INTRODUCTION
Sponges (phylum Porifera) have long been considered ‘living hotels’ due to the great diversity and abundance of other taxonomic groups that are often found in association with them (Pearse, Reference Pearse1950; Klitgaard, Reference Klitgaard1995; Ribeiro et al., Reference Ribeiro, Omena and Muricy2003). These associations represent a wide range of ecological interactions, facultative or obligatory, that range from mutualism to parasitism; however, the exact nature of many associations remains unclear (Wulff, Reference Wulff2006). Because sponges have bodies composed of an intricate network of canals, associated organisms may find substrate and shelter inside them (Çinar et al., Reference Çinar, Katagan, Ergen and Sezgin2002; Huang et al., Reference Huang, McClintock, Amsler and Huang2008). As sponges are important components of benthic communities and interact with a wide range of organisms (Wulff, Reference Wulff2006; Becerro, Reference Becerro2008), they are considered to be important reservoirs of marine biodiversity (Cerrano et al., Reference Cerrano, Calcinai, Pinca, Bavestrello, Suzuki, Nakamori, Hidaka, Kayanne, Casareto, Nadao, Yamano and Tsuchiya2006).
Previous studies of sponge-associated fauna have been carried out in the North Atlantic Ocean (Frith, Reference Frith1976; Biernbaum, Reference Biernbaum1981; Peattie & Hoare, Reference Peattie and Hoare1981; Klitgaard, Reference Klitgaard1995; Huang et al., Reference Huang, McClintock, Amsler and Huang2008; Fiore & Jutte, Reference Fiore and Jutte2010), the Caribbean (Pearse, Reference Pearse1950; Villamizar & Laughlin, Reference Villamizar, Laughlin, Reitner and Keupp1991), the Mediterranean (Rützler, Reference Rützler1976; Koukouras et al., Reference Koukouras, Voultsiadou-Koukouras, Chintiroglou and Dounas1985, Reference Koukouras, Russo, Voultsiadou-Koukouras, Dounas and Chintiroglou1992, Reference Koukouras, Russo, Voultsiadou-Koukouras, Arvanitidis and Stefanidou1996; Ilan et al., Reference Ilan, Ben-Eliahu and Galil1994; Çinar et al., Reference Çinar, Katagan, Ergen and Sezgin2002), the Pacific Ocean (Long, Reference Long1968; Magnino et al., Reference Magnino, Pronzato, Sarà and Gaino1999; Beaulieu, Reference Beaulieu2001; Skilleter et al., Reference Skilleter, Russell, Degnan and Garson2005; Cerrano et al., Reference Cerrano, Calcinai, Pinca, Bavestrello, Suzuki, Nakamori, Hidaka, Kayanne, Casareto, Nadao, Yamano and Tsuchiya2006), and the Indian Ocean (Abdo, Reference Abdo2007). Only four studies have been performed in the South Atlantic Ocean: one in Argentina (Cuartas & Excoffon, Reference Cuartas and Excoffon1993) and three in Brazil (Duarte & Nalesso, Reference Duarte and Nalesso1996; Ribeiro et al., Reference Ribeiro, Omena and Muricy2003; Stofel et al., Reference Stofel, Canton, Antunes and Eutrópio2008). Other studies along the Brazilian coast have described associations between sponges and particular groups of organisms: parasitic crustaceans (Duarte & Morgado, Reference Duarte and Morgado1983), decapods (Bezerra & Coelho, Reference Bezerra and Coelho2006), gammarids and caprellids (Serejo, Reference Serejo1998), copepods (Johnsson, Reference Johnsson1998, Reference Johnsson2000, Reference Johnsson2002; Bispo et al., Reference Bispo, Johnsson and Neves2006), and polychaetes (Neves & Omena, Reference Neves and Omena2003).
