Román-Contreras and Romero-Rodríguez2005; Romero-Rodríguez & Román-Contreras, Reference Romero-Rodríguez and Román-Contreras2013). Its distribution extends from the coast of North Carolina and Florida, USA (Markham, Reference Markham1985) to the coast of the states of Espírito Santo, São Paulo and Santa Catarina, Brazil (Lemos de Castro & Brasil-Lima, Reference Lemos de Castro and Brasil-Lima1980). But in Mexico it has been reported only in Laguna de Términos, south-western Gulf of Mexico (Román-Contreras & Romero-Rodríguez, Reference Román-Contreras and Romero-Rodríguez2005; Romero-Rodríguez & Román-Contreras, Reference Romero-Rodríguez and Román-Contreras2014).
Diversity among taxa of crustaceans is not limited to external morphological structures; there is also heterogeneity in their internal organization (McLaughlin, Reference McLaughlin, Bliss and Mantel1983). Wilson (Reference Wilson, Bauer and Martin1991) and Warburg (Reference Warburg2011) noted that even though isopod crustaceans have wide morphological diversity and their sexual adaptations have been addressed in several studies, the structure and function of their copulatory organs and reproductive systems are still poorly understood.
Multiple abiotic or biotic factors influence the reproductive patterns in isopod species, e.g. both temperature and photoperiod can regulate the onset of reproduction, mainly in terrestrial species. Population density can also affect fecundity since crowding shortens the reproductive period, slows down the rhythm of laying and reduces the number of eggs (Caubet et al., Reference Caubet, Juchault and Mocquard1998). Presence of males in a population of the terrestrial isopod Armadillidium vulgare (Latreille, 1804) stimulated female reproductive onset by reducing the duration of parturial intermoult by nearly 40%, from 54 days in lone females to 32 days in those paired with a male; this can also be appointed as a delay in the ovarian development of unpaired females (Jassem et al., Reference Jassem, Juchault and Mocquard1982; Lefebvre & Caubet, Reference Lefebvre and Caubet1999).
The male influence on female reproductive onset in A. vulgare may be caused by physical stimulation of the female genital apparatus (Lefebvre & Caubet, Reference Lefebvre and Caubet1999). However, if several females of the same species are grouped in a particular place this, too, may stimulate reproduction so its preparturial intermoult period is reduced. Further work is required to identify the cause of this stimulation (Caubet et al., Reference Caubet, Juchault and Mocquard1998), and to ascertain whether this trait is also present in other isopods, as has been suggested for the bopyrid isopod Probopyrus ringueleti Verdi & Schuldt, 1988 (Schuldt, Reference Schuldt1993).
In contrast to the abundance of reports on bopyrid taxonomy and the damage caused by bopyrids to their hosts, knowledge about their reproductive organs is scant, probably because the small body size of most of these parasites inhibits study of their internal anatomy (Hiraiwa, Reference Hiraiwa1934).
Among the first authors who studied the internal anatomy of bopyrid isopods, Rathke (Reference Rathke1837) and Cornalia & Panceri (Reference Cornalia and Panceri1858) described the internal organization of both sexes in Bopyrus squillarum (Latreille, 1802) and Gyge branchialis Cornalia & Panceri, 1861, respectively; however, description of the gonads was limited to their position and aspect. Kossmann (Reference Kossmann1881) noted that the appearance of the female gonads of Bopyrina ocellata (Czerniavsky, 1868) (referred to as B. virbii) is modified during growth.
A detailed description of the reproductive organs of female and male Parapenaeon japonica (Thielemann, 1910) (as Epipenaeon japonica) defined the shape and development of the gonads and provided diagrams of the germ cells (Hiraiwa, Reference Hiraiwa1933, Reference Hiraiwa1934). A microanatomical study of the ovaries of P. ringueleti revealed a gradient of dorsoventral laminar maturation of the germ cells. The development of the region of vitellogenesis II in the ovary appeared to be related to the presence of the male over the female's abdomen, since vitellogenesis II was not seen in oocytes of lone mature females (Schuldt, Reference Schuldt1993).
Oliveira & Masunari (Reference Oliveira and Masunari1998) did not record lone immature females of Aporobopyrus curtatus (Richardson, 1904), i.e. all these females were paired with a cryptoniscus larva or a bopyridium, and they proposed that the presence of an immature female accelerates the development of the larvae into males. Similarly, Romero-Rodríguez & Román-Contreras (Reference Romero-Rodríguez and Román-Contreras2014) reported ovigerous females of Bopyrinella thorii (Richardson, 1904) of smaller size than unpaired immature females and suggest that the establishment of pairs of parasites encourages the sexual maturation of both individuals. The present study aims to determine the influence of the male on the gonadal maturation in the female of Bopyrina abbreviata.
