Introduction
The striped red mullet Mullus surmuletus (Linnaeus, 1758) (Osteichthyes, Perciformes, Mullidae) is distributed in the Mediterranean and Black Sea and in the eastern Atlantic, from the North Sea as far as Senegal (Hureau, Reference Hureau, Whitehead, Bauchot, Hureau, Nielsen and Tortonese1986). It is a demersal species inhabiting mainly rough substrates (Hureau, Reference Hureau, Whitehead, Bauchot, Hureau, Nielsen and Tortonese1986) with the highest abundance between 100–200 m of depth (Tserpes et al., Reference Tserpes, Fiorentino, Levi, Cau, Murenu, Zamboni and Papaconstantinou2002). Mullus surmuletus exhibits a pattern of ‘inter-depth’ migration related to reproduction, meaning that it recruits in shallower habitats over Posidonia oceanica seagrass beds (Garcia-Rubies & Macpherson, Reference Garcia-Rubies and Macpherson1995), spawns in deeper habitats and, after reproduction, it continues dispersing into deeper waters (Machias et al., Reference Machias, Somarakis and Tsimenides1998). Although small individuals are commonly found in shallow and warm waters (Machias et al., Reference Machias, Somarakis and Tsimenides1998), the occurrence of post-larval and juvenile stages of M. surmuletus in offshore oceanic waters has also been reported (Deudero, Reference Deudero2002). Concerning its feeding behaviour, M. surmuletus is an opportunistic benthivorous species, preying exclusively on benthic organisms and mainly Crustacea, Polychaeta, Mollusca, Echinoderma and small fish (Gharbi & Ktari, Reference Gharbi and Ktari1981; Golani & Galil, Reference Golani and Galil1991; Vassilopoulou et al., Reference Vassilopoulou, Papaconstantinou and Christides2001).
Both morphological (Fage, Reference Fage1909; Benzinou et al., Reference Benzinou, Carbini, Nasreddine, Elleboode and Mahé2013; Mahé et al., Reference Mahé, Villanueva, Vaz, Coppin, Koubbi and Carpentier2014) and genetic studies (Mamuris et al., Reference Mamuris, Stamatis and Triantaphyllidis1999; Galarza et al., Reference Galarza, Turner, Macpherson and Rico2009; Matić-Skoko et al., Reference Matić-Skoko, Šegvić-Bubić, Mandić, Izquierdo-Gomez, Arneri, Carbonara, Grati, Ikica, Kolitari, Milone, Sartor, Scarcella, Tokaç and Tzanatos2018) have shown that M. surmuletus tends to form distinct populations within its distribution range, although genetic panmixia has also been reported at a small geographic scale (Apostolidis et al., Reference Apostolidis, Moutou, Stamatis and Mamuris2009). This emphasizes the need for collecting basic biological information about the species at regional level, i.e. reproductive biology, spawning period, length at first maturity as well as age and growth that can be considered in fish stock assessments and population dynamics. To the best of our knowledge, this information is very limited for the Hellenic waters, despite M. surmuletus being one of the main target species of the demersal fisheries exploited by more than one gear type (Stergiou et al., Reference Stergiou, Petrakis and Papaconstantinou1992; Tserpes et al., Reference Tserpes, Fiorentino, Levi, Cau, Murenu, Zamboni and Papaconstantinou2002). The data date back to the 1990s, when preliminary biological information about the species was reported from the central Aegean Sea (Vassilopoulou & Papaconstantinou, Reference Vassilopoulou and Papaconstantinou1992), the Thermaikos Gulf and the Thracian Sea (Papaconstantinou et al., Reference Papaconstantinou, Politou, Caragitsou, Stergiou, Mytilineou, Vassilopoulou, Fourtouni, Karkani, Kavadas, Petrakis, Siapatis, Chatzinikolaou and Giagnisi1994), and the Cretan Sea (Machias et al., Reference Machias, Somarakis and Tsimenides1998).
