INTRODUCTION
Crangon crangon, the common brown shrimp, settles sedimentary and coastal areas at 0–20 m, but including maximum depth to 130 m. The brown shrimp popularly is found in the north-eastern Atlantic, in the Baltic Sea, Mediterranean Sea and in the Black Sea (Holthuis, Reference Holthuis1980). The maximum size is 89 mm, but mainly shrimps are 30–40 mm in length. (Tiews, Reference Tiews1970). Reproduction occurs several times a year, to maximum age 3–5 years (Luttikhuizen et al., Reference Luttikhuizen, Campos, van Bleijswijk, Peijnenburg and van der Veer2008). It is an important taxon, ecologically, commercially and economically (Luttikhuizen et al., Reference Luttikhuizen, Campos, van Bleijswijk, Peijnenburg and van der Veer2008). Crangon crangon is the major predator of shallow marine ecosystem (Van der Veer and Bergman, Reference Van der Veer and Bergman1987) and is prey of few larger fish (Amara et al., Reference Amara, Laffargue, Dewarumez, Maryniak, Lagardère and Luczac2001). Crangon crangon is a commercially fished resource, and is food for many marine animals, being the major food for flatfish (Pleuronectes platessa), crabs (Carcinus maenas), seals (Phoca vitulina), various waders (Limicolae), seagulls (Laridae) and auks (Alcidae) (Jung & Zauke, Reference Jung and Zauke2008). This benthic organism, with Carcinus maenas, represents 90% of macrocrustaceans in the shallow marine ecosystem (Selleslagh & Amara, Reference Selleslagh and Amara2008). Muscles (tails), heads and shells of Crangon crangon are rich in proteins, calcium, vitamins, flavour components, natural antioxidants (Heu et al., Reference Heu, Kim and Shahidi2003) and lipids like fatty acids (FAs) and essential fatty acids (Holub, Reference Holub2002). Moreover, recent research suggests the possibility of the shrimp shells application to ‘transform production of biodiesel fuel into a faster, less expensive and more environmentally friendly’ process. Chitin only represents 69% dry weight of shrimp shell, and in appropriate conditions chitin can be transformed into saccharides, which have relatively high catalytic performance for biodiesel production, exhibiting interesting acid catalytic properties (Yang et al., Reference Yang, Zhang and Zheng2009).
Crangon crangon is the main supplier of essential fatty acids (Holub, Reference Holub2002). The concentration of two main essential fatty acids in C. crangon amounted to about 300 mg/100 g, and it is comparable with the concentration of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in cod (Holub, Reference Holub2002).
Lipids play an important role in the organism: as a source of energy, precursors of steroid hormones, intercellular mediatiors, compounds of phospholipids and sphingolipids, molecules are responsible for increasing protein barrier integrity and, most importantly, dietary lipids are essential representatives of every living cell (Zhao et al., Reference Zhao, Jia, Gao, Li, Lu, Li and Xu2008). Lipids are important in parasites (Mika et al., Reference Mika, Gołębiowski, Szafranek, Rokicki and Stepnowski2010), insects (Gołębiowski, Reference Gołębiowski2012; Gołębiowski et al., Reference Gołębiowski, Boguś, Paszkiewicz, Wieloch, Włóka and Stepnowski2012, Reference Gołębiowski, Cerkowniak, Boguś, Włóka, Dawgul, Kamysz and Stepnowski2013), marine organisms (Mika et al., Reference Mika, Gołębiowski, Skorkowski and Stepnowski2012) as well as in mammals (Green et al., Reference Green, Martinez-Coria, Khashwji, Hall, Yurko-Mauro, Ellis and LaFerla2007). It is impossible for humans to synthesize some lipids, which are needed for different life processes. The new function of two main acids (EPA, DHA) is ‘immunonutrients’—with a role in beneficial effects on inflammatory bowel diseases and autoimmune disease (Alexander, Reference Alexander1998; Stulnig, Reference Stulnig2003), a reduction of cancer effect (Holub, Reference Holub2002; Logan, Reference Logan2004) and an increase of intensity of the immune system (Sijben & Calder, Reference Sijben and Calder2007; Zhao et al., Reference Zhao, Jia, Gao, Li, Lu, Li and Xu2008). Marine organisms—fish, mussels and crustaceans—are rich in polyunsaturated fatty acids (PUFAs), especially the fatty acids omega-3 (Bragagnolo & Rodriguez-Amaya, Reference Bragagnolo and Rodriguez-Amaya2001; Garofalaki et al., Reference Garofalaki, Miniadis-Meimaroglou and Sinanoglou2006). These organisms have important commercial nutritional and economic consequences (Garofalaki et al., Reference Garofalaki, Miniadis-Meimaroglou and Sinanoglou2006). The higher levels of sterols (especially cholesterol) with highly unsaturated fatty acids are important components of biological membranes (Le Nechet et al., Reference Le Nechet, Dubois, Gouygou and Berge2007), are the intermediaries in the biosynthesis of ecdysteroids (Boutry et al., Reference Boutry, Saliot and Barbier1979) and are important in reproductive biochemistry (Allen, Reference Allen1999). The concentration and amount of fatty acids and sterols, and also the content of unsaturated fats, depend on many factors. The main reasons for change in the composition of the fatty acids (FAs) are: access to food and direct access to essential fatty acids omega-3, as well as to their precursors, linoleic acid 18: 2 n-6 and α-linolenic18: 3 n-3, which create the endogenous fatty acids (Maazouzi et al., Reference Maazouzi, Masson, Soledad Izquierdo and Pihan2007). However, the compositions of fatty acids and sterols are also dependent on weather conditions, climate (Limbourn & Nichols, Reference Limbourn and Nichols2009), seawater temperature (Philips et al., Reference Philips, Jeffs, Melville-Smith, Chubb, Nelson and Nichols2006), and season (Kasai & Sakai, Reference Kasai and Sakai2004; Garofalaki et al., Reference Garofalaki, Miniadis-Meimaroglou and Sinanoglou2006). Their composition changes with the availability of plankton (Limbourn & Nichols, Reference Limbourn and Nichols2009) and directly with the type of the analysed tissue (Perez-Velazquez et al., Reference Perez-Velazquez, Gonzalez-Felix, Lawrence and Gatlin III2003). The lowest-energy calorific and poorly energetic components are present in the winter–spring period (November–March) (Campos et al., Reference Campos, Van der Veer, Freitas and Kooijman2009).