MATERIALS AND METHODS
Individuals of the caridean Hippolyte zostericola parasitized by Bopyrina abbreviata were collected during February and April 2010 with a Colman–Seagrove sledge net in meadows of Thalassia testudinum Banks ex König, 1805 on the inner margin of Isla del Carmen, Términos lagoon, Campeche, Mexico (19°10′–18°05′N 92°12′–91°10′W). For a more detailed description of the study area see Yáñez-Arancibia et al. (Reference Yáñez-Arancibia, Lara-Domínguez, Chavance, Flores-Hernández, Yáñez-Arancibia and Day1988) and Raz-Guzmán & Sánchez (Reference Raz-Guzmán, Sánchez, Kuo, Phillips, Walker and Kirkman1996).
The biological material was fixed with Davidson's solution, a suitable fixative for histological techniques in crustaceans (Bell & Lightner, Reference Bell and Lightner1988), by a period of 72–100 h to ensure that the fixative could pervade the internal organs of the parasites (Bortolini & Álvarez, Reference Bortolini and Álvarez2008). Afterwards, the material was washed with running water for 6 h and transferred into 70% ethanol until histological processing.
Because collection and subsequent handling of the biological material might damage the gill chamber of the host, thereby risking loss of the parasites settled therein, we considered only B. abbreviata females that were completely covered by the intact branchiostegite of the host. The total length (TL) of each B. abbreviata was recorded: in symmetric females, TL was measured from the anteromedial margin of the head to the posterior margin of the pleon; in asymmetric females, TL was measured from the posterior margin of the pleotelson to the edge of the first thoracic somite of the longer side (Cash & Bauer, Reference Cash and Bauer1993; Romero-Rodríguez & Román-Contreras, Reference Romero-Rodríguez and Román-Contreras2008).
Developmental stages were defined according to the criteria proposed by Beck (Reference Beck1980), McDermott (Reference McDermott1998) and Masunari et al. (Reference Masunari, Da Silva and Oliveira2000) for other bopyrids: (a) Immature females – symmetric or asymmetric body, oostegites absent or moderately developed, coxal plates absent or poorly developed in pereomeres 1–3 of the longer side of the body; (b) Mature females – clearly asymmetric body, oostegites fully developed, coxal plates developed in pereomeres 1–3 of the longer side of the body; and (c) Ovigerous females – similar to mature females but with eggs or epicaridium larvae in the marsupium. Presence or absence of male or cryptoniscus larvae in the marsupium was recorded so that the immature and mature females could be grouped as lone or paired.
Females of every group and stage of development were processed in sequential dehydration, leaving the specimens for 30 min in each step. Tissue samples were cleared in xylene for 30 min before embedding in paraplast with a melting point of 56–58°C. Sections 7 µm thick were obtained with a rotary microtome Leica RM212RT and stained with standard haematoxylin and eosin, an overall staining technique that allows a broad overview of tissue (Torres & Bortolini, Reference Torres, Bortolini, Escobar-Briones and Álvarez2002; Bortolini & Álvarez, Reference Bortolini and Álvarez2008; Álvarez et al., Reference Álvarez, Bortolini and Høeg2010). Finally, samples were mounted in synthetic resin for later observation by optical microscopy.
Ovarian maturation in B. abbreviata was recorded according to the scale of sexual maturity proposed by Schuldt (Reference Schuldt1993) for Probopyrus ringueleti, which recognizes four stages of maturity: (a) Incipient – ovary poorly developed, in cross section occupies less than 30% of the surrounding loose connective tissue, germ cells at the end of vitellogenesis I, therefore plaques of eosinophilic yolk are not present (Figure 1A, D); (b) Medium – ovary more developed, oocytes in vitellogenesis II occupy most of the available frame (Figure 1E); (c) Advanced – ventrolaterally the mass of oocytes in vitellogenesis II almost reaches the body wall, oocyte size varies from one organism to another; and (d) Ovulation or post-spawn – the follicular cavity or cisterna follicularis appears empty or with some residual oocytes in advanced or total vitellogenesis (Figures 2D & 3D).