With the exception of two studies that included hexactinellid sponges (Beaulieu, Reference Beaulieu2001; Fiore & Jutte, Reference Fiore and Jutte2010), almost all studies of sponge-associated fauna focused on the class Demospongiae. Only one study, conducted in Hampshire, UK, has investigated the associated fauna of a calcareous sponge (Frith, Reference Frith1976). This study, however, found no fauna associated with either Sycon ciliatum (Fabricius, 1780) or Grantia compressa (Fabricius, 1780) and did not describe any organisms found with Leucosolenia botryoides (Ellis & Solander, 1786) (Frith, Reference Frith1976).
Paraleucilla magna Klautau et al., Reference Klautau, Monteiro and Borojevic2004 is a calcareous sponge found along the Brazilian coast (adjacent to the Rio de Janeiro, São Paulo and Santa Catarina states) and in the Mediterranean (along the southern coast of Italy and around Malta). In both regions, it is considered to be a non-native species, although its origin is still unknown (Klautau et al., Reference Klautau, Monteiro and Borojevic2004; Longo et al., Reference Longo, Mastrototaro and Corriero2007; Zammit et al., Reference Zammit, Longo and Schembri2009; Gravili et al., Reference Gravili, Belmontea, Cecere, Denitto, Giangrande, Guidetti, Longo, Mastrototaro, Moscatello, Petrocelli, Piraino, Terlizzi and Boero2010). It lives attached to hard substrates in photophilous or sciaphilous conditions and in pristine or polluted waters (Klautau et al., Reference Klautau, Monteiro and Borojevic2004; Longo et al., Reference Longo, Mastrototaro and Corriero2007; Gravili et al., Reference Gravili, Belmontea, Cecere, Denitto, Giangrande, Guidetti, Longo, Mastrototaro, Moscatello, Petrocelli, Piraino, Terlizzi and Boero2010). This species has a leuconoid aquiferous system with a large atrial cavity and many canals that can be easily occupied by other organisms. In the original description of P. magna, crustaceans, echinoderms, and polychaetes were described as associating with this species (Klautau et al., Reference Klautau, Monteiro and Borojevic2004); however, there has been no subsequent research on its associated fauna. Therefore, to gain knowledge about the associated macrofauna of calcareous sponges, we investigated the composition of macrofauna inhabiting P. magna over the course of one year. The objectives of this study were to: (1) describe the species composition of the associated macrofauna of P. magna in Rio de Janeiro, south-western Atlantic; (2) investigate the influence of sponge volume on these associations; and (3) analyse possible seasonal variations of these associations.
MATERIALS AND METHODS
Sampling
Five specimens of P. magna (Figure 1B) were collected monthly throughout 2005 (except in February, when only four individuals were collected, and in April, when no collection occurred), totalling 54 specimens. All specimens were collected at Vermelha Beach (22°57′18″ S–43°09′42″ W), in Rio de Janeiro, Brazil (Figure 1A; Lanna et al., Reference Lanna, Monteiro, Klautau, Custódio, Lôbo-Hajdu, Hajdu and Muricy2007). Specimens were collected by snorkelling at 0–4 m depth and were removed from the substrate with a knife. While underwater, each specimen was bagged individually (to avoid the escape of associated organisms) and then fixed and preserved in 93% ethanol. At the laboratory, the volume of each sponge was calculated by liquid displacement in a graduated cylinder (see Ribeiro et al., Reference Ribeiro, Omena and Muricy2003; Lanna et al., Reference Lanna, Monteiro, Klautau, Custódio, Lôbo-Hajdu, Hajdu and Muricy2007). Sponge specimens were then carefully fragmented under a stereomicroscope to remove the macrofauna (>1 mm) that remained inside. Associated organisms of each sponge specimen were separated by morphotype within higher taxa and then identified to the lowest possible taxonomic level with the help of specialists.
