Introduction
Proper evaluation of natural and anthropogenic contributions of trace metals to surface sediments and seawater is challenging given their intrinsically variable concentrations associated with the fluctuating conditions in coastal environments. The analysis of environmental matrices such as water or sediment provides a picture of the total contaminant load rather than the fraction with direct ecotoxicological relevance. The use of biomonitors eliminates the need for complex studies on the chemical speciation of aquatic contaminants and facilitates the evaluation of biologically available levels of contaminants in aquatic ecosystems or their effects on living organisms (Phillips & Segar, Reference Phillips and Segar1986; Huerta-Díaz et al., Reference Huerta-Díaz, De León-Chavira, Lares, Chee-Barragán and Siqueiros-Valencia2007; Ackali & Kucuksezgin, Reference Akcali and Kucuksezgin2011).
Marine organisms are commonly used as bioindicators of trace metal contamination (Volterra & Conti, Reference Volterra and Conti2000; Hernández-Almaraz et al., Reference Hernández-Almaraz, Méndez-Rodríguez, Zenteno-Savin, O'Hara, Harley and Serviere-Zaragoza2016). Algae and molluscs are among the organisms most used for this purpose (Rainbow, Reference Rainbow1995). Macroalgae accumulate trace metals, reaching concentrations that are thousands of times higher than levels in seawater (Rai et al., Reference Rai, Gaur and Kumar1981; Bryan & Langston, Reference Bryan and Langston1992). Algae bind only free metal ions, the concentrations of which depend on the nature of the suspended particulate matter; this, in turn, comprises both organic and inorganic complexes (Seeliger & Edwards, Reference Seeliger and Edwards1977; Luoma, Reference Luoma1983; Volterra & Conti, Reference Volterra and Conti2000). Marine macroalgae are regarded as suitable indicators due to being widely distributed in aquatic environments, sessile, easy to collect and identify, and for bioaccumulating metals in concentrations that can be several orders of magnitude higher than those in the surrounding seawater (Sánchez-Rodríguez et al., Reference Sánchez-Rodríguez, Huerta-Díaz, Shoumilin, Holguín-Quiñones and Zertuche-González2001; Conti & Cecchetti, Reference Conti and Cecchetti2003; Khaled et al., Reference Khaled, Hessein, Abdel-Halim and Morsy2014; Bonanno et al., Reference Bonanno, Veneziano and Piccione2020).
It has been shown that metal concentrations in macroalgae are proportional to the respective dissolved concentration of each in the surrounding water (Huerta-Díaz et al., Reference Huerta-Díaz, De León-Chavira, Lares, Chee-Barragán and Siqueiros-Valencia2007). Various macroalgae groups have been shown to be good indicators of environmental conditions. For example, green algae such as Ulva lactuca (Linnaeus 1753) have a high affinity for manganese (Mn), iron (Fe), copper (Cu), zinc (Zn) and lead (Pb) (Ryan et al., Reference Ryan, McLoughlin and O'Donovan2012). The brown macroalgae Fucus vesiculosus Linnaeus (Giusti, Reference Giusti2001; Ryan et al., Reference Ryan, McLoughlin and O'Donovan2012), Ascophyllum nodosum (Linnaeus) (Le Jolis) 1863, Laminaria digitata (Hudson) J.V. Lamouroux (Stengel et al., Reference Stengel, Macken, Morrison and Morley2004), Lessonia trabeculata Villouta & Santelices 1986, and L. nigricens Bory (Contreras et al., Reference Contreras, Mella, Moenne and Correa2009; Sáez et al., Reference Sáez, Lobos, Macaya, Oliva, Quiroz and Brown2012) have been used to monitor metal contamination as well as temporal and intraspecific fluctuations in metal concentrations in coastal waters. Evans & Edwards (Reference Evans and Edwards2011) also documented that the brown alga Macrocystis pyrifera can be used to measure Cu and Zn concentrations in seawater because tissue concentrations follow those in seawater, although there are interactions between both metals (Cu may inhibit Zn uptake). Perhaps more importantly, once these metals are taken up, tissue concentrations fluctuate temporally and the alga can release them (depuration) when seawater concentrations drop. The red macroalgae Spyridia filamentosa Wulfen (Harvey) 1833, Gelidium floridanum W.R.Taylor 1943 and Polysiphonia lanosa Linnaeus (Tandy) 1931 have also been useful for detecting various trace and heavy metals in the environment (Rodríguez-Castañeda et al., Reference Rodríguez-Castañeda, Sánchez-Rodríguez, Shumilin, Sapozhnikov, Anderson, Brodie, Onsoy and Critcheley2006; Ródenas de la Rocha et al., Reference Ródenas de la Rocha, Sánchez-Muniz, Gómez-Juaristi and Marín-Larrea2009; Ryan et al., Reference Ryan, McLoughlin and O'Donovan2012; dos Santos et al., Reference dos Santos, Schmidt, de, Polo, Kreusch, Pereira, Costa, Simioni, Chow, Ramlov, Maraschin and Bouzon2014; Farias et al., Reference Farias, Hurd, Eriksen and Macleod2018).