Fig. 1. Location, aspect and ovary development of Bopyrina abbreviata Richardson, 1904. (A) paired and compacted gonads of lone immature female, longitudinal section; (B) ovary detail of lone immature female; (C) development of lateral protuberance at distal sides in ovary of lone immature female, longitudinal section; (D) ovary on incipient stage of development of immature female paired with a cryptoniscus larva, transverse section; (E) ovary in advance stage of development of a mature female settle on host left side, transverse section. (F) dorsal lamina germinal layer detail of mature female, transverse section. (Ov, ovary; Hp, hepatopancreas; Dt, digestive tract; black arrow, oogonia II; white arrow, oocytes in vitellogenesis I; clear arrow, oocytes in vitellogenesis II; *, dorsal lamina germinal layer; D, dorsal; V, ventral; P, posterior; A, anterior).

Fig. 2. Female Bopyrina abbreviata Richardson, 1904 paired with a cryptoniscus larva or a male. (A) immature female with a cryptoniscus larva, transverse section; (B) ovigerous female with a mature male, longitudinal section; (C) mature male detail, longitudinal section; (D) ovigerous female with ovary in post-spawn stage, longitudinal section. (Cr, cryptoniscus larva; Imf, immature female; Mtf, mature female; Mtm, mature male; Mg, male gonad; Or, oocytes in reabsorption; black arrow, embryos in egg stage; white arrow, host branchiostegite).

Fig. 3. Maturation of germ cells in female Bopyrina abbreviata Richardson, 1904. (A) ovarian detail of immature female, transverse section; (B) oocytes in vitellogenesis II of mature female, longitudinal section; (C) oocytes in vitellogenesis II of lone mature female, transverse section; (D) ovary in post-spawn stage, longitudinal section; (E) detail of ovary in post-spawn stage with cells in reabsorption and new oocytes generation, longitudinal section; (F) detail of ovary in advance development stage, transverse section. (OoI, oogonia I; Ov, ovary; Hp, hepatopancreas; Dt, digestive tract; Ov I, oocytes in vitellogenesis I; Ov II oocytes in vitellogenesis II; Or, oocytes in reabsorption; black arrow, oogonia II; white arrow, follicular cells; *, dorsal lamina germinal layer).
RESULTS
Of the 604 Bopyrina abbreviata females collected, 255 met the criterion of protection by the intact branchiostegite of the host. Of the 124 classified as immature, 48 were unpaired, 54 were paired with a cryptoniscus larva and 22 were paired with a male. Only one female classified as mature was lone, whilst 29 were paired with a male but without embryos in the marsupium and 101 mature females paired with a male were ovigerous. No mature female was paired with a cryptoniscus larva. Each of these groups was represented in the 21 females of different sizes that were selected for the histological treatment (Table 1).
Table 1. Sizes of female Bopyrina abbreviata Richardson, 1904 selected for histological analysis.
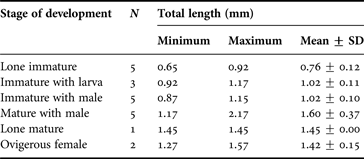
N, number; SD, standard deviation.
Ovarian characterization of Bopyrina abbreviata
The ovary of B. abbreviata lies in the pereon and is a paired structure that runs lengthwise over the digestive tract. In young females the ovaries extend between the first and fifth pereomere, are tubular and compact carrying oogonia and oocytes, mainly in prophasic stage (Figure 1A, B).
Ovarian structure changed throughout female development; laterally, several lateral protrusions evolved discontinuously over the distal margin of each gonad, giving each an undulate appearance (Figure 1C). The ovaries progressively wrap dorsoventrally round the digestive organs (Figure 1D), which in some sections are completely surrounded by the gonads. This was observed mainly in some females larger than 2.0 mm TL (Figure 1E).
Relative ovarian size reflects the asymmetry of the whole body, which in turn is related to which of the branchial chambers of Hippolyte zostericola the female occupies, i.e. if a female B. abbreviata is attached to the right branchial chamber of its host, the parasite and its ovary are smaller on the right, and vice versa (Figure 1E). In a mature female the ovary length extends from the middle part of its head to the first pleomere.