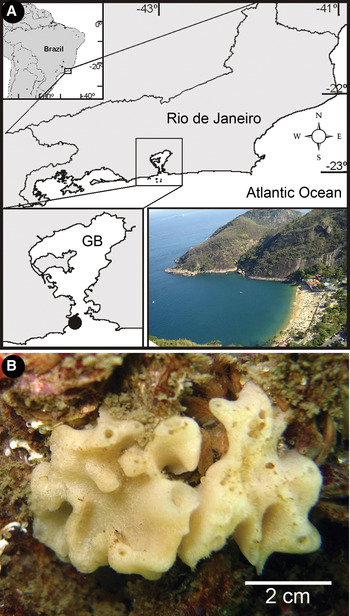
Fig. 1. (A) Location and aerial view of the study area. Vermelha Beach is located at the entrance of the eutrophic Guanabara Bay (GB) (black dot at inferior left corner) (map source: DIVA-GIS, Vermelha Beach photograph: F. Azevedo); (B) in vivo photograph of Paraleucilla magna.
Data analysis
We counted the total number of associated individuals and the total number of taxa to calculate species richness, frequency, abundance, density, diversity (H′), and Pielou's evenness (J′) (Ludwig & Reynolds, Reference Ludwig and Reynolds1988). To investigate whether the total volume of P. magna specimens collected each month (i.e. the sum volume of the five analysed individuals) could predict species richness, abundance, diversity and evenness, we performed linear regressions (Sokal & Rohlf, Reference Sokal and Rohlf1995). The values of species richness, frequency, abundance, density, diversity (H′), and Pielou's evenness (J′) obtained for each month were used as replicates to test whether these attributes of the associated fauna varied between the dry (April to September) and rainy (October to March) seasons. The rainy season in Rio de Janeiro usually starts in September (Dereczynski et al., Reference Dereczynski, Oliveira and Machado2009), however, we have considered it as starting in October for our analyses because in 2005 (when the specimens were collected) the rainy period started only in that month (AlertaRio, Reference AlertaRio2011). All data were tested for normality and homoscedasticity prior to performing analyses of variance (ANOVAs). Temporal patterns in the community of associated fauna were assessed by means of a principal component analysis (PCA), in which the dimensionality of 21 species (the number of species that occurred in more than one month) was reduced to only two components (latent variables) representing the primary temporal patterns of dominant species. As most species were rare, and because many zeros were present in the data set (see Table 1), we applied a Hellinger transformation prior to analysis (see Legendre & Gallager, Reference Legendre and Gallagher2001). PCA scores obtained for each month were used as replicates for the ANOVA to test whether these attributes of the associated fauna varied between the dry and rainy seasons (Jassby & Powell, Reference Jassby and Powell1990).
Table 1. Variation of the number of taxa associated with Paraleucilla magna. Colonies of Hydrozoa were not quantified, and their presence is marked with ‘P’. Total number of individuals/colonies for each taxon and for each month and the total number of taxa of each phylum (within parentheses) are provided. (Por – Porifera, Cni – Cnidaria, Pla – Plathyhelminthes, Nem – Nematoda, Ann – Annelida, Art – Arthropoda, Mol – Mollusca, Bry – Bryozoa, Ech – Echinodermata, Asc – Ascidiacea). (*) indicatess presence of juveniles.

RESULTS
Associated macrofauna
A total of 349 individuals, representing 51 species and 10 phyla, were identified living in association with the 54 analysed specimens of P. magna (Table 1). The mean species richness of associated taxa was 11.9 species/month (±4.4; Table 2). Arthropoda (mostly Crustacea) showed the highest species richness (17 species); followed by Annelida, with 11 taxa of polychaetes; and Mollusca, with nine species (Table 1). The species diversity of the total associated macrofauna was high (H′ = 3.0), but the total evenness was low (J′ = 0.4) (Table 2).
Table 2. Summary of the ecological data collected each month.