The primary aim of the current research is the determination of variation in total lipids, lipid classes and fatty acids composition, particularly the fatty acid profiles in the triacylglycerols (TAGs), as well as the sterols profiles. We examined quantitative lipid composition in the abdomen muscle of C. crangon in winter and spring.
MATERIALS AND METHODS
Chemicals and reagents
Chemicals were obtained as follows: dichloromethane (high-performance liquid chromatography (HPLC) grade), ethanol (99.8%), methanol (HPLC grade), aceton (HPLC), n-hexane (HPLC), acetonitrile (HPLC), isooctane (analytically pure), 0.9% NaCl from Chempur (Piekary Śląskie, Poland), 2,5-dihydroxybenzoic acid (DHB) (HPLC grade) from Sigma-Aldrich (Poznań, Poland), and NaCl (0.9%) from Fresenius-Kabi (Kutno, Poland). Ethanol (99.8%), sodium hydrate (pills) were purchased from POCH S.A. (Gliwice, Poland) and BSTFA + TMCS (99:1) (BSTFA (N, O-bis(trimethylsilyl)trifluoroacetamide) and TMCS (trimethylchlorosilane)) was obtained from Supelco (Bellefonte, USA).
Biological material
Baltic shrimps were caught in the coastal area of the Gulf of Gdansk near Sobieszewo Island on 22 December 2010 (seven adults, weight 2236 mg) and 6 April 2011 (6 adults, weight 1953 mg) at 54°35′88″N 18°83′16′′E. Shrimps were collected from a depth between 80 and 100 m both in the winter and spring. The Crangon crangon were identified, sexed, measured, recorded and sacrificed shortly after collection. Only non-ovigerous females were used for the analysis. Their abdominal muscles were dissected, separated from the cuticles and frozen at −80°C in glass tubes. Before the analytical procedure the abdominal muscles were weighted frozen and then water was removed from the examined tissues by lyophilization. The tissues were homogenized (dissected completely) in physiological saline (0.9% NaCl) and lipids were extracted by using 20 ml dichloromethane for 20 min. The low weight of shrimps during these two periods was determined and the low number of marine organisms.
HPLC analyses
The solvents of all the extracts were evaporated in tared flasks on a roto evaporator and under a stream of nitrogen. Lipid extracts were separated into several classes using high performance liquid chromatography with laser light scattering detector (HPLC–LLSD) (Shimadzu, Japan) in the normal-phase on an analytical column (250 × 4.6 mm) filled with Econosil Silica (particle size = 5 µm). The mobile phase consisted of hexane (Solvent A) and dichloromethane with the addition of 15% acetone (Solvent B). The gradient was programmed linearly from A to B within 20 min.
GC–MS analyses
The free fatty acids (2) and sterols (3) fractions separated by HPLC–LLSD were silylized by treatment with 50 µl of 1% bis(trimethylsilyl)acetamide and 99% chlorotrimethylsilane (Sigma Aldrich), and heated at 100°C for 1 h. Triacylglycerols (1) and polar lipids (PL) (4) (detected in lipids of spring period only) were hydrolysed by heating in 100 µl of a methanolic solution of 0.5 M KOH for 3 h at 70°C in sealed ampoules. Analyses of the lipid derivatives were carried out by gas chromatography mass spectrometry (GC–MS) on a Hewlett Packard GC-MS 5890 (Finnigan Mat) equipped with a fused silica capillary column HP-5, 30 m × 0.25 mm i.d., and with film of thickness of 0.25 µm. The GC–MS was programmed from 60°C to 300°C at the rate of 4°C/min, with helium carrier gas at the column head pressure of 60 kPa.