In Mexico, coastal lagoons are affected by anthropogenic activities in their associated watersheds, such as industry, mining, aquacultural and agricultural expansion, sewage discharges, dredging, and accelerated population growth; metal contamination gradients and high metal concentrations are usually related to these activities (Marín-Guirao et al., Reference Marín-Guirao, Lloret and Marin2008; Vizzini et al., Reference Vizzini, Costa, Tramati, Gianguzza and Mazzola2013; Hatje et al., Reference Hatje, de Souza, Ribeiro, Eça and Barros2016; Páez-Osuna et al., Reference Páez-Osuna, Álvarez-Borrego, Ruiz-Fernández, García-Hernández, Jara-Marini, Bergés-Tiznado, Piñón-Gimate, Alonso-Rodríguez, Soto-Jiménez, Frías-Espericueta, Ruelas-Inzunza, Green-Ruiz, Osuna-Martínez and Sánchez-Cabeza2017). Metal concentrations have been measured in macroalgae from the east coast of the Gulf of California over several years (Páez-Osuna et al., Reference Páez-Osuna, Ochoa-Izaguirre, Bojórquez-Leyva and Michel-Reynoso2000; Jara-Marini et al., Reference Jara-Marini, Molina-García, Martínez-Durazo and Páez-Osuna2020), as well as in macroalgae from the west coast of the Baja California peninsula (Shumilin et al., Reference Shumilin, Kalmykov, Sapozhnikov, Nava-Sánchez, Gorsline, Godinez-Orta and Rodríguez-Castañeda2000; Sánchez-Rodríguez et al., Reference Sánchez-Rodríguez, Huerta-Díaz, Shoumilin, Holguín-Quiñones and Zertuche-González2001; Rodríguez-Castañeda et al., Reference Rodríguez-Castañeda, Sánchez-Rodríguez, Shumilin, Sapozhnikov, Anderson, Brodie, Onsoy and Critcheley2006; Huerta-Díaz et al., Reference Huerta-Díaz, De León-Chavira, Lares, Chee-Barragán and Siqueiros-Valencia2007). Most studies analysing metals in algae have been carried out as one-time investigations, using a single sample of algae, and in just one survey into the region (Rodríguez-Castañeda et al., Reference Rodríguez-Castañeda, Sánchez-Rodríguez, Shumilin, Sapozhnikov, Anderson, Brodie, Onsoy and Critcheley2006); just a few have monitored different seasons over one year, but using a single species (Rodríguez-Figueroa et al., Reference Rodríguez-Figueroa, Shumilin and Sánchez-Rodríguez2009). Previous studies conducted in Guaymas Bay and La Paz Bay involving organisms such as clams, urchins and fish, as well as sediments, measured Cd, Cu, Pb, Zn, Ni and Mn concentrations. Until the past decade, these metals in macroalgae still maintained levels considered typical of non-polluted sites, despite some of these study sites being known for heavy anthropogenic activities that could potentially release toxic wastes into the environment (Méndez et al., Reference Méndez, Salas-Flores, Arreola-Lizarraga, Alvarez-Castañeda and Acosta2002, Reference Méndez, Acosta, Arreola-Lizárraga and Padilla2004, Reference Méndez, Palacios, Acosta, Monsalvo-Spencer and Álvarez-Castañeda2006; Hernández-Almaráz et al., Reference Hernández-Almaraz, Méndez-Rodríguez, Zenteno-Savín, García-Domínguez, Vázquez-Botello and Serviere-Zaragoza2014, Reference Hernández-Almaraz, Méndez-Rodríguez, Zenteno-Savin, O'Hara, Harley and Serviere-Zaragoza2016; Serviere-Zaragoza et al., Reference Serviere-Zaragoza, Lluch-Cota, Mazariegos-Villarreal, Balart, Valencia-Valdez and Méndez-Rodríguez2021).
The present study was conducted in Guaymas Bay and La Paz Bay, located on opposite sides of the Gulf of California, to compare metal concentrations in three macroalgae species and how these relate to the anthropogenic activities in adjacent areas. The three algae species studied belong to the Rhodophyta, Chlorophyta and Ochrophyta and are cosmopolitan, abundant and usually found year-round. Around the globe, seaweeds have been included as key organisms for determining and monitoring the ecological status of coastal ecosystems (Ballesteros et al., Reference Ballesteros, Torras-Bouldú, Pinedo, García, Mangialajo and de Torres2007; Juanes et al., Reference Juanes, Guinda, Puente and Revilla2008). For example, the brown algae Padina gymnospora (Kützing) Sonder 1871 and Dictyota bartayresiana J. V. Lamoroux 1809, and the green algae Ulva lactuca and Enteromorpha intestinalis (Linnaeus) Nees 1820 were suggested as biomonitors for Cd, Ni, Cr, Cu, Zn and Pb in the Gulf of Kutch (India) (Chakraborty et al., Reference Chakraborty, Bhattacharya, Singh and Maity2014), as well as Gracilaria conferuoides for As, Cu, Pb, Cr and Fe in Liusha Bay, South China Sea (Caixue et al., Reference Caixue, Fujin, Xingli and Chun-Liang2010). Pan et al. (Reference Pan, Wernberg, de Bettignies, Holmer, Li, Wu and Xiao2018) monitored macroalgae in the East China Sea; in their study, macroalgae were suggested as good quality biomonitors of heavy metals in the environment. The European Water Framework has also included macroalgae as biomonitors (Ballesteros et al., Reference Ballesteros, Torras-Bouldú, Pinedo, García, Mangialajo and de Torres2007; Juanes et al., Reference Juanes, Guinda, Puente and Revilla2008).
The objectives of the present study were the following: (1) determine whether Spyridia filamentosa, Padina mexicana and Ulva ohnoi could be good indicators of the presence of metals in two bays of the Gulf of California; (2) identify seasonal variability patterns in metal concentrations in the cosmopolitan macroalgae studied; and (3) determine whether metal concentrations are associated with the main anthropogenic activities at each bay.
Materials and methods
Study area
La Paz Bay, located on the south-western coast of the Gulf of California, is a locality where various anthropogenic activities take place, including aquatic tourism and aquaculture. Although no industries are currently operating in the area, mining activities take place at San Juan de la Costa, in the western coast of the La Paz Bay, related to the extraction of phosphorite from a deposit that has been exploited for nearly 30 years (Rodríguez-Castañeda et al., Reference Rodríguez-Castañeda, Sánchez-Rodríguez, Shumilin, Sapozhnikov, Anderson, Brodie, Onsoy and Critcheley2006; Servicio Geológico Mexicano, 2008). Guaymas Bay, on the central eastern coast of the Gulf of California, is an important port with intense industrial activity mainly associated with sardine canneries, particularly in the site known as El Paraje (Méndez et al., Reference Méndez, Salas-Flores, Arreola-Lizarraga, Alvarez-Castañeda and Acosta2002; Rodríguez-Castañeda et al., Reference Rodríguez-Castañeda, Sánchez-Rodríguez, Shumilin, Sapozhnikov, Anderson, Brodie, Onsoy and Critcheley2006; Osuna-Ramírez et al., Reference Osuna-Ramírez, Arreola, Padilla-Arredondo, Mendoza-Salgado and Méndez-Rodríguez2017). From October to August, local fishmeal plants process 600 000 t year−1 of sardine and discharge 20 Mm3 year−1 of wastewater with high organic matter content, fatty acid saturation from fish oils and acidic pH (García-Sifuentes et al., Reference García-Sifuentes, Pacheco-Aguilar, Valdez-Hurtado, Márquez-Ríos, Lugo-Sánchez and Ezquerra-Brauer2009; Osuna-Ramírez et al., Reference Osuna-Ramírez, Arreola, Padilla-Arredondo, Mendoza-Salgado and Méndez-Rodríguez2017).