The most abundant higher taxa were Arthropoda (54%), Mollusca (21%), and Bryozoa (9%) (Figure 2), while the most frequent were Arthropoda, Annelida (Polychaeta), Mollusca, and Bryozoa, present in 72.2%, 57.4%, 48.2%, and 40.7% of the sponges, respectively (Figure 3). Chordata (Ascidiacea), Cnidaria (Hydrozoa), and Echinodermata were found less frequently (present in 22.2%, 14.8%, and 12.9% of the sponges, respectively), while Platyhelminthes, Nemertea, and Porifera were found in only 1.8% of specimens (Figure 3). The density of associated individuals was highest in November and June (3.1 and 2.8 ind.cm−3) and lowest in February and January (0.2 and 0.3 ind.cm−3). This variation was not significantly different between the dry and rainy seasons (Table 3A).

Fig. 2. Proportion of the higher taxa associated with Paraleucilla magna.

Fig. 3. Frequency (%) of the phyla associated with Paraleucilla magna.
Table 3. Summary of the ANOVA testing the influence of seasonality (dry versus rainy seasons) on community descriptors during the study period (df = degrees of freedom; Sum Sq = sum of squares; Mean Sq = mean of squares; Pr > F = P value associated with the F statistic; *P < 0.05).

Juvenile representatives of Crustacea (Mithrax sp.), Polychaeta (Sabellidae sp., Syllidae spp.), Mollusca (Mytilidae sp.), and Echinodermata (Lytechinus variegatus) were found living associated with P. magna. In addition, pregnant crustacean females were also frequently observed.
Volume
Total sponge volume (i.e. the sum volume of sponges collected each month; Table 2) did not differ between seasons (Table 3B) but was significantly correlated to both species diversity (H′) (R2 = 0.43, df = 10, P = 0.027, Figure 5A) and the number of taxa (species richness) (R2 = 0.37, df = 10, P = 0.04, Figure 5B), indicating that larger sponges contained a higher variety of taxa and a higher diversity of species. Nonetheless, regression analyses indicated that the total volume in each month did not correlate to either the Pielou evenness index (J′) (R2 = 0.04, df = 10, P = 0.52, Figure 5C) or the total number of associated individuals (abundance) (R2 = 0.03, df = 10, P = 0.56, Figure 5D).

Fig. 4. Monthly variation of the number of species and individuals associated with Paraleucilla magna.

Fig. 5. Quantitative analyses of the macrofauna associated with Paraleucilla magna. Linear regression between sponge volume and (A) species diversity (H′); (B) number of taxa; (C) evenness (J′); (D) number of individuals. The dotted lines indicate the 95% confidence intervals.
Seasonality
The periods of lowest and highest species richness (February = 4; January = 20, respectively) coincided with the months of lowest and highest diversity (H′) (February – H′ = 1.4; January – H′ = 2.7) (Table 2). Abundance (i.e. the number of associated individuals) was lowest in February (only three individuals), and highest in November (66 individuals) (Table 1; Figure 4). The evenness of associated macrofauna tended to be high, being highest in February and March (J′ = 1.0) and lowest in June (J′ = 0.6) (Table 2). None of these community descriptors differed significantly between the dry and rainy seasons (Table 3C–F).
Seasonal changes in the community of macrofauna associated with P. magna were analysed using biplots based on PCA (Figure 6A). The total amount of variation explained by the first two scores (corresponding to the first two principal components) was 56.9%. The PCA biplot did not show a clear seasonal difference between the dry and rainy seasons. Nevertheless, three groups of species were partially distinguished by the analysis:
Group A (formed mainly by the bryozoan Scrupocellaria aff. reptans (Linnaeus, 1758) and the ascidians Didemnum sp. 1 and Bugula neritina (Linnaeus, 1758)), which appeared between February and June;
Group B (formed mainly by the mollusc Bivalvia sp.1 and the crustaceans Pachycheles laevidactylus Ortmann, 1892 and Cymadusa filosa Savigny, 1816) that appeared from July to November;
Group C (formed mainly by the ophiuroid Ophiactis lymani Ljungman, 1872) comprised only one species and was found exclusively in January and December.