RESULTS
Total lipid content and lipid classes
The chemical composition of lipids showed three classes during the winter period and four lipid classes during the spring (Figure 1). The following lipid classes were present in the winter extract: triacylglycerols (TAG); free fatty acids (FFA); and sterols. The polar fraction (PL) was present only in the spring extract. Table 1 summarizes differences not only in the number of classes but also the domination of classes and quantitative composition. Considerable difference was seen in the total lipid content. Level of lipids was three times higher during the spring period. The total lipid content of Crangon crangon abdomen muscle ranged from 1.1% during the winter to 3.2% of total dry mass in the spring. The percentage contents of particular fractions were as follows: TAGs 20.5% (winter) and 10.0% (spring), FFAs 44.5% (winter) and 63.0% (spring), sterols 35.0% (winter—only cholesterol) and 17.0% (spring), and PLs detected only in spring, with the same value as the TAG fraction, 10.0%. However, in both cases, the FFAs were the major lipid class and the next dominated group was sterols (Table 1).
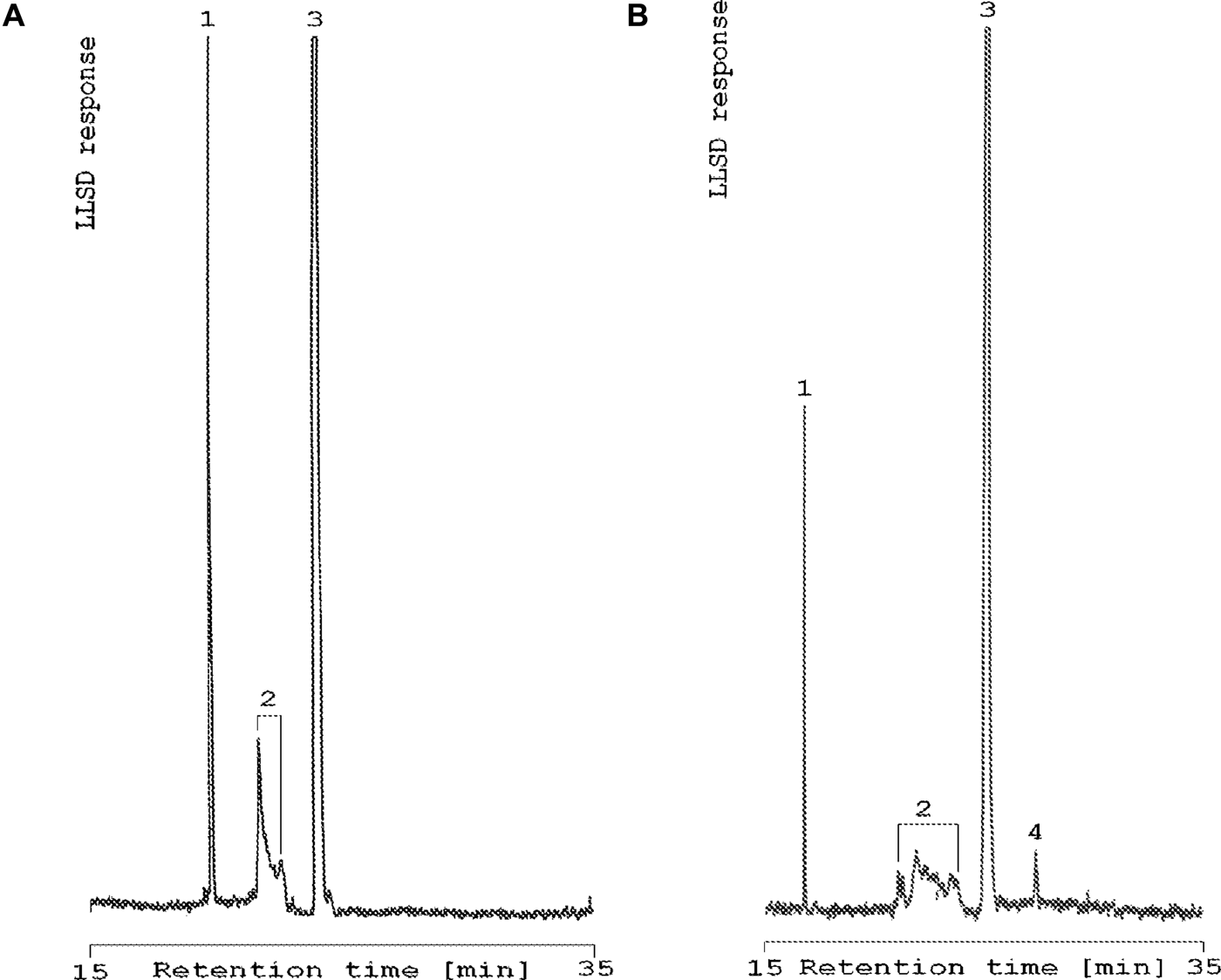
Fig. 1. Separation of lipid classes of abdomen's muscle of Crangon crangon during winter (A) and spring period (B) using a normal-phase HPLC method and the LLSD detector. 1, Triacylicerols; 2, free fatty acids; 3, sterols; 4, polar lipids fraction.
Table 1. Lipid content and class composition of adult Crangon crangon determined during winter and spring period. Number of measurements N = 3.