The ovarian cells originate from a dorsal lamina germinal layer that extends dorsally (Figure 1F, 3D, E) and leads to the following dorsoventral maturation sequence of the germ cells: Oogonia I and follicular cells. The oogonia I, 2.5–3.6 µm, exhibit a clear nucleus, round or ovoid and about 0.9–1.1 µm in diameter and granular chromatin (Figure 3E). The follicular cells, 2.3–2.6 µm, have an ovoid nucleus with a diameter of 0.6–0.8 µm (Figure 3B, C). Next, there is a layer of oogonia II (4.1–6.2 µm) with a round nucleus 1.8–3.0 µm in diameter, and a small number of mesodermal cells (Figure 3A, F). Further on, towards the ventral region, oocytes in vitellogenesis I (10.8–13.2 µm) have a nucleus of 2.7–3.7 µm diameter, and basophilic and granular cytoplasm (Figure 3A, F). At the end of the maturation sequence are found oocytes in vitellogenesis II, and this forms the most massive ventral layer in a mature ovary. These oocytes, 40.7–68.6 µm, have a nucleus diameter from 8.7 to 11.7 µm, cytoplasmic vacuolations of 8–10 µm and eosinophilic droplets of about 1 µm (Figure 3B, C). The yolk is made up of plates of 10–13 µm. Some oocytes in vitellogenesis I and II had processes of cellular reabsorption, similar to the oocytes in vitellogenesis II showed in Figure 3D.
Effect of male presence on ovarian development of Bopyrina abbreviata
Ovarian development of B. abbreviata was classified as incipient in all lone immature females analysed. Most of these females had a tubular ovary (Figure 1A, B), but even when it was more developed it never filled more than 30% of the internal anatomy of the female (Figure 1D). The germ cells in the gonads of these immature females were mainly oogonia, but in some cases it was possible to observe some oocytes in vitellogenesis I.
Despite the difference in size of the lone immature females, their external morphology was similar. In only one of these females (67 mm TL) the pleomeres were well defined on both sides of the pleon, while in the remaining females the pleomeres were moderately fused onto one of the pleon edges. The greatest difference among these lone immature females was in the two largest (0.91 and 0.92 mm TL), whose bodies were moderately asymmetrical due to the poor development of the coxal plates in the first three pereomeres and whose dorsal facie was slightly pigmented.
In the three immature females paired with a cryptoniscus larva the degree of ovarian development was classified as medium, since among the germinal cells set on the gonads oocytes in vitellogenesis II were observed with a cell size of 45 µm and the yolk was in drops of medium size. Furthermore, the ovarian dorsoventral development exceeded 50% of the internal space available but in no cases reached the abdominal body wall of the female. The largest immature female paired with a cryptoniscus larva had a bulging white abdomen, an appearance attributable to a further ovarian development. The coxal plates in two of these immature females were poorly developed in the first three pereomeres, and in the other one only the first pereomere had a coxal plate. The degree of fusion of the pleomeres on one of the two pleon edges ranged from medium to completely fused.
The degree of development of the ovaries in immature females paired with a male was variable. In the smallest of these females (Table 1) paired with an immature male of 0.5 mm TL, the ovary was classified as incipient since it had characteristics similar to those described for the lone immature females. In the two larger females of this group, one of 1.12 mm TL paired with a mature male of 0.65 mm TL, and another of 1.15 mm TL accompanied by an immature male of 0.45 mm TL, the ovaries contained oocytes in vitellogenesis II and due to their dorsoventral development the gonads were very close to the female abdominal wall but without reaching it, so ovarian development was classified as medium. Immature females paired with a male had a bulging white abdomen, and a body with a slightly more pronounced asymmetry that the previous group of females owing to the further development of the coxal plates in pereomeres 2–3. Except for the smallest of these females, all had the pleomeres fused on the shorter side margin of the pleon.
The ovaries of all mature females paired with a male were at an advanced stage of ovarian development. The germ cell stratification in the gonads of these females showed a great number of oocytes in vitellogenesis II very close to each other, giving them a rectangular or trapezoidal appearance (Figure 1D). These oocytes in vitellogenesis II ranged from 49 to 65 µm.
In mature females with a male, the ovaries occupied almost the entire internal space available, so other organs were compressed anteriorly. The largest mature females paired with a male (Table 1) had a whitish bulging abdomen; a large number of oocytes were visible dorsally through the exoskeleton, which may be an indication that egg laying was imminent. Morphologically, mature females paired with a male were all very similar, with coxal plates well developed in the first three pereomeres, the pleomeres fused on the shorter lateral margin of the pleon and the dorsal body surface unpigmented.
The lone mature female was very similar morphologically and histologically to those accompanied by a male described previously. The ovaries of this female were in advanced development, the oocytes in vitellogenesis II being rectangular and 45–65 µm; however, some of them were observed with process of reabsorption, especially in those nearer to the ventral wall of the female (Figure 3C), which may indicate that they would not be released into the marsupium. The ovarian size of this lone mature female caused its abdomen to bulge appreciably. The first three pereomeres had well developed coxal plates, while the pleomeres on the shorter side margin of the pleon were completely fused and the dorsal part of the female was unpigmented.