Fig. 6. Principal component analysis (PCA) of the associated fauna of Paraleucilla magna: (A) biplot representation of the PCA showing both observations (months) and variables (species) in the same graph. The left and bottom axes use the unity for observations, while the top and right axes are graduated according to the first two principal components of the original variables. PC1 accounts for 38.7% of the total variation, while PC2 accounts for 18.2%. Months are represented by upper case letters (Species: bi1, Bivalvia sp. 1; bi3, Bivalvia sp. 3; bot, Botrylloides giganteum; bug, Bugula neritina; cal, Calyptraeidae; cre, Crepidula sp.; cym, Cymadusa filosa; di1, Didemnum sp. 1; di2, Didemnum sp. 2; ela, Elasmopus pectenicrus; mel, Melitidae sp.; mic, Micropanope nuttingi; myt, Mytilidae sp.; opl, Ophiactis lymani; ops, Ophiactis savignyi; pac, Pachycheles laevidactylus; pod, Podceridae sp.; qua, Quadrimaera quadrimana; scr, Scrupocellaria aff. reptans; sy2, Syllidae sp. 2; sy3, Syllidae sp.); (B and C) box plots of the scores of (B) the first principal component (PC1) and (C) the second principal component (PC2) in the dry and rainy seasons. Each box displays the median, upper and lower quartiles of the distribution of sponge volume per month. Box whiskers represent the maximum and minimum range, while empty circles show outliers.
Scores of the first component (PC1), which account for 38.7% of the variation, did not differ significantly between the dry and rainy seasons (Figure 6B; Table 4A). However, the scores of the second component (PC2), which account for 18.2% of the variation, were significantly different between these seasons (Figure 6C; Table 4B).
Table 4. Summary results of the analysis of variance carried out on the scores of the two main principal components testing the seasonality (dry versus rainy seasons) of the associated fauna during the studied period (df = degrees of freedom; Sum Sq = sum of squares; Mean Sq = mean of squares; Pr > F = P value associated with the F statistic; *P < 0.05).

DISCUSSION
Paraleucilla magna exhibited moderate-to-low richness of associated macrofauna (51 species) relative to all other sponge species investigated to date (48 Demospongiae and two Hexactinellida). Demosponges, for example, yielded an average of 95.5 associated taxa per species (±162.2 of standard deviation), with a minimum of two and a maximum of 809 taxa (e.g. Westinga & Hoetjes, Reference Westinga and Hoetjes1981; Villamizar & Laughlin, Reference Villamizar, Laughlin, Reitner and Keupp1991; Cuartas & Excoffon, Reference Cuartas and Excoffon1993; Klitgaard, Reference Klitgaard1995; Koukouras et al., Reference Koukouras, Russo, Voultsiadou-Koukouras, Arvanitidis and Stefanidou1996; Betancourt-Lozano et al., Reference Betancourt-Lozano, Gonzalez-Farias, Gonzalez-Acosta, Garcia-Gasca and Bastida-Zavala1998; Magnino et al., Reference Magnino, Pronzato, Sarà and Gaino1999; Çinar et al., Reference Çinar, Katagan, Ergen and Sezgin2002; Neves & Omena, Reference Neves and Omena2003; Ribeiro et al., Reference Ribeiro, Omena and Muricy2003; Abdo, Reference Abdo2007; Huang et al., Reference Huang, McClintock, Amsler and Huang2008). In P. magna, Crustacea was the most abundantly represented group of associated organisms (54%), followed by Mollusca (21%), and Bryozoa (9%). In other studied sponges, Crustacea was also one of the two most abundantly represented groups, being present in 80% of the sponge species examined, followed by Polychaeta (60%) and Echinodermata (24%). Molluscs were the second most abundant group in P. magna (21%); however, this is not a common occurrence, as they have been identified as a dominant group in only a few species of sponges (8% of those examined so far; Long, Reference Long1968; Peattie & Hoare, Reference Peattie and Hoare1981; Kligaard, 1995; Koukouras et al., Reference Koukouras, Russo, Voultsiadou-Koukouras, Arvanitidis and Stefanidou1996). The same pattern occurs with Bryozoa, which was the third most abundant taxon in P. magna but is not considered to be among the two most abundant organisms in other studied sponges. However, bryozoans were the second most dominant group (12.8% of the total number of taxa) found in demosponges of the Faroe Island, north-eastern Atlantic (Klitgaard, Reference Klitgaard1995) and, as in the present study, Klitgaard (Reference Klitgaard1995) also found that most of the bryozoans were attached to the outer surface of the sponges. Associations between sponges and bryozoans may be related to the fact that sponges may provide suitable substrate to bryozoans in habitats of otherwise limited substrate availability, as noted by Klitgaard (Reference Klitgaard1995).