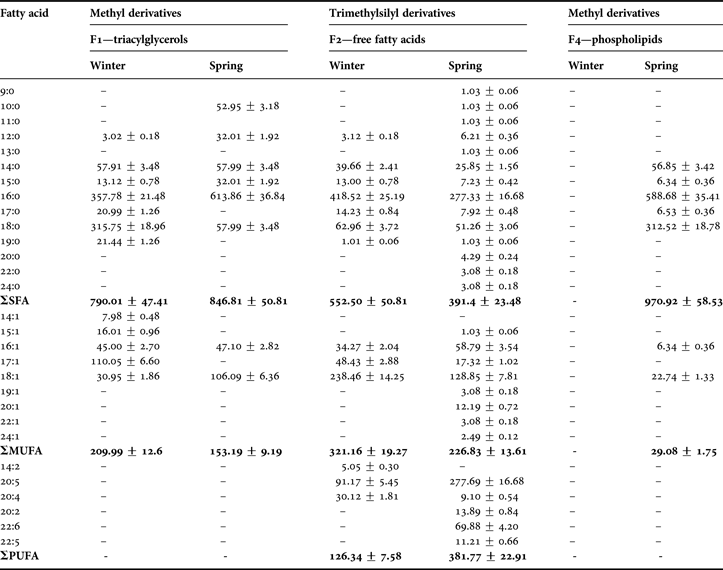
The fatty acids composition
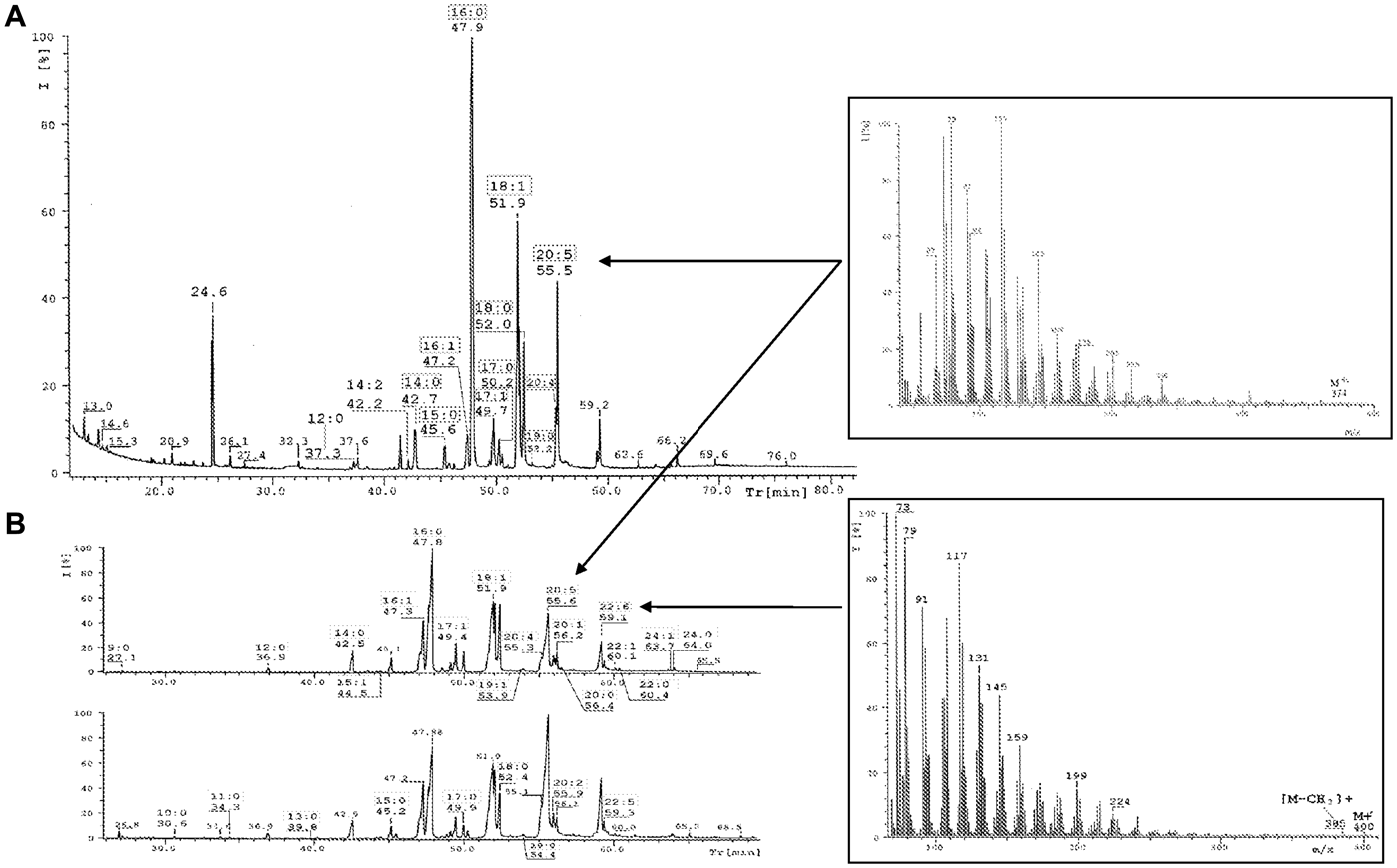
Table 2 summarizes the results obtained during the identification and quantification of fatty acids (FAs) in the lipid fractions of C. crangon abdomen muscle. Among the FFAs were 13 FAs in winter (seven saturated (SFA), three monounsaturated (MUFA) and three polyunsaturated (PUFA) fatty acids) and 27 FAs in spring (14 SFAs, eight MUFAs and five PUFAs). Among TAGs there was a higher diversity in winter. 12 FAs were identified, including seven SFAs and five MUFAs, with no PUFAs present. A similar profile was identified in spring, where among eight FAs, only two were unsaturated, with one double bond. Seven FAs identified (as fatty acids methyl esters (FAMEs)) in the PL fraction were the same as in the TAG fraction: only SFAs (7) and MUFAs (2). Only one essential fatty acid (EFA)—EPA; 20:5 n-3—was identified during winter time (Figure 2). In the second period DHA; n−3 was identified additionally (Figure 2).