La Paz Bay (LP)
La Paz Bay is located on the south-western littoral of the Gulf of California, at 24°06′–24°47′N 110°18′–110°45′W. It is the largest bay on the eastern coast of the Baja California peninsula; water exchange between the bay and the Gulf of California occurs through the North Mount (350 m depth) and the San Lorenzo Channel (10 m depth) (Obeso-Nieblas et al., Reference Obeso-Nieblas, Shirasago, Sánchez-Velasco and Gaviño-Rodríguez2004). It comprises an area of ~1200 km2 and is bordered by the Baja California peninsula and the Espiritu Santo Island complex. It has an elongated shape 90 km long by 30 km wide, with a well-defined channel between 220 and 350 m depth; two sections can be distinguished, a shallow area to the south and a deeper section to the north. Four water masses are present in the bay: Surface Tropical Water, Surface Equatorial Water, Gulf of California Water and Subtropical Subsurface Water (Cervantes-Duarte et al., Reference Cervantes-Duarte, González-Rodríguez, Funes-Rodríguez, Ramos-Rodríguez, Torres-Hernández and Aguirre-Bahena2021). The local climate is arid dry (BWh), with annual evaporation (215 mm) exceeding precipitation (180 mm) (Monreal-Gómez et al., Reference Monreal-Gómez, Molina-Cruz and Salas-de León2001). Three distinctive seasons can be recognized – cold, dry and rainy seasons – based on patterns of air temperature (15.2–19.3°C from November to February; 17.4–27.1°C from March to June; and 24.4–30.1°C from July to October) and precipitation (maximum precipitation from July to October: 2.1–102.3 mm month−1) (INEGI, 2010). These data were used to select months representative of each season to conduct the sampling: January (cold season), May (dry season) and October (rainy season) of 2016. Based on a preliminary survey of all shallow areas of the bay, three sampling locations with the conspicuous presence of benthic macroalgae and contrasting characteristics and nutrient concentrations were selected (Table 1). The substrate at San Juan de la Costa (SJC, 24°22′30″N 110°42′00″W) consists of boulders and sandy patches; a major mining company operates at this site extracting phosphorite, which is then transported by sea elsewhere for processing (Mesa-Zavala, Reference Mesa-Zavala2013). Casa del Marino (CM, 24°10′20.8″N 110°18′33.3″W) is a protected shallow area located at the southern part of the bay, in the waterfront of the city of La Paz (250,000 inhabitants). This site is dredged regularly, usually harbours local fishing boats, and constantly receives sewage from the adjacent city. The sandy bottom is covered by boulders, shells and coral remains (Chávez-Sánchez, Reference Chávez-Sánchez2012). The third site, El Tecolote (TE, 24°20′9″N 110°19′00″W), is located near the San Lorenzo Channel; its southern portion is uninhabited, characterized by a hard-rock platform with sandy patches (Figure 1).

Fig. 1. La Paz Bay and Guaymas Bay. SJC, San Juan de la Costa; CM, Casa del Marino; TE, El Tecolote; AL, Almagre Grande; PA, El Paraje; CP, Cerro Pastel.
Table 1. Main characteristics and mean concentration of dissolved inorganic nitrogen (DIN μM) and phosphate (PO4), as recorded at the study sites between 2013 and 2016

SJC, San Juan de la Costa; CM, Casa del Marino; TE, El Tecolote; AL, Almagre Grande; PA, El Paraje; CP, Cerro Pastel.
Mean values of Dissolved Inorganic Nitrogen (DIN. μM) and PO4 (μM) at each site and season obtained from Chávez-Sánchez et al. (Reference Chávez-Sánchez, Piñón-Gimate, Serviere-Zaragoza, López-Bautista and Casas-Valdez2018) and this study.
Guaymas Bay (GU)
Guaymas Bay is a coastal lagoon located at 27°51′8″N 110°49′51″W on the central east coast of the Gulf of California. The lagoon stretches across 33.6 km2, its depth varies between 5–8.5 m, and connects with the sea through a 1.2 km wide, 8 m deep mouth. The local climate is warm dry, with a mean annual temperature over 22°C and a summer rainy season. Based on its geomorphological characteristics and water exchange with the ocean, GU is classified as a restricted coastal lagoon (Kjerfve & Magill, Reference Kjerfve and Magill1989) permanently connected to the sea. It has three entry channels and a well-defined tidal circulation, being a vertically very-well mixed system (Figure 1). It receives sewage discharges of 63 effluents from the city of Guaymas (150,000 inhabitants) and industrial facilities, including a thermal power plant, a cement factory and shipyards. Additionally, seven fish-processing facilities discharge their wastewater into the bay (Osuna-Ramírez et al., Reference Osuna-Ramírez, Arreola, Padilla-Arredondo, Mendoza-Salgado and Méndez-Rodríguez2017). A pier for loading and unloading hydrocarbons, copper and pesticides is located at the inner bay (Méndez et al., Reference Méndez, Salas-Flores, Arreola-Lizarraga, Alvarez-Castañeda and Acosta2002). Table 1 shows the main characteristics and concentrations of dissolved inorganic nitrogen (DIN) and phosphate (PO4) at GU.
Three sampling locations with the conspicuous presence of benthic macroalgae and contrasting anthropogenic activities were selected at GU. Almagre Grande (AL, 27°54′25.3″N 110°52′22.9″W) is a site where port operations take place, including copper loading and unloading. El Paraje (PA, 27°52′51.1″N 110°51′22.9″W) holds the Fisheries Industrial Park of the city of Guaymas, including seven fishmeal processing plants that operate during the sardine fishing season (October–August). Cerro Pastel (CP, 27°56′8.5″N 110°59′33.3″W), a small island within San Carlos Bay, is a pristine area of sandy bottom and patches of rocky coral surrounded by oceanic water (Figure 1).
Sample collection
Samples were collected in January, May and October in both La Paz Bay and Guaymas Bay. Three macroalgae species, known to be the most abundant ones in the region, were collected at each site: Spyridia filamentosa (Rhodophyta), Ulva ohnoi M. Hiraoka & S. Shimada (Hiraoka et al., Reference Hiraoka, Shimada, Uenosono and Masuda2004; Melton et al., Reference Melton, Collado-Vides and López-Bautista2016) (Chlorophyta) and Padina mexicana E.Y. Dawson 1944 (Ochrophyta). In the field, macroalgae samples were collected manually by freediving during low tide on each date (dry, rainy, cold seasons) between 0.5 and 1 m depth. Different algal morphotypes were sorted from the samples, and at least 100 g of each morphotype was collected. This material was rinsed in the field with seawater to remove sediments and epibionts and was transported to the laboratory for analysis. In the laboratory, a portion of each macroalgae sample was fixed with 4% formaldehyde seawater for taxonomic identification using the keys for the region by Norris (Reference Norris2010, Reference Norris2014); current species names were reviewed in AlgaeBase (Guiry & Guiry, Reference Guiry and Guiry2021). The identified material was then rinsed with deionized water, oven-dried for 48 h at 60°C to constant weight and stored in labelled sterile plastic bags for further processing.