A study of the associated fauna of the demosponge Mycale microsigmatosa Arndt, 1927 was performed at the same location of the present study (Ribeiro et al., Reference Ribeiro, Omena and Muricy2003). Both P. magna and M. microsigmatosa exhibit associated macrofauna of similar species richness (51 and 75 species, respectively) and composition. However, the differences observed in taxonomic composition between these two sympatric species can be explained by the different sample sizes of each study. In the present study, we analysed 54 specimens of P. magna, while Ribeiro et al. (Reference Ribeiro, Omena and Muricy2003) analysed 19 specimens of M. microsigmatosa. Species diversity was the same in P. magna and M. microsigmatosa (H′ = 3.0), while evenness was lower in P. magna (J′ = 0.4, versus J′ = 0.7 for M. microsigmatosa). The difference in evenness values between both species may be also due to sampling differences. In the present work, several collections throughout the year were made, while Ribeiro et al. (Reference Ribeiro, Omena and Muricy2003) made only one collection. The most striking difference between these two species is in the total number of associated individuals (abundance): P. magna was associated with 349 individuals (0.9 ind.cm−3), while M. microsigmatosa was associated with 2235 (13 ind.cm−3). If we consider that both sponges have the same type of aquiferous system (leuconoid), we could expect similar internal canals and, consequently, similar associated macrofauna. Nonetheless, P. magna has a large atrium, while M. microsigmatosa has only canals, and whereas P. magna is massive, M. microsigmatosa is an incrustant sponge. In addition, the external surface of P. magna is full of folds, while M. microsigmatosa has a smoother surface. Despite these morphological characteristics that seem to characterize P. magna as a better host, M. microsigmatosa is host to more associated organisms. A possible explanation for this difference in macrofauna abundance is the presence of chemicals that might reduce predation in M. microsigmatosa and, consequently, provide more protection for its associated macrofauna. Although this hypothesis has not been tested, M. microsigmatosa does produce a series of compounds, some of which inhibit microorganism proliferation (Compagnone et al., Reference Compagnone, Oliveri, Piña, Marques, Rangel, Dagger, Suárez and Gómez1999; Marinho et al., Reference Marinho, Moreira, Pellegrino, Muricy, Bastos, Santos, Giambiagi-deMarval and Laport2009, Reference Marinho, Muricy, Silva, deMarval and Laport2010; Santos et al., Reference Santos, Pontes, Santos, Muricy, Giambiagi-deMarval and Laport2010). The potential importance of sponge allelochemicals in influencing the composition and abundance of associated fauna has already been pointed out (Koukouras et al., Reference Koukouras, Russo, Voultsiadou-Koukouras, Dounas and Chintiroglou1992; Skilleter et al., Reference Skilleter, Russell, Degnan and Garson2005). A good example can be found in the work of Betancourt-Lozano et al. (Reference Betancourt-Lozano, Gonzalez-Farias, Gonzalez-Acosta, Garcia-Gasca and Bastida-Zavala1998), which describes a significant relationship between inquilinism and the antibiosis activity of Aplysina fistularis (Pallas, 1766) in Mexico.