Fig. 2. The total ion current chromatogram of fatty acid trimethylsilyl ethers and mass spectrum of the TMS of EPA and DHA during winter (A) and spring period (B) detected by GC–MS.
Table 2. Main fatty acids in polar and neutral lipid fractions of abdomen muscle's of adult Crangon crangon detected by GC–MS methods during winter and spring period (µg/g dry mass). Number of measurements N = 3.

The major FAs in the TAG fraction (winter period) were: 16:0 (35.8%), 18:0 (31.6%), 17:1 (11.0%), 14:0 (5.8%) and 16:1 (4.5%). The similar composition dominated the FAs recorded in the spring period, but their percentage composition differed significantly. The amount of acid 16:0 was almost 62%, the amount of 18:0 was much lower (only 5.8%) and acids 14:0 and 16:1 had almost the same percentage composition (5.7% and 4.7%, respectively) (Table 2). TAG fraction was more abundant in unsaturated FAs in winter than in spring. Additionally, the acids 14:1, 15:1, 17:1 and two saturated with 17 and 19 carbon atoms were present. In the spring sample, in the FFA fraction was noticed diversity. Nonetheless the percentage content of fatty acids was very different. Lipids contained 19 FAs present in concentrations from 0.1% to 1.4%. In this range, the dominant fatty acids were 20:2 (1.4%) and 22:5 (1.1%). In this fatty acids profile were detected acids: 22:6 n-3 (7%) and 20:5 n-3 (27.8%). 20:5 acid was the major compound also in the second period (9%). Additionally, arachidonic acid 20:4 n-6 was detected at 3% in winter and 0.9% during the spring. In this fraction, similar to the TAG spring fraction, acids 16:0 (42% and 27.8%) and 18:1 (24% and 13%) dominated in winter and spring, respectively. Acid 14:0 was abundant in other acid profiles, but in the FFA fraction was present only in smaller percentages.
Among polar fraction in spring time, dominant saturated acids are mainly 16:0 (59%) and 18:0 (more than 31%), as well as acid 14:0 (5.7%—percentage content very similar to the remaining profiles of FAs). The common feature of all fatty acid profiles was dominance of SFAs as the result of organic material which originated from fresh phytoplankton food (spring) and phytodetritus (Maazouzi et al., Reference Maazouzi, Masson, Soledad Izquierdo and Pihan2007). The following typical SFAs were present: 12:0, 14:0, 15:0, 16:0 and 18:0 and even 17:0 (absent in the TAG spring sample) (Table 2). These acids almost always were characteristic in all lipid profiles for Baltic marine shrimp during the two periods. The next feature was the presence of MUFAs, mostly 16:1 and 18:1 in all fractions and 17:1 during the winter period (in TAG fraction acid comprising 11% of total FAs). In the polar lipids and TAG fractions EPA and DHA acids were not detected. While the FFA fraction was dominant in the spring sample, this class was two times more abundant in PUFAs than the other fractions in this sample and in winter time.