Metal analyses
Each macroalgae sample was processed in triplicate. An ~0.5 g sample of dried material was digested in a 3:1 nitric acid:hydrogen peroxide solution (reagent grade, Avantor Performance Material, Central Valley, PA) in a microwave oven (Mars 5X, CEM, Matthews, NC). Concentrations of cadmium, lead, copper, zinc, nickel, manganese and iron in the digested samples were quantified by flame atomic absorption spectrophotometry using an air-acetylene flame (Avanta, GBC Scientific Equipment, Braeside, VIC, Australia) (Méndez et al., Reference Méndez, Palacios, Acosta, Monsalvo-Spencer and Álvarez-Castañeda2006; Hernández-Almaraz et al., Reference Hernández-Almaraz, Méndez-Rodríguez, Zenteno-Savín, García-Domínguez, Vázquez-Botello and Serviere-Zaragoza2014). The accuracy of the analytical methods was validated as per the International Atomic Energy Agency (Reference Material IAEA-392 – trace, minor and major elements in algae). Recovery percentages were all above 95% (Table 2). Metal concentrations are expressed in μg g−1 dry weight. The detection limits were Cd, 0.01; Pb, 0.017; Zn, 0.07; Mn, 0.04, Ni, 0.03; and Fe, 0.07 (μg g−1).
Table 2. Analysis of the standard reference material IAEA-392 (μg g−1) (International Atomic Energy Agency, trace, minor and major elements in algae)

Bioaccumulation coefficient and enrichment factor
To examine whether macroalgae showed enrichment related to their local environment, for each collection site we calculated the bioaccumulation coefficient in each macroalgae species relative to seawater and the Enrichment Factor (EF) in macroalgae relative to sediments. The bioaccumulation coefficient was calculated as the ratio of macroalgae concentration to the concentration in coastal water (to this end, we used the concentration reported for North Pacific Waters; Nozaky, Reference Nozaki1997). The Enrichment Factor is defined as the ratio between the concentration of one contaminant in the indicator organism and the environment (water or sediment) relative to a natural reference value (background level) that had been previously reported for sediments (e.g. Loska et al., Reference Loska, Cebula, Pelczar, Wiechuła and Kwapuliński1997; Volterra & Conti, Reference Volterra and Conti2000). The application of EF allowed comparing metal concentrations in macroalgae vs marine sediments.
The Enrichment Factor (EF) has been based on the normalization of a tested element against a reference element with low occurrence variability (e.g. Chakraborty et al., Reference Chakraborty, Bhattacharya, Singh and Maity2014). In the study area, the data on the concentrations of metals in water, sediments or algae are insufficient for normalization; thus, we used the element proposed by Pérez-Tribouillier (Reference Pérez Tribouillier2014). In La Paz Bay, this author found that Li was the least variable metal, and using it allowed normalizing all other elements in his study. Besides, metal concentrations in sediments were used as a baseline for calculating the Enrichment Factor used in the comparisons.
The Enrichment Factor (EF) was calculated by normalizing the terrigenous element Li, which was the most efficient normalizer (Pérez-Tibouillier et al., Reference Pérez-Tribouillier, Shumilin and Rodríguez-Figueroa2015), as follows:
where Mm denotes the concentration of element M (Metal) in macroalgae (m) at each site; Li, the concentration of lithium at each site (in this case, La Paz Bay); Mcrust, the concentration of element M in the crust, and Licrust, the concentration of lithium in the upper continental crust (Wedepohl, Reference Wedepohl1995).
Five contamination categories are recognized based on the Enrichment Factor (Buat-Menard & Chesselet, Reference Buat-Menard and Chesselet1979). The values are interpreted as follows: EF < 2, deficiency to mineral enrichment; EF = 2–5, moderate enrichment; EF = 5–20, significant enrichment; EF = 20–40, very high enrichment; EF > 40, extremely high enrichment (Chakraborty et al., Reference Chakraborty, Bhattacharya, Singh and Maity2014).
Statistical analyses
All data were tested for homogeneity and homoscedasticity prior to analysis. MANOVAs, followed by a Tukey's multiple comparison test, as appropriate, were used to explore differences in mean metal concentrations between species, sites, and seasons of the year, separately for each metal (Zar, Reference Zar2010). Pearson's correlation coefficient was used to assess the correlation between metal concentrations separately for each lagoon. A significance level (α) of 0.05 was used for all tests; all statistical tests were carried out using the software STATISTICA 8 (StatSoft Inc., 2007).
Results
Metal concentrations in algae
Metal concentrations in algae showed significant differences between species, sites and seasons of the year (Table 3). The species Spyridia filamentosa showed significantly higher (P < 0.05) Fe, Ni and Cu concentrations relative to the other two species examined, as well as higher (P < 0.05) Zn and Pb concentrations than Ulva ohnoi. However, Padina mexicana and U. ohnoi showed higher Mn and Cd concentrations (P < 0.05; Table 3). There were significant differences between sites (P < 0.05). Algae from SJC had significantly higher Mn and Cd concentrations; algae from AL had higher Fe, Cu, Zn and Pb levels; and Ni was higher in algae from SJC and AL (Table 3). As regards seasons, Cu, Ni, Pb and Fe concentrations in algae were significantly higher (P < 0.05) during the cold season, while Mn and Cd showed no seasonal differences (Table 3).
Table 3. Mean (± std error) metal concentration (μg g−1) by species, site and season of the year (different superscripts denote significant differences)

Considering the three variables combined (i.e. site, species and season), significant differences in metal concentrations were also evident. The highest Fe concentration was found in Spyridia filamentosa from AL and PA in the cold season; Mn concentration was higher in S. filamentosa from SJC in the dry and rainy seasons and in Padina mexicana in the rainy season (Figure 2A, B). The highest Cu concentration was recorded in S. filamentosa from AL in the cold season. The highest concentration of Zn was found in S. filamentosa and P. mexicana from AL and PA in the three seasons (Figure 2C, D). The highest Ni concentrations were observed in S. filamentosa from AL, CP and PA in the cold season and in Ulva ohnoi from SJC in the cold season. The highest Cd concentrations were recorded in S. filamentosa and P. mexicana from SJC in the three seasons. The concentration of Pb was highest in S. filamentosa and U. ohnoi from AL and CP in the cold season (Figure 2E–G).