Paraleucilla magna shares with M. microsigmatosa at least three associated species, two of which (the ophiuroids Amphipholis squamata and Ophiactis savignyi) occur commonly in other sponge species (Table 5). Although echinoderms have been found in only 12.9% of the analysed specimens of P. magna, they (particularly Ophiuroidea) are commonly found in demosponges (Wendt et al., Reference Wendt, van Dolah and O'Rourke1985; Duarte & Nalesso, Reference Duarte and Nalesso1996; Betancourt-Lozano et al., Reference Betancourt-Lozano, Gonzalez-Farias, Gonzalez-Acosta, Garcia-Gasca and Bastida-Zavala1998; Ribeiro et al., Reference Ribeiro, Omena and Muricy2003; Clavico et al., Reference Clavico, Muricy, da Gama, Batista, Ventura and Pereira2006; Abdo, Reference Abdo2007) and other benthic organisms, such as bryozoans (Morgado & Tanaka, Reference Morgado and Tanaka2001). Associations of Ophiactis savigny and O. lymani with marine organisms are apparently common. For example, both species have been described as common epifauna on the tubes of the polychaete Phyllochaetopterus socialis Claparède, 1869 (Nalesso et al., Reference Nalesso, Duarte, Pierozzi and Enumo1995), on the octocoral Carijoa riisei (Duchassaing & Michelotti, 1860) (Neves et al., Reference Neves, Lima and Pérez2007), and on algae (Mladenov & Emson, Reference Mladenov and Emson1988). The frequent association of these ophiuroid species with varied taxa (algae, polychaetes, corals and sponges) may indicate that these associations (including with P. magna) are only occasional or opportunistic. These ophiuroids may seek out these organisms only for protection or food (Klitgaard, Reference Klitgaard1995).
Table 5. Species associated with Paraleucilla magna that were already found associated with other sponge species. 1 – Mycale microsigmatosa (Rio de Janeiro, Brazil; Ribeiro et al., Reference Ribeiro, Omena and Muricy2003); 2 – Mycale angulosa (São Paulo, Brazil; Duarte & Nalesso, Reference Duarte and Nalesso1996); 3 – Dysidea robusta Vilanova & Muricy, 2001 (Rio de Janeiro, Brazil; Serejo, Reference Serejo1998); 4 – Topsentia sp. (south-eastern United States; Fiore & Jutte, Reference Fiore and Jutte2010); 5 – Ircinia campana (Lamarck, 1814) (south-eastern United States; Fiore & Jutte, Reference Fiore and Jutte2010); 6 – Sarcotragus foetidus (Turkish Aegean coast; Çinar et al., Reference Çinar, Katagan, Ergen and Sezgin2002); 7 – Aplysina lacunosa (Pallas, 1766) (Venezuelan Caribbean; Villamizar & Laughlin, Reference Villamizar, Laughlin, Reitner and Keupp1991); 8 – Sarcotragus fasciculatus (Pallas, 1766) (North Aegean Sea; Koukouras et al., Reference Koukouras, Voultsiadou-Koukouras, Chintiroglou and Dounas1985); 9 – Sidonops corticostylifera (Hajdu, Muricy, Custodio, Russo & Peixinho, 1992) (Rio de Janeiro, Brazil; Clavico et al., Reference Clavico, Muricy, da Gama, Batista, Ventura and Pereira2006); 10 – Halichondria panicea (Pallas, 1766) (Menai Strait, UK; Peattie & Hoare, Reference Peattie and Hoare1981); 11 – Ircinia strobilina (Lamarck, 1816) (Bimini, Bahamas; Pearse, Reference Pearse1950); 12 – Geodia macandrewii Bowerbank, 1858 (Faroe Islands; Klitgaard, Reference Klitgaard1995); 13 – Cliona varians (Duchassaing & Michelotti, 1864) (Stofel et al., Reference Stofel, Canton, Antunes and Eutrópio2008).