DISCUSSION
Ovarian characterization of Bopyrina abbreviata
The internal organization of Bopyrina abbreviata agrees with the description by Wägele (Reference Wägele, Harrison and Ruppert1992) for isopods in general, and it also supports the organ arrangement described by Kossmann (Reference Kossmann1881) for Bopyrina ocellata (as B. virbii) and by Hiraiwa (Reference Hiraiwa1933, Reference Hiraiwa1934) for Parapenaeon japonica (as Epipenaeon japonica).
The paired ovaries lie in the pereon and run lengthwise over the digestive organs, with oviducts that end in a small cleft-like ventral gonopore near the ventral margin of the insertion of the fifth pereiopod; the gonads are connected to the wall of the digestive organs and to the inner wall of the body by suspensory ligaments (Kossmann, Reference Kossmann1881; Hiraiwa, Reference Hiraiwa1933; Wägele, Reference Wägele, Harrison and Ruppert1992).
The shape and position of the ovaries of immature females of B. abbreviata coincide with the descriptions of the gonads of young females of B. ocellata (see Kossmann, Reference Kossmann1881), P. japonica (see Hiraiwa, Reference Hiraiwa1934) and Probopyrus rigueleti (see Schuldt, Reference Schuldt1993). In these species, including B. abbreviata, the increase in female size is accompanied by a rapid extension of the ovaries. Kossmann (Reference Kossmann1881) noted that with lateral development of the ovary in B. ocellata it twists and adopts a zigzag form with follicular protuberances, a feature also observed in immature females of B. abbreviata.
Likewise, Hiraiwa (Reference Hiraiwa1934) described in the ovaries of young female P. japonica the presence of a laminar structure with various projections or branches that extends from the first anterior thoracic segment to the posterior region of the abdomen and is occupied by oogonia and oocytes. Wägele (Reference Wägele, Harrison and Ruppert1992) noted that in the ovaries of isopods, in general, the different cells (epithelial cells, follicular cells, oogonia and oocytes) originate from a lateral germinal zone that forms a band along the ovary. For P. japonica, Hiraiwa (Reference Hiraiwa1934) described germ cells generated in the ovary wall and running inwards in a parallel arrangement. The present observations in B. abbreviata differ, since these germ cells originate from a dorsal laminar layer that extends horizontally, from which the maturation gradient occurs towards the ventral region; this agrees with observations of P. ringueleti (Schuldt, Reference Schuldt1993).
Oogonia have been observed in the ovaries of P. ringueleti from very early sizes (Schuldt, Reference Schuldt1993), and they persist through the four stages of maturity established for this species. Hiraiwa (Reference Hiraiwa1934) noted that even when some oocytes have accumulated yolk and develop to occupy most of the space available within the ovary, it is possible to find oocytes at early stages of development within the same gonad. Both patterns are consistent with that observed in B. abbreviata because even when the gonads of some females, mainly mature females, were filled with oocytes in vitellogenesis II, there were some ‘nodes’ in the laminar layer where oogonia and/or oocytes were in early vitellogenesis. The process of reabsorption observed in various kinds of germ cells throughout the oogenic cycle of B. abbreviata coincides with that reported in P. ringueletii, and has been proposed as a regulatory factor of fecundity, i.e. the number of oocytes is adjusted to the real spawning possibilities of the species (Schuldt, Reference Schuldt1993).
The asymmetry in extension of the ovaries caused by the increase in size of females of B. abbreviata is similar to that reported by Schuldt (Reference Schuldt1993) in P. ringueleti, although it is not mentioned in the anatomical description of other species such as B. ocellata and P. japonica (Kossmann, Reference Kossmann1881; Hiraiwa, Reference Hiraiwa1933, Reference Hiraiwa1934). In agreement with the description of P. japonica by Hiraiwa (Reference Hiraiwa1934), when the ovary in B. abbreviata does not occupy the entire body of the female, in immature females mainly, the available space is occupied by a mass of connective tissue formed by small irregular cells that surround the internal organs, which are enveloped and compressed by the mass of developing oocytes.