Sterols
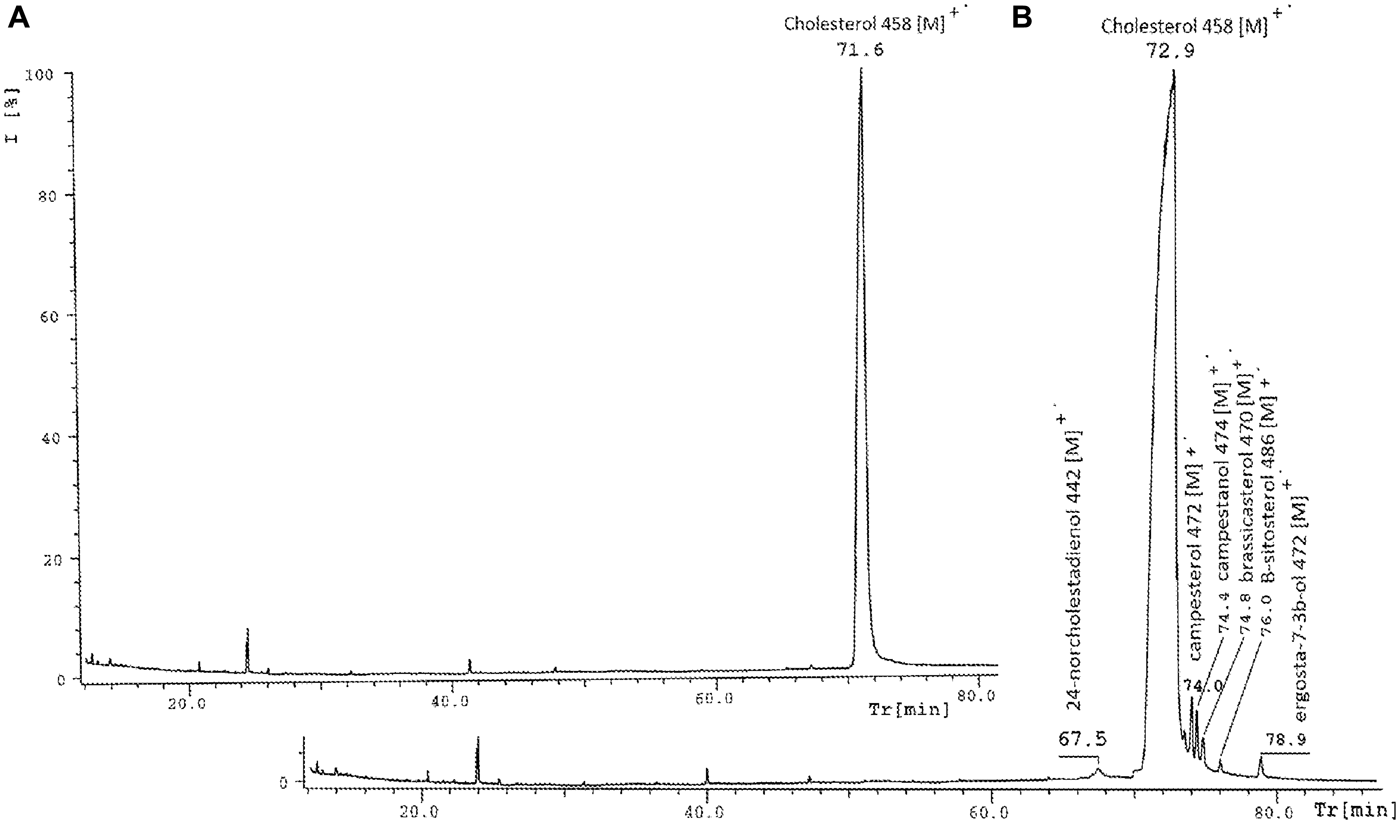
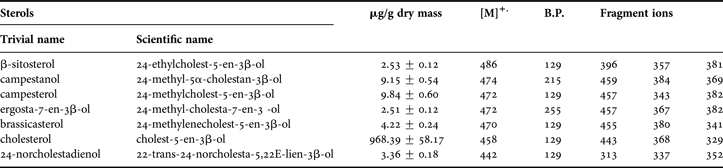
The bigger diversity was in sterol fractions. The sterols composition of C. crangon abdomen muscle is shown in Table 3. The spring sample was very rich. The following sterols were identified: 24-norcholestadienol (retention time (RT) 67.5 min); cholesterol (RT 71.9 min); campesterol (RT 74.0 min); campestanol (RT 74.4 min); brassicasterol (RT 74.8 min); β-sitosterol (RT 76.0 min); and ergosta-7-en-3β-ol (RT 78.9 min) (Figure 3). Table 3 shows the characteristic ions of sterol trimethylsilyl ethers such as: 24-norcholestadienol—m/z 442, 313, 337, 352, cholesterol—m/z 458, 443, 368, 329, campesterol—m/z 472, 457, 343, 382, campestanol—m/z 474, 459, 384, 369, brassicasterol—m/z 470, 455, 380, 341, β-sitosterol—m/z 486, 396, 357, 381 and ergosta-7-en-3β-ol—m/z 472, 457, 367, 382. Characteristic ion for all sterols, except of campestanol (m/z 215) and ergosta-7-en-3β-ol (m/z 255) was m/z 129 peak. In the winter time only cholesterol was identified. Among eight sterols identified in this study, seven representatives appeared in traces. Only campesterol and campestanol had percentage content close to 1% (campesterol 1.0% and campestanol 0.9%). The other sterols ranged from 0.4% to 0.2%. Cholesterol was predominant among the group of sterols and amounted to 97% (Table 3).

Fig. 3. Mass spectra of the TMS derivatives of sterols in abdomen's muscle of Crangon crangon during winter (A) and spring period (B) detected by GC–MS.
Table 3. Molecular masses and major fragmentation ion of TMS ether derivatives of each peak of Crangon crangon collected in GC–MS during spring period. Number of measurements N = 3.