The life-history traits of the species, concerning its reproduction and growth, have been studied mostly in the eastern Atlantic Ocean (Bay of Biscay: N'Da, Reference N'Da1992; Pajuelo et al., Reference Pajuelo, Lorenzo, Ramos and Villamil-Mata1997; N'Da et al., Reference N'Da, Déniel and Yao2006; eastern English Channel–southern North Sea: Mahé et al., Reference Mahé, Coppin, Vaz and Carpentier2013) and the western Mediterranean Sea (Alboran Sea: Lamrini, Reference Lamrini2010; off Majorca: Morales-Nin, Reference Morales-Nin1991, Reference Morales-Nin1992; Reñones et al., Reference Reñones, Massutí and Morales-Nin1995; Catalan Sea: Sánchez et al., Reference Sánchez, Morales-Nin and Martin1983; Morales-Nin, Reference Morales-Nin1986; Gulf of Lion: Campillo, Reference Campillo1992). Fewer studies exist for the central Mediterranean (Southern Tyrrhenian and Ionian Seas: Andaloro, Reference Andaloro1982; Strait of Sicily: Andaloro & Giarritta, Reference Andaloro and Giarritta1985; off Tunisia: Gharbi & Ktari, Reference Gharbi and Ktari1981; Jabeur, Reference Jabeur1999) and the eastern Mediterranean (central Aegean Sea: Vassilopoulou & Papaconstantinou, Reference Vassilopoulou and Papaconstantinou1992; NE Aegean Sea: Ilhan et al., Reference Ilhan, Akalin, Özaydin, Tosunoğlu and Gurbet2009; Arslan & İşmen, Reference Arslan and İşmen2013; Torcu-Koç et al., Reference Torcu-Koç, Erdoğan, Üstün and Joksimović2015; Egyptian waters: Hashem, Reference Hashem1973; Mehanna, Reference Mehanna2009).
The aim of this study was to investigate the main biological features of M. surmuletus in the south Aegean Sea (eastern Mediterranean), and specifically the spawning season, length at first maturity, length–weight relationship, age and growth. Validation of the periodicity of growth increment deposition was also performed by applying marginal increment analysis, as in previous studies (Reñones et al., Reference Reñones, Massutí and Morales-Nin1995; Pajuelo et al., Reference Pajuelo, Lorenzo, Ramos and Villamil-Mata1997; Mahé et al., Reference Mahé, Coppin, Vaz and Carpentier2013; Bakali et al., Reference Bakali, Talbaoui and Bendriss2016), and complementary methods (otolith edge analysis, assessment of the reproductive period and length–frequency distribution modes of the species) for the first time. Furthermore, the results of previous findings for the Mediterranean and the Atlantic were compared following an extensive literature review. Overall, this study attempts to provide valuable data for the stock assessment of M. surmuletus in one of the major Hellenic fishing grounds.
Materials and methods
Sampling procedure
Sampling was carried out at the market or on board fishing vessels in the south Aegean Sea (Figure 1) between February and December 2016 using a rule of 5 individuals per length class of 10 mm interval, according to the National Data Collection Framework Program. For each specimen, total length (L T) was recorded to the nearest millimetre (mm), while total weight (W T) and eviscerated weight (W E) were recorded to the nearest gram (g). Sex was determined by macroscopic observation of the gonads in all individuals. Sexual maturity stages were assessed according to Nikolsky's scale (Reference Nikolsky1963): I: immature, II: resting, III: developing, IV: maturing, V: mature, and VI: spent.

Fig. 1. Map indicating the sampling location (red dashed frame) of Mullus surmuletus in the south Aegean Sea. The map was prepared in ArcMap v10.4.
Sex ratio
Sex ratio was calculated by size and month. The samples were adjusted for possible unbalances between the numbers of individuals per size class. The chi-square test (χ2, Zar, Reference Zar1996) was used to examine the differences between the observed and the expected ratio of 1:1.
Somatic indices
The gonadosomatic index (I G) was calculated according to the equation: I G = (W G/W E) × 100, where W G is the gonad weight and W E the eviscerated weight of the specimens, all recorded in grams. The condition factor was calculated by sex as: K = (W E/L T3) × 100, where W E is the eviscerated weight in g and L T the total length in cm (Ricker, Reference Ricker1975). In this way, K is not affected by the maturity condition and the level of stomach fullness, and better attributes the physical condition of fish and its seasonal change (Nikolsky, Reference Nikolsky1963). Both I G and K were calculated per sex, maturity stage and month. Non-parametric statistical methods were used to test for significant differences in the median values of I G and K between sexes (Mann–Whitney Wilcoxon-test, W) and among maturity stages (Kruskal–Wallis test, KW). All statistical analyses were implemented in STATGRAPHICS Centurion XVI.