Fig. 2. Mean concentrations (μg g−1) of Fe, Mg, Cu, Zn, Ni, Cd and Pb in tissues of three macroalgae species, Spyridia filamentosa, Padina mexicana and Ulva ohnoi from different sites in three contrasting seasons of the year 2016. SJC, San Juan de la Costa; CM, Casa del Marino; TE, El Tecolote; AL, Almagre; PA, El Paraje; CP, Cerro Pastel; C, cold season; D, dry season; R, rainy season.
Bioaccumulation coefficient and enrichment factor
The bioaccumulation coefficient for each species showed values in excess of 50,000 for Fe, followed by values in the order of thousands for Mn and Pb, between 40–300 for Cu, Zn and Cd, and attained the lowest values for Ni at between 17–26. Spyridia filamentosa showed the highest bioaccumulation coefficient for all metals (Figure 3).
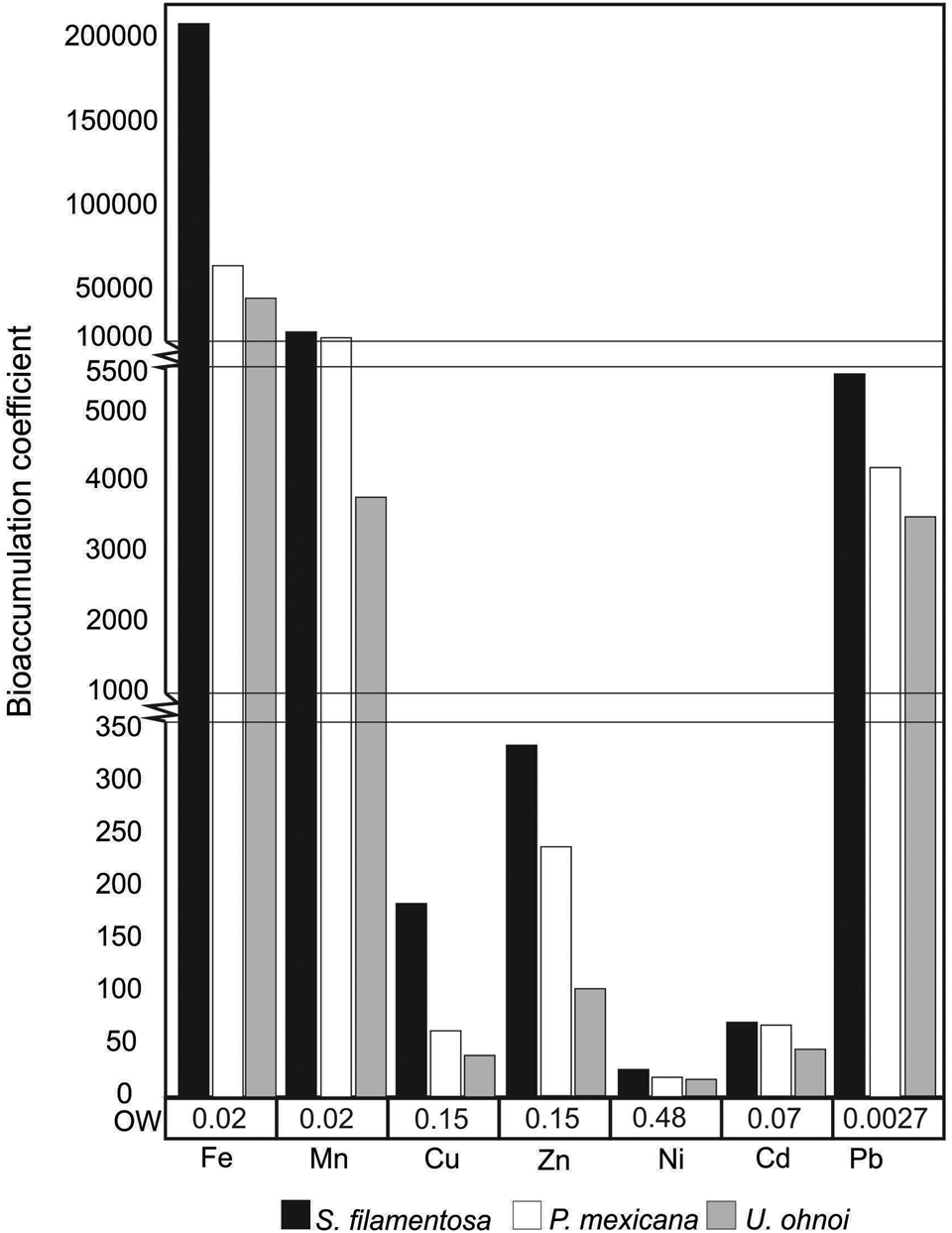
Fig. 3. Bioaccumulation coefficient of each macroalgae species in relation to water concentrations for each metal. OW = Oceanic Water concentrations (μg l−1).
In general, the Enrichment Factor attained values within the category Deficiency to Mineral Enrichment (<2) for all metals. The exceptions were Cu in Almagre Grande (Moderate Enrichment, 2–5) and Cd in SJC, with EF values ranging from Very High Enrichment (20–40) to Extremely High Enrichment (>40) (Figure 4).

Fig. 4. Enrichment Factor (EF) in macroalgae at each sampling site for each metal, in relation to sediment concentrations. * Sediment EF values from Pérez-Tiboullier (Reference Pérez Tribouillier2014). Note that some values were calculated specifically for this study, so they are approximate values. EF could not be calculated for iron. SJC, San Juan de la Costa; CM, Casa del Marino; TE, El Tecolote; AL, Almagre Grande; PA, El Paraje; CP, Cerro Pastel. Cc, sediment metal concentration of the continental crust (μg g−1).
Correlation between metal concentrations
Significant positive correlations were found between Fe-Mn, Fe-Cu, Fe-Zn, Fe-Ni, Fe-Cd, Fe-Pb, Mn-Ni, Mn-Cd, Cu-Zn and Zn-Pb at La Paz Bay. In contrast, Mn-Pb and Cd-Pb showed significant negative correlations. At Guaymas Bay, significant positive correlations were found between Fe-Mn, Fe-Cu, Fe-Zn, Fe-Ni, Fe-Pb, Mn-Zn, Mn-Cd, Cu-Zn, Cu-Ni, Cu-Pb, Zn-Pb and Ni-Pb (Table 4).