DISCUSSION
Winter and spring periods differed considerably in total lipids content, lipid classes, fatty acids and sterols composition. It was caused particularly by adaptation to various conditions of weather, temperature of water (in lower temperature shrimp are going to in lower level of sea and settling in the sand or muddy bottom (Holthuis, Reference Holthuis1980)). The second main case is the lack of food, especially of zooplankton and phytoplankton or other sources of nutrition (Donaldson, Reference Donaldson1976). The muscle lipid of the abdomen of Crangon crangon in winter and spring time amounted to 24.0 ± 1.29 mg/g dry weight (1.1% dry mass) and 63.0 ± 4.08 mg/g dry weight (3.2% dry mass), respectively. This lipid content of the abdomen's muscle was similar to another crustacean Palinurus vulgaris (1.0–2.6%) (Garofalaki et al., Reference Garofalaki, Miniadis-Meimaroglou and Sinanoglou2006). The lower values of total lipid content are dependent upon the degree of development of shrimp—the larval stage contained much more lipid than the adult stage (Allen, Reference Allen1999), which the shrimp used for growth and moulting (Tocher, Reference Tocher2003), survival and pigmentation success (Coutteau & Mourente, Reference Coutteau and Mourente1997).
During spring four lipid classes (TAGs, FFAs, sterols and PLs) were detected. The PLs are the major part of the lipid composition (Garofalaki et al., Reference Garofalaki, Miniadis-Meimaroglou and Sinanoglou2006; Limbourn & Nichols, Reference Limbourn and Nichols2009). In our study, the content of PLs was the same as the TAG content (10% dry mass) and this group was diverse and abundant (Table 2). Small amounts or the lack of PL detection may be dependent upon the detection limit of liquid chromatography to this type of compound (Allen, Reference Allen1999) and method used for separation of the abundant lipid group. In our research, HPLC–LLSD was used. Triacylglycerols occur as an energy source in storage lipids in adult shrimp (Allen, Reference Allen1999), but PLs contained much more PUFAs than TAGs. Moreover, neutral lipids are transformed to PLs, transported to ovaries and exploited during development, reproduction and maturation (Mourente & Rodriquez, Reference Mourente and Rodriquez1991). Polar lipids, as easily emulsified, are better sources of PUFAs than neutral lipids (TAGs or wax esters) (Limbourn & Nichols, Reference Limbourn and Nichols2009). In our work, among all determined lipid fractions during the spring period, the highest diversity characterized FFAs and sterols. The sterols fractions were determined as follows: 4-norcholestadienol, cholesterol, campesterol, campestanol, brassicasterol, β-sitosterol, and ergosta-7-en-3β-ol, which are the characteristic sterols of crustaceans. They lack the capacity to synthesize sterols, and among 16 known sterols, none is synthesized (Kanazawa, Reference Kanazawa2001), so all sterols come from dietary sources (Virtue et al., Reference Virtue, Nicol and Nichols1993; Kanazawa, Reference Kanazawa2001). Sterols are converted by crustaceans into the requisite cholesterol (Virtue et al., Reference Virtue, Nicol and Nichols1993). In turn, amongst the FFA samples, 27 acids with from nine to 24 carbon atoms were determined. There were saturated acids and also monounsaturated, polyunsaturated and highly unsaturated fatty acids. Approximate composition was noted in other studies (Jeffs et al., Reference Jung and Zauke2002; Limbourn & Nichols, Reference Limbourn and Nichols2009). As reported by some authors (Jeffs et al., Reference Jeffs, Phleger, Nelson, Mooney and Nichols2002), SUFA dominated in spring period, which agrees with our results. Monounsaturated fatty acids were the second most popular subclass of FAs, which was a surprise because PUFAs occurred in the lowest numbers. Additionally, in our study, short-chain fatty acids (>9:0) and 23:0 were detected. The rest of the fatty acids composition was the same (Table 2 and Jeffs et al., Reference Jeffs, Phleger, Nelson, Mooney and Nichols2002).
In winter three groups of lipids (TAGs, FFAs and sterols) were identified and the least abundant was the sterols fraction, where only cholesterol occurred at levels above the detection limit. The great variety of marine sterols is dependent on human production. Some drugs accumulate in the marine environment, and a drug used in human and veterinary medicine blocks sterol synthesis by inhibiting cytochrome P450-dependent 14α-demethylases (Stenersen, 2004). Cholesterol is synthesized in animals via the 14α-demethylase substrate lanosterol (Porsbring et al., 2009), clotrimazole exposure decreases the level of cholesterol and its derivatives: 22-dehydrocholesterol and desmosterol (Porsbring et al., 2009). It is probably the cause of the low level or absence of the other sterols during the winter period. On the other hand, high variability of crustaceans sterols is related to nature of the environment and adaptation to difficult environmental conditions (Boutry et al., Reference Boutry, Saliot and Barbier1979). Moreover, crustaceans cannot synthesize sterol de novo, and the basic level of their sterols is lower and they must have dietary source of sterols. Therefore crustaceans collect these lipids' elements from chemoautotrophic and phytodetrital source of nutrition (Allen, Reference Allen1999). Membrane permeability, cell plasticity and the exchange of solutes depends on the variations of the side chain structures (Boutry et al., Reference Boutry, Saliot and Barbier1979), hence there is such a large number of all sorts of steroidal compounds at living organisms. It is difficult to say much about the dominant lipid fraction from the analysed percentage content of individual factions from the winter sample, because percentage value of each fraction was very similar. During spring the situation was similar, percentage contents of TAGs, sterols and PLs were similar (10%, 17%, 10%, respectively), and only FFAs occurred in higher amounts (63%). Donaldson also described smaller values of lipids in winter and spring, and indicated this period as richer than during other seasons of the year (Donaldson, Reference Donaldson1976).