Spawning period
The spawning period was determined by identifying monthly changes in the proportion of maturity stages and I G. Additionally, in order to assess the effect of body size on the progress of reproductive maturation, mature (stages III–VI) female and male individuals were divided into two size groups (111–180 and 181–320 mm L T) and their percentage was examined per month. The criterion for the selection of the two size groups was that above 180 mm L T all individuals were considered mature regardless of sex.
Length at 50% maturity
Length at 50% maturity (L 50) was determined by fitting of maturity ogives. The proportions of mature (stages III–VI) vs immature (stages I–II) individuals within length classes of 10 mm were estimated per sex for the observed period of reproduction. A logistic curve was fitted to the data and the length at which 50% of the individuals are sexually mature was calculated following the equation: P = 1/[1 + e(α + bLT)], where P is the proportion of mature individuals in each length interval, and α and b are the fitted parameters (King, Reference King1995). The length at 50% maturity was calculated as: L 50 = α/b (Sparre & Venema, Reference Sparre and Venema1992).
Age estimation and validation
Sagittal otoliths were removed from the cranial cavity, placed in water to remove surrounding membranes, cleaned and stored dry. Age estimation was based on counting macroscopically the alternating opaque and translucent zones along the left sagittal otolith axis, from the core to the post-rostrum edge two different times by the same expert. Each left otolith was observed under transmitted light against a black background. Otoliths showing deformation or an indistinct annulus pattern were excluded from the ageing procedure. The birth-date of M. surmuletus was assumed to be 1 January. To minimize any possible source of bias, all readings were performed with a time interval of 3 months between them and without prior knowledge of the specimen's length, sex or previous count. When the two successive age counts differed, the final choice was based on a third age reading. Considering the ageing results, an age-length key was constructed for combined sexes. Individuals that were out of the main bulk of the L T range of each age group were re-examined and excluded from further analysis only in cases where the otolith image was less unclear.
The individual left otolith radius (R in mm) was measured and power regression analysis, based on the r 2 statistic value, was used to describe the fish L T–R relationship following the equation: L T = αRb (Zar, Reference Zar1996) by sex. All otolith measurements were taken in mm using the Image-Pro Plus v4.5.1.22 software. Analysis of covariance (ANCOVA) (Zar, Reference Zar1996) was used to test for between-sex differences by comparing the slopes of the aforementioned regressions.
To validate the periodicity of growth increment formation, Marginal Increment Analysis (MIA) was carried out for specimens with 1, 2 and 3 annual rings by calculating the monthly marginal increment, i.e. the distance between the otolith edge and the last growth ring following the formula: MI = R − R i, where R is the otolith radius and R i is the distance between the edge and the last growth ring (Bagenal & Tesch, Reference Bagenal, Tesch and Bagenal1978). Complementary information to validate the periodicity of growth increment formation was used and derived from: (a) the qualitative description of each otolith edge by recording the presence or absence of a translucent ring and describing the level of its formation; (b) the peak of the reproductive period; (c) the length–frequency distribution of the population per 10 mm of L T during the period of annulus formation to identify discrete length modes, following Bhattacharya's method (Reference Bhattacharya1967), which was incorporated in the FISAT software (Gayanilo et al., Reference Gayanilo, Sparre and Pauly2006), assuming that each mode in the overall size–frequency distribution represented a cohort. To apply this method, a larger dataset was used that was obtained from the trawl fishery data collected under the Hellenic National Data Collection Framework Program (Anonymous, 2017); (d) the comparison of the average length of the mode of the YOY identified by Bhattacharya's method (Reference Bhattacharya1967) when the smallest marginal increment was found with the mean fish length at which the first translucent annulus is deposited that was back-calculated using Campana's formula (Reference Campana1990): TLi = TLc + (TLc − TLo) × (OLi − OLc)/(OLc − OLo), where TLi and OLi are fish length and otolith length, respectively, at age 1; TLc and OLc are fish length and otolith length, respectively, at capture; TLo and OLo are fish length and otolith length, respectively, at hatching (fish hatch length was 2.83 mm according to Russell, Reference Russell1976); and (e) comparison of the mean value of the first annulus radius (R 1) to the otolith radius of the young of the year (YOY), as estimated by the L T–R equation and using as L T the average length of the mode of the YOY identified by Bhattacharya's method (Reference Bhattacharya1967) for the period of annulus formation (obtained from the marginal and edge analyses). Regarding edge analysis, five categories of otolith edge type were used (Figure 2): (1) beginning of formation of a non-continuous translucent ring at the otolith edge (Type A); (2) continuous and thin (narrow) translucent ring at the otolith edge (Type B); (3) continuous and thick (wide) translucent zone at the otolith edge (Type C); (4) continuous and thick translucent zone followed by a non-continuous thin and weak opaque zone at the edge (Type D); and (5) continuous and thick translucent zone surrounded by a continuous and fully formed opaque zone (Type E) (Figure 2).