Table 4. Correlations between metal concentrations recorded in algae from La Paz Bay and Guaymas Bay in 2016

Values in bold are significantly different from zero.
Discussion
Previous studies reported significant differences in metal concentrations between sites and between species; this information is summarized in Table 5. From these studies, authors have found that, in general, brown algae showed the highest metal concentrations, followed by red and green algae (Sánchez-Rodríguez et al., Reference Sánchez-Rodríguez, Huerta-Díaz, Shoumilin, Holguín-Quiñones and Zertuche-González2001), while in the continental portion of the Gulf of California, green algae showed the highest concentrations (Páez-Osuna et al., Reference Páez-Osuna, Ochoa-Izaguirre, Bojórquez-Leyva and Michel-Reynoso2000). However, other studies have found no significant differences in metal concentrations between macroalgae species or groups (Rodríguez-Castañeda et al., Reference Rodríguez-Castañeda, Sánchez-Rodríguez, Shumilin, Sapozhnikov, Anderson, Brodie, Onsoy and Critcheley2006; Huerta-Diaz et al., Reference Huerta-Díaz, De León-Chavira, Lares, Chee-Barragán and Siqueiros-Valencia2007). These findings suggest that any of the macroalgae species could be used as a good indicator of metal concentrations. Further, longer-term studies examining particular species at different sites or focusing on physiological aspects of some of these species are needed to elucidate why some species accumulate more metals than others under certain conditions, as observed in the present study.
Table 5. Comparison of metal concentrations (μg g−1) recorded in different macroalgae species from the Pacific Coast and the Gulf of California

Metal concentrations in seaweed species may reflect their particular morphology, as those with a larger surface area have higher metal contents in tissues. Growth rates can also affect bioaccumulation patterns, with faster-growing specimens showing lower concentrations (Lobban & Harrison, Reference Lobban and Harrison1994). It has also been found that different seaweed species have distinct affinities for different heavy metals. This may reflect competition between metals for binding or uptake sites in the seaweed (Akcali & Kucuksezgin, Reference Akcali and Kucuksezgin2011). For example, in U. lactuca, trace elements can be adsorbed on the surface or stored in the cells within the cytosol (Turner et al., Reference Turner, Pedroso and Brown2008). Metal surface complexation prompts adsorption, whereas internalization can occur as a result of surface complexation or autonomously as a passive process in the case of lipophilic metal complexes (Mason et al., Reference Mason, Reinfelder and Morel1996; Bonanno et al., Reference Bonanno, Veneziano and Piccione2020). Brown algae are highly selective for bivalent metals, which they take up from seawater (Volterra & Conti, Reference Volterra and Conti2000).
Differences in metal concentrations in algae between sites can be found because of the source of metals and background metal concentrations, such as discharges of household and industrial effluents (Osuna-López et al., Reference Osuna-López, Páez-Osuna, Marmolejo-Rivas and Ortega-Romero1989; Páez-Osuna et al., Reference Páez-Osuna, Ochoa-Izaguirre, Bojórquez-Leyva and Michel-Reynoso2000) that influence metal bioavailability (Marín-Guirao et al., Reference Marín-Guirao, Lloret and Marin2008; Vizzini et al., Reference Vizzini, Costa, Tramati, Gianguzza and Mazzola2013; Hatje et al., Reference Hatje, de Souza, Ribeiro, Eça and Barros2016; Páez-Osuna et al., Reference Páez-Osuna, Álvarez-Borrego, Ruiz-Fernández, García-Hernández, Jara-Marini, Bergés-Tiznado, Piñón-Gimate, Alonso-Rodríguez, Soto-Jiménez, Frías-Espericueta, Ruelas-Inzunza, Green-Ruiz, Osuna-Martínez and Sánchez-Cabeza2017). For example, algae from SJC and AL generally showed the highest metal concentrations (Mn, Cd and Ni in SJC and Fe, Cu, Zn, Pb and Ni in AL); both sites are influenced by anthropogenic activities (one site is located in the vicinity of a mine and the second at the cargo port) and phosphorite mining operations at SJC might increase minerals draining off from the mine to coastal water during the extraction processes (Föllmi et al., Reference Föllmi, Schöllhorn, Ulianov, Adatte, Spangenberg, de Kaenel and Garrison2019).
No seasonal monitoring studies have been carried out previously in this region. Some studies have shown that metal concentrations in macroalgae vary with the season of the year (Páez-Osuna & Marmolejo, Reference Páez-Osuna and Marmolejo-Rivas1990; Jara-Marini et al., Reference Jara-Marini, Tapia-Alcaraz, Dumer-Gutiérrez, García-Rico, García-Hernández and Páez-Osuna2013a, Reference Jara-Marini, Tapia-Alcaraz, Dumer-Gutiérrez, García-Rico, García-Hernández and Páez-Osuna2013b, Reference Jara-Marini, Molina-García, Martínez-Durazo and Páez-Osuna2020). High metal concentrations recorded in the spring in several studies have been related to high photosynthetic rates as macroalgae biomass increases (Strezov & Nonova, Reference Strezov and Nonova2003). Seasonal differences in algal metabolism explain the variations observed in metal concentrations in algal tissue (Vasconcelos & Leal, Reference Vasconcelos and Leal2001).
High Fe concentrations in macroalgae, relative to other heavy metals, are associated with the role of this metal in organisms and high photosynthesis rates in tropical coastal habitats (Chakraborty et al., Reference Chakraborty, Bhattacharya, Singh and Maity2014). The high Fe concentrations recorded (2000 and 13,000 μg g−1) in the macroalgae studied were far above those recorded in other studies in the same region. Concentrations between 2000–7200 μg g−1 have been reported in the region (Páez-Osuna et al., Reference Páez-Osuna, Ochoa-Izaguirre, Bojórquez-Leyva and Michel-Reynoso2000; Sánchez-Rodríguez et al., Reference Sánchez-Rodríguez, Huerta-Díaz, Shoumilin, Holguín-Quiñones and Zertuche-González2001; Table 5). However, Fe accumulation by macroalgae could be up to three orders of magnitude higher when they grow in polluted environments (Gosavi et al., Reference Gosavi, Sammut, Gifford and Jankowski2004); this might be the case in AL and PA, particularly during the cold season, when these sites are exposed to more activity from the fishing season and increased port activities (Table 1). Other studies have related high Fe concentrations in macroalgae to industrial activities, as most macroalgae species bioaccumulate Fe from the surrounding environment (Storelli et al., Reference Storelli, Storelli and Marcotrigiano2001).