Effect of male presence on ovarian development of Bopyrina abbreviata
Overall, two reproductive patterns are known in isopods: discrete or seasonal and continuous or non-seasonal (Warburg, Reference Warburg2013). They can be influenced by the environment through abiotic factors such as temperature, photoperiod or humidity and/or by biotic factors such as population density, crowding, sex ratio or the presence/absence of the male (Caubet et al., Reference Caubet, Juchault and Mocquard1998). Jassem et al. (Reference Jassem, Juchault and Mocquard1982, Reference Jassem, Juchault, Souty-Grosset and Mocquard1991) noted that temperature and photoperiod determine the beginning of the breeding season of Armadillidium vulgare, and also reported that the reproductive onset of females of this species is accelerated by the presence of males; this ‘male effect’ is characterized by the reduction in duration of the preparturial intermoult and of the secondary vitellogenesis period. This effect is produced by a physical (tactile) and/or chemical stimulus, requiring direct physical contact between male and female, in which the male copulatory organs come into contact with the female genital apparatus. Whether or not insemination occurs, this stimulates the neurosecretory cells on the protocerebrum that control synthesis of the hormone that inhibits vitellogenesis; the hormone concentration is lowered and this decline allows rapid synthesis of vitellogenin thus reducing the period of vitellogenesis (Picaud et al., Reference Picaud, Souty-Grosset and Martin1989; Caubet et al., Reference Caubet, Juchault and Mocquard1998; Lefebvre & Caubet, Reference Lefebvre and Caubet1999).
In bopyrids this ‘male effect’ has been reported only in P. ringueleti (Schuldt, Reference Schuldt1993), a species that has shown an absence or scant presence of oocytes in vitellogenesis II in mature females unpaired with a male. The pattern observed in B. abbreviata contrasts with that reported by Schuldt (Reference Schuldt1993), since the only difference recorded in ovarian development between the lone mature female and those paired with a male was that the lone female showed evidence of cellular reabsorption in some oocytes with secondary vitellogenesis. Nevertheless, because the ovary of immature females of similar sizes was more developed when they were paired with a male, it could be suggested that the presence of a cryptoniscus larva or a male between the pleopods should trigger the gonadal maturation process of the B. abbreviata female. Therefore, if the pairing occurs when the female is small, it may still be relatively small when reproduction begins. This would explain the presence of ovigerous females of B. abbreviata of lower TL than that of unpaired females (Romero-Rodríguez & Román-Contreras, Reference Romero-Rodríguez and Román-Contreras2013), as has been reported for Bopyrinella thorii (Romero-Rodríguez & Román-Contreras, Reference Romero-Rodríguez and Román-Contreras2014). These authors suggested that despite the absence of a male the female is capable of producing oocytes, since they recorded lone mature females of B. thorii with ovaries filled with oocytes, which were visible through the exoskeleton. The present results support this, because oocytes of lone mature and immature females of B. abbreviata reached the stage of vitellogenesis II, although oocytes of the immature females were much less numerous than those recorded in mature females.
Because the shape of the gonads is related to the size of the female and as through its development it shows external morphological variations, we may assume that if the presence of a male promotes accelerated development of the ovaries it will also influence the morphology of the female. This could explain the differences between lone and paired immature females in body symmetry, the development of coxal plaques in pleomeres 1–3, and the degree of fusion of pleomeres at any edge of the pleon, since such features were more developed in females paired with a male. However, it is necessary to perform more detailed studies in order to confirm the possible relationship between the degree of ovarian development and the morphological characteristics of females of Bopyrina abbreviata. Although this study suggests that the male influences the ovarian development some females were able to produce oocytes without a male presence, so it is important to describe if the influence of the male on the ovarian development is just mechanical or by a humoral factor which also could determine the release of the oocytes into the marsupium.
ACKNOWLEDGEMENTS
The authors express their gratitude to the following: H. Álvarez-Guillén and J. Reda-Deara (Estación El Carmen, ICMyL-UNAM) for their support with fieldwork; M. Martínez-Mayén (Unidad de Ecología y Biodiversidad Acuática, ICMyL-UNAM) for assistance in the field and laboratory; A.I. Bieler-Antolín (Laboratorio de Microcine, Facultad de Ciencias, UNAM) for aid with the microphotograph images; Ann Grant for valuable comments and improvements to the English version; and three anonymous referees and the editor for their comments and suggestions that improved our manuscript.
FINANCIAL SUPPORT
We thank Consejo Nacional de Ciencia y Tecnología (CONACyT) for the scholarship granted (47889/170315) to the first author throughout his postgraduate studies; and the ICMyL-UNAM, for economic support of this study as part of Project 136: Biología, sistemática y ecología de crustáceos decápodos de México: Laguna de Términos, Golfo de Mexico, in charge of the second author.