Fig. 2. Different types (A–E) of otolith edge based on the degree of formation of the translucent zone in Mullus surmuletus in the south Aegean Sea; each growth increment zone is represented by a red dot; the total length (L T) and capture date of each corresponding individual are also given; beginning of formation of a non-continuous translucent zone at the edge of the otolith (Type A); continuous and thin (narrow) translucent zone at the edge of the otolith (Type B); continuous and thick (wide) translucent zone at the edge of the otolith (Type C); continuous and thick translucent zone followed by a non-continuous thin and weak opaque zone at the edge (Type D); continuous and thick translucent zone surrounded by a continuous and fully formed opaque zone (Type E). Photos by Vasiliki Kousteni.
Length–weight relationship
Power regression analysis was used to describe the length–weight relationship according to the equation W T = αL Tb (Ricker, Reference Ricker1975) following the least square method applied to the log-transformed data for females and males as: logW T = loga + blogL T, where W T is the total weight in g, L T the total length in cm, α the intercept and b the slope of the regression. Slope b of the regressions was tested against the isometric slope standard of 3 by sex and overall with Student's t-test (Zar, Reference Zar1996). Analysis of covariance (ANCOVA) (Zar, Reference Zar1996) was used to test the between-sex differences by comparing the slopes of the aforementioned regressions.
Growth modelling
The von Bertalanffy growth function (VBGF) was used and growth parameters were estimated for sexes combined and for females alone (since no large males were included in the samples) according to the equation: L t = L inf × [1 − e−k(t−t0)], where L t is the predicted length at age t in mm, L inf the mean theoretical asymptotic length in mm, k a growth rate parameter in year−1 and t 0 the theoretical age at zero length in years (Von Bertalanffy, Reference Von Bertalanffy1938). Longevity was estimated according to the equation t max = 3/k, where k is the growth rate per year (Pauly, Reference Pauly1984).
Results
Sex ratio
The total sample consisted of 1032 individuals. This sample included 279 individuals whose sex could not be identified due to the bad condition of their gonads; they were either infected by sea lice or immature. The dominance of females was statistically significant in every sampling month (χ2 test: P χ2 < 0.05 in all cases), except in June (χ2 = 2.450, df = 1, P χ2 = 0.05) (Figure 3a). Sex ratio by length is presented in Figure 3b. No males were recorded in the 101–120, 241–250 and 271–320 mm L T size classes, while a decreasing trend was obvious for males measuring 130–270 mm L T (F = 32.02, df = 13, P ANOVA < 0.01). Significant differences in the sex ratio with female dominance were observed in all size classes, except the 121–150 and 171–190 mm L T size classes (121–130 mm L T: χ2 = 1.263, df = 1, P χ2 > 0.05; 131–140 mm L T: χ2 = 1.358, df = 1, P χ2 > 0.05; 141–150 mm L T: χ2 = 1.358, df = 1, P χ2 > 0.05; 171–180 mm L T: χ2 = 1.577, df = 1, P χ2 > 0.05; 181–190 mm L T: χ2 = 2.512, df = 1, P χ2 > 0.05).

Fig. 3. Sex ratio of Mullus surmuletus by month (a) and size (L T) (b) in the south Aegean Sea.