As Mn participates in macroalgae metabolism, it is likely to be found at higher tissue concentrations relative to other metals (Strezov & Nonova, Reference Strezov and Nonova2003). Similar to Fe, Mn values recorded in the present study were higher than those previously reported in the literature (Table 5). Such high Mn concentrations, particularly in SJC, may be related to the mining operations, regardless of the season. Rodríguez-Figueroa et al. (Reference Rodríguez-Figueroa, Shumilin and Sánchez-Rodríguez2009) found Mn concentrations of 20–860 μg g−1 in macroalgae from Santa Rosalía, which were related to mining extraction operations (Table 5). The correlations between the concentration of Fe, Mn, Cu and Zn at La Paz Bay and between Fe, Mn, Cu, Zn and Ni at Guaymas Bay suggest that all these metals come from common sources.
In Guaymas Bay, as already mentioned, there are inputs of various pollutants, particularly at AL from port operations such as loading and unloading of substances and materials, that cause spills of fuels, pesticides and additives with high contents of heavy metals such as Cu, Pb, Ni and Mn, in addition to the incidental release of copper (Méndez et al., Reference Méndez, Palacios, Acosta, Monsalvo-Spencer and Álvarez-Castañeda2006). At this site, Cu concentrations in macroalgae reached peak levels. Cu levels ranging from 200–300 μg g−1 have been recorded in species from polluted areas (Hawk et al., Reference Hawk, Melsom and Omang1974). However, this was the case only for AL since Cu concentrations at the other sites were lower than levels indicating polluted areas. Cu concentrations in macroalgae collected in other shipping ports have also been related to oil pollution and airborne pollutants released by commercial vessels (Chakraborty et al., Reference Chakraborty, Bhattacharya, Singh and Maity2014). Biggs & D'Anna (Reference Biggs and D'Anna2012) found that Cu concentrations in water increased soon after boats were introduced into marinas, as the primary sources of Cu were leachates from Cu-based paints from boats. Today, more than 1000 different types of boats and vessels arrive at Guaymas Bay (Méndez et al., Reference Méndez, Salas-Flores, Arreola-Lizarraga, Alvarez-Castañeda and Acosta2002).
According to Lobban & Harrison (Reference Lobban and Harrison1994), enzyme cofactors may regulate the concentration of Mn, Cu, Zn and Ni, whereas activators of dehydrogenases and enzymes involved in protein synthesis may also regulate Zn concentration. Elements such as Fe, Cu, Mn, Zn and Co (in vitamin B12) may accumulate in algal tissue as they are important nutrients (Huerta-Díaz et al., Reference Huerta-Díaz, De León-Chavira, Lares, Chee-Barragán and Siqueiros-Valencia2007). However, environmental concentrations are also important for determining the source of metals.
Nickel is an element associated with oil refining and combustion and marine traffic (Brito et al., Reference Brito, de Souza, Costa, Moura and Korn2016). We recorded Ni concentrations higher than those reported elsewhere, suggesting Ni contamination in our study areas, particularly at SJC, AL and PA. Similarly high Ni concentrations have been recorded in the marine seagrass Halodule wrightii Ascherson 1868 (9.56 μg g−1) from a site near the shipping routes of Todos os Santos Bay, Brazil (Brito et al., Reference Brito, de Souza, Costa, Moura and Korn2016), suggesting that algae growing in sites near shipping routes may show high Ni concentrations.
In benthic macrophytes in general, Zn levels below 100 μg g−1 are suggested as a benchmark for non-polluted areas (Moore & Ramamurti, Reference Moore and Ramamurti1987). Say et al. (Reference Say, Burrows, Whitton, McLusky, de Jonge and Pomfret J1990) proposed three concentration ranges (dry weight) of Zn in Ulva (as Enteromorpha) as an indication of the degree of pollution in estuaries: 50 μg g−1 for non-polluted areas, 50–150 μg g−1 for moderately polluted areas and 150 μg g−1 for heavily polluted areas. In our study, Zn concentrations in Ulva were all below 50 μg g−1; however, since we recorded higher values in Spyridia filamentosa (up to 73 μg g−1), we can assume that this indicates moderately polluted conditions, particularly at AL and PA. In the Gulf of Kutch in India, sources of Zn have been associated with effluents from the cement industry and a thermal power plant, as shown by the various algae species sampled (Chakraborty et al., Reference Chakraborty, Bhattacharya, Singh and Maity2014). In the surroundings of AL there is also an operating cement plant which can influence high Zn concentrations at this site. In contrast, the low Zn concentrations found at El Tecolote and Cerro Pastel indicate little or no influence from human activities. This is consistent with the results from a study on four macroalgae species from the Strait of Magellan, Chile, where Zn concentration was 41.76 μg g−1, well within the range considered indicative of unpolluted water (Astorga-España et al., Reference Astorga-España, Calisto-Ulloa and Guerrero2008).
Cadmium and Pb are toxic heavy metals that pose a global environmental issue. These heavy metals are non-essential, non-biodegradable and are bioaccumulated by absorption or uptake (Sarada et al., Reference Sarada, Prasad, Kumar and Murthy2014). Like the other metals, high Cd concentrations were found at San Juan de la Costa. In addition to mining, in this zone phosphate rocks are also primary sources of Pb, As, Cd and Zn (Nziguheba & Smolders, Reference Nziguheba and Smolders2008). Cadmium concentrations in algae suggest an evident variation in uptake levels across sites, showing that bioaccumulation largely depends on environmental pollution levels (Hashim & Chu, Reference Hashim and Chu2004; Chakraborty et al., Reference Chakraborty, Bhattacharya, Singh and Maity2014).