In each lipid sub-sample the same composition of typical acids is repeated: 14:0, 15:0, 16:1, 16:0, 18:1 and 18:0. Similarly as described by Clarke, the major fatty acids in all neutral and polar lipid fraction were 14:0, 16:1, 16:0, 18:0, 18:1, 20:5, 22:6, characteristic compounds for benthic crustaceans (Clarke, Reference Clarke1979; Virtue et al., Reference Virtue, Nicol and Nichols1993). In our study, only the spring sample of FFAs contained 14:0 and 18:0. Polyunsaturated fatty acids descended from another source of food—phytoplankton. Main acids: EPA and DHA are biomarkers for phytoplankton, of which a significant amount was recorded during spring (Allen, Reference Allen1999; Lee et al., Reference Lee, Hagen and Kattner2006). Additionally, PUFAs are needed for working membranes in a deep-sea environment. For example, long-chain unsaturated fatty acids of shrimps (e.g. acid 18:1) can derive from bacteria, which are characteristic of deeper areas (Allen, Reference Allen1999). The major differences were observed between the TAG fraction during winter and spring. The winter sample of TAGs was much more varied; however, not in the number of PUFAs, but only in number of SFAs and MUFAs. Monounsaturated fatty acids are used by crustaceans as a primary source of energy (Limbourn & Nichols, Reference Limbourn and Nichols2009). Thus, the main food source in winter is phytodetritus, which is rich in these components (Maazouzi et al., Reference Maazouzi, Masson, Soledad Izquierdo and Pihan2007).
The following factors are decisive in the amounts and diversity of FAs: the weather; the temperature of water; and the habitat. The PUFA content is correlating with the life cycle of C. crangon—during spring and summer the development and the growth of young larval stages occurs (Abad et al., Reference Abad, Ruiz, Martinez, Mosquera and Sánchez1995). Simultaneously, the lowest contents of energy elements and the lowest values of the energy are noted in the period November–March (Campos et al., Reference Campos, Van der Veer, Freitas and Kooijman2009), which explains the low level or the lack of PUFA in autumn and winter (Mika et al., Reference Mika, Shorkowski, Stepnowski and Gołębiowski2013 and Table 2). The next factor—habitat—is directly connected with the type of food taken up. The bigger quantity of omega-3 FA comes from fresh organic matter, whose source is the phytoplankton inhabiting the water depths. Algae and a waterside flora are rich in acids omega-6, as well as detritus, which provides SFAs additionally (Abad et al., Reference Abad, Ruiz, Martinez, Mosquera and Sánchez1995; Maazouzi et al., Reference Maazouzi, Masson, Soledad Izquierdo and Pihan2007). The next factor is the duration of solar exposure of water, causing an increase of saturated acids (Kasai & Sakai, Reference Kasai and Sakai2004). Concurrently type of the analysed tissue is also deciding on the kind and amounts of PUFA. Muscles are the store of essential fatty acids and sterols. Muscular tissue has considerable affinity for concentrating these lipid associations in the form of phospholipids. Phospholipids are a better store for PUFA than neutral lipids (Perez-Velazquez et al., Reference Perez-Velazquez, Gonzalez-Felix, Lawrence and Gatlin III2003; Limbourn and Nichols, Reference Limbourn and Nichols2009) and they play a role also as a main component of cell membranes. High concentration of PUFA in organisms influences their survival and their tolerance to changes of salinity. The presence of these acids modifies the permeability of the cell membrane and modulates the activity of the sodium–potassium pump, as an essential osmoregulatory mechanism (Maazouzi et al., Reference Maazouzi, Masson, Soledad Izquierdo and Pihan2007).
CONCLUSIONS
For the first time the lipid content of the abdomen muscle of benthic taxon of adult stage Crangon crangon is determined. As the result of these studies the composition of lipid classes, and the fatty acid profiles in all fractions have been determined, and also the composition of marine sterols in C. crangon. The composition of lipids did not differ widely in comparison to the other crustaceans (Allen, Reference Allen1999). Minor differences are noticeable in fatty acids composition, and depended on the season, weather conditions, availability of nutrients, age (Çelik et al., Reference Çelik, Türeli, Çelik, Yanar, Erdem and KüÇükgülmez2004) and sex of the organisms—rapid gonad maturation and maximum concentration of lipid during the spring period (March–April). Dietary composition of nutrients much more affected the PUFA content than temperature and weather condition (Abad et al., Reference Abad, Ruiz, Martinez, Mosquera and Sánchez1995). Consumption of shrimps increases not only because of taste, but also due to the usefulness of shrimps as a supply of healthy essential fatty acids (Holub, Reference Holub2002). Despite the small size of C. crangon it stands out in accessibility of biological material. It is worthwhile to continue study of these organisms and consider them as PUFAs source.
FINANCIAL SUPPORT
Financial support was provided by the Polish Ministry of Research and Higher Education under grants BW/8430-5-0453-0 and DS 530-8110-D195-12.