The volume of P. magna was positively related only to species diversity and number of taxa (richness). These relationships have already been observed in other sponge species: S. foetidus (for species diversity) and M. microsigmatosa, M. angulosa, S. foetidus, and Spheciospongia vesparium (Lamarck, 1815) (for richness) (Westinga & Hoetjes, Reference Westinga and Hoetjes1981; Duarte & Nalesso, Reference Duarte and Nalesso1996; Çinar et al., Reference Çinar, Katagan, Ergen and Sezgin2002; Ribeiro et al., Reference Ribeiro, Omena and Muricy2003). In P. magna, higher volumes can reflect a diverse array of microhabitats inside the sponge, such as more and larger folds, or larger atria and oscula, which could accommodate larger organisms and, consequently, a higher diversity of taxa. On the other hand, no relationship between volume and number of individuals was observed in P. magna, and this relationship has also not been observed in several demosponge species (four from the Aegean Sea, Koukouras et al., Reference Koukouras, Russo, Voultsiadou-Koukouras, Dounas and Chintiroglou1992; and two from Australia, Skilleter et al., Reference Skilleter, Russell, Degnan and Garson2005). In P. magna, large volumes might provide habitat for other species that could then compete with the fauna that live in smaller sponges. The fact that we found associated organisms in a great variety of sponge volumes (from 0.3 cm3 to 37 cm3) suggests that this species is rapidly colonized by organisms in the environment.
In the present study, no significant seasonal variation in community descriptors of the fauna associated with P. magna (species richness, number of individuals, species diversity (H′), and evenness index (J′)) was detected. This lack of seasonal variation can be explained, in part, by the relationship of some of these descriptors with sponge volume (as described above). As neither sponge volume nor the community descriptors exhibit variation between the dry and rainy seasons (see Table 2), the absence of any seasonal trend could be expected. However, it is important to consider that sample size, differences in the sponges volume collected each month and a possible atypical year could have influenced these results.
Although the PCA biplot (Figure 6A) suggests no seasonal variation between the dry and rainy seasons, the second component (PC2) scores differed significantly between seasons. This latter result indicates that some environmental change (in features such as salinity, temperature, or food availability) might influence the composition of the associated fauna community. However, the causes of variation explained by the first component (PC1) are unknown and not likely to be correlated with season. On the other hand, we observed three groups of species that occupied P. magna in temporal succession (Groups A, B, and C). The establishment of these groups may reflect the life cycle of the associated organisms.
We frequently found pregnant crustacean females and juveniles of several taxa (molluscs, crustaceans, echinoderms, and polychaetes) inhabiting P. magna that probably used their host as a temporary shelter during vulnerable periods of their life cycle (i.e. reproductive or juvenile stages). This kind of relationship can be characterized as opportunistic. Ribeiro et al. (Reference Ribeiro, Omena and Muricy2003) and Abdo (Reference Abdo2007) also found pregnant females, juveniles or reproductively active individuals associated with M. microsigmatosa and two Haliclona species in Brazil and Australia, respectively.
These findings suggest that sponges may be important shelters during some stages of the life cycle of many invertebrates, enhancing their survival. All of these aspects regarding the role of sponges in the community reiterate a previous proposal (Cerrano et al., Reference Cerrano, Calcinai, Pinca, Bavestrello, Suzuki, Nakamori, Hidaka, Kayanne, Casareto, Nadao, Yamano and Tsuchiya2006) namely, that sponges are important reservoirs of biodiversity and that the phylum Porifera should be seriously considered in conservation programs.
ACKNOWLEDGEMENTS
We thank the taxonomy specialists for helping us with identifications of the associated fauna: Luciana Muguet (Bryozoa), Cléo Oliveira (Mollusca), Juliana Bahia (Platyhelminthes), Carlos Renato R. Ventura and Fernanda Viana (Echinodermata), Daniela Barbosa (Ascidiacea), Paulo Paiva (Polychaeta) and Tereza G. Silva (Crustacea). We are thankful to Baslavi Condor and Paulo Paiva for their help in data analysis and to the anonymous referees for their suggestions that improved manuscript quality. We thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), the Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) (E-26/111.541/2008), and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq–480368/2008 2; CNPq/PIBIC–480368/2008 2) for grants and fellowships during this project.