Although Pb was found in tissue from macroalgae collected in our study sites, Pb concentrations were within the range of the average abundance in the Earth's crust (3–30 μg g−1) (Smith et al., Reference Smith, Cameron, McDowell, Niemeyer and Sampson1996; Soto-Jiménez et al., Reference Soto-Jiménez, Hibdon, Rankin, Aggarawl, Ruiz-Fernandez, Páez-Osuna and Flegal2006). Burdon-Jones et al. (Reference Burdon-Jones, Denton, Jones and McPhic1975) and Agadi et al. (Reference Agadi, Bhosle and Untawale1978) reported that Pb levels greater than 100 μg g−1 (dry weight) are related to contaminated water (Storelli et al., Reference Storelli, Storelli and Marcotrigiano2001; Akcali & Kucuksezgin, Reference Akcali and Kucuksezgin2011). However, the Pb concentrations recorded in the present study were below this limit; therefore, our study sites can be considered non-polluted by Pb. As for the other metals quantified, the levels in macroalgae associated with seawater pollution are yet to be determined.
In the Gulf of California, metal concentrations in the water column have been determined, but data are still scarce; some of the available data are summarized in Table 6. According to these data, oceanic water levels in the North Pacific are low, while seawater concentrations in samples from areas with anthropogenic activities are higher. The values recorded in macroalgae in our study are several orders of magnitude higher than the values reported in seawater, consistent with previous literature reports suggesting that macroalgae bioaccumulate metals present in the surrounding water.
Table 6. Comparison of metal concentrations recorded in different sites influenced by various anthropogenic activities from the Pacific Coast and Gulf of California (sediment or water)

Metal concentrations in sediments obtained for locations resembling our sampling sites were similar to the levels recorded in macroalgae (Table 6), also indicating the relationship with a pristine site or a site with anthropogenic activity, as discussed above. Metal concentrations in macroalgae vary greatly between sites and between seasons, and there are also insufficient data on metal concentrations in seawater and sediments for the study area. Consequently, it was impossible to conclude whether macroalgae are effectively bioaccumulating elements or are suitable bioindicators of environmental conditions. For this reason, we used the Enrichment Factor, which has been used for sediments and describes a range of environmental conditions, from mild to heavily polluted by metals (Loska et al., Reference Loska, Cebula, Pelczar, Wiechuła and Kwapuliński1997; Kaushik et al., Reference Kaushik, Satya and Naik2009). Enrichment Factor values for Mn, Zn and Pb in macroalgae were lower than in sediments, except for Mn at SJC and Pb at ET. In contrast, Cu levels were higher at Almagre Grande and El Paraje, while Ni and Cd showed high concentrations in all sites. This suggests that macroalgae are suitable indicators of both metals and anthropogenic activities, as the high values observed in each site were consistent with anthropogenic activities. These findings agree with the observations reported by Pérez-Tribouillier (Reference Pérez Tribouillier2014) for La Paz Bay, where conditions ranged from unpolluted to low mineral levels. Chakraborty et al. (Reference Chakraborty, Bhattacharya, Singh and Maity2014) found different enrichment values for several metals in sediments, depending on the anthropogenic activities in the vicinity of the sites studied. In the present study, metal concentrations in macroalgae were higher than metals concentrations in seawater in all cases (Figure 3) and, occasionally, metal concentrations in algae exceeded those in sediments, except for sites on the coast of Sonora subjected to higher impacts from anthropogenic activities (Table 6). In a study by Pan et al. (Reference Pan, Wernberg, de Bettignies, Holmer, Li, Wu and Xiao2018), the bioaccumulation coefficient for metals in macroalgae was tens- to thousands-fold higher relative to metal levels in seawater, which is consistent with the observations in the present study. These findings support the conclusion that the Enrichment Factor and the bioaccumulation coefficient estimated in macroalgae are good indicators of metal concentrations in the environment where macroalgae thrive.
Our findings suggest that the macroalgae species examined might be suitable indicators of marine contamination since they are easy to collect and provide detailed information on metals in their local environment. Of the species studied, Spyridia filamentosa could be the best indicator for Fe, Ni, Cu, Zn and Pb, and P. mexicana and U. ohnoi for Mn and Cd concentrations. In this ranking order, any of the three species can be used as an indicator of metal concentrations. These species may be sampled year-round in most coastal regions during low tide; sorting macroalgae samples into species is strongly recommended, assisted with particular keys according to the region (e.g. Norris, Reference Norris2010, Reference Norris2014) or using the tool AlgaeBase (Guiry & Guiry, Reference Guiry and Guiry2021). This approach would allow any investigator or environmental manager to collect macroalgae species to monitor environmental metal concentrations, and is also cost-effective since macroalgae can be collected from the shore in some regions. Monitoring activities in coastal regions are relevant given the growing human populations in these areas, leading to increased anthropogenic pressure on marine environments.
Conclusions
Spyridia filamentosa concentrated the largest amounts of metals and is therefore regarded as the best indicator, followed by P. mexicana and U. ohnoi. Our findings indicate that the three species studied are suitable for monitoring metal concentrations in marine environments. The bioaccumulation coefficient and the Enrichment Factor showed that macroalgae are suitable indicators of seawater quality and metal concentrations in the surrounding environment. Metal concentrations in the three macroalgae species studied varied between sites according to environmental pollution derived from anthropogenic activities during the cold season. The site with the highest Cd and Mn concentrations was San Juan de la Costa (SJC); the highest Fe, Zn, Cu, Pb and Ni concentrations were recorded in algae collected from Almagre Grande (AL). The high metal concentrations in macroalgae from SJC are related to mining activities, while those found at AL are related to industrial activities such as canneries and maritime traffic, among others. Metal concentrations in macroalgae indicate that environmental concentrations are highly variable in both bays. This was expected since anthropogenic activities, including maritime traffic, sardine fishing, industrial activities (especially canneries), mining, and port operations, are also highly variable in terms of quantity, intensity and season when such activities take place.
Acknowledgements
We thank Efrain López Montaño and Edgar Alcántara for their assistance in the field with sample collection and Baudilio Acosta Vargas and Griselda Peña Armenta for analytical work. We also thank Mr Refugio López Tapia from the Laboratorio de Química of CIBNOR for his technical assistance. This study was funded by Consejo Nacional de Ciencia y Tecnología (funder Id: http:/dx.doi.org/10.13039/501100003141) through research grant CONACYT-CB154415, by CIBNOR through grant PC 0.5, and by SEP-CONACyT through grant A1-S-26700. Piñón-Gimate, Cervantes-Duarte and Casas-Valdez thank the Instituto Politécnico Nacional (funder Id: http:/dx.doi.org/10.13039/501100003069) for Estímulo al Desempeño a la Investigación (EDI) and Comisión de Fomento a las Actividades Académicas (COFAA) scholarships. María Elena Sánchez-Salazar edited the English version of the manuscript.