Somatic indices
The I G ranged from 0.03 to 11.11% (mean ± SD = 2.19 ± 2.66% I G, N = 523) in females and from 0.04 to 3.78% (mean ± SD = 0.81 ± 0.66% I G, N = 243) in males. The median value of I G was significantly higher in females compared with males (W = 54,624.0; P W < 0.01). In both sexes, I G increased significantly between successive maturity stages (KW = 359.779, P KW < 0.01 and KW = 93.1195, P KW < 0.01, respectively). The highest mean value of I G was observed in stage V in both sexes (mean ± SD = 6.32 ± 1.45% I G, N = 109 in females; mean ± SD = 2.11 ± 0.73% I G, N = 20 in males) when the gonads reached maximum maturity (Figure 4).

Fig. 4. Gonadosomatic index (I G %) and condition factor (K) in each maturity stage of female and male Mullus surmuletus in the south Aegean Sea; grey area, 50% of the values; asterisk (+), mean; horizontal line, median; notch, 95% confidence level for median; vertical lines, minimum and maximum.
The study of I G per month showed that in mature females, maximum I G was found in March (mean ± SD = 6.47 ± 1.15% I G, N = 11), while in mature males, maximum I G was found in February (3.01%), thus revealing that the peak of maturity is reached earlier in males compared with females (Figure 5). Following these months, where the highest mean I G value was recorded, a significant decrease in mean I G values was observed for both sexes. A second lower peak in I G was observed in July in both sexes (mean ± SD = 2.02 ± 2.14% I G, N = 43 in females; mean ± SD = 0.54 ± 0.47% I G, N = 20 in males) reflecting a secondary peak in species reproductive activity in mid-summer.

Fig. 5. Mean values of gonadosomatic index (I G %) and condition factor (K) with standard error bars of mature female and male Mullus surmuletus in the south Aegean Sea by month.
The K value ranged from 0.84 to 1.55 (mean ± SD = 1.20 ± 0.10 K, N = 593) in females and from 0.93 to 1.43 (mean ± SD = 1.18 ± 0.09 K, N = 279) in males. The median value of K was significantly higher in females compared with males (W = 69,605.5; P W < 0.01). The K value did not differ significantly among maturity stages in both females (KW = 3.67512, P KW > 0.05) and males (KW = 10.8697, P KW = 0.05). The highest mean value of K was observed in maturity stages II and III in both sexes (mean-II ± SD = 1.21 ± 0.11 K, N = 148 and mean-III ± SD = 1.21 ± 0.07 K, N = 6 in females; mean-II ± SD = 1.20 ± 0.09 K, N = 75 and Mean-III ± SD = 1.20 ± 0.08 K, N = 37 in males) (Figure 4).
The examination of K by month revealed that the maximum K value was observed during winter in both sexes, and specifically in February in females (mean ± SD = 1.28 ± 0.06 K, N = 9) and in December in males (mean ± SD = 1.25 ± 0.04 K, N = 9) (Figure 5).
Spawning period
The distribution of each maturity stage of females and males per month is presented in Figure 6. Immature individuals (stages I–II) were present on a monthly basis, regardless of sex. Females with developing gonads (stage III) were recorded only in February, April and October, while maturing females (stage IV) were found between February and July. Spawning females (stage V) were found between March and July and spent females (stage VI) were found from May to December. In the case of males, developing individuals (stage III) were found from March to June and from October to December, while maturing individuals (stage IV) were found between November and May. Spawning males (stage V) were found between March and July, as for females, while spent males (stage VI) were found from April to December. The examination of the percentage of mature female and male by month for two size groups indicated that large (>180 mm L T) females and males reached a peak in spring (between March and April). Smaller females (<180 mm L T) seemed to mature in early summer (June), while smaller males (<180 mm L T) in early spring and summer (Figure 7).

Fig. 6. Frequency (%) of female and male Mullus surmuletus maturity stages in the south Aegean Sea by month according to Nikolsky's scale (Reference Nikolsky1963): I: immature, II: resting, III: developing, IV: maturing, V: mature and VI: spent.

Fig. 7. Frequency (N) of mature female and male Mullus surmuletus for two size groups (<180 mm and >180 mm L T) in the south Aegean Sea.
Length at 50% maturity
The smallest spawning female and male (stage V) reached 132 and 152 mm L T, respectively. The size of mature females (stages III–VI) ranged from 132 to 320 mm L T (mean ± SD = 200.9 ± 34.16 mm L T) and that of mature males (stages III–VI) ranged from 130 to 262 mm L T (mean ± SD = 181.6 ± 28.36 mm L T). At lengths greater than 180 mm L T, all females were mature, while all males were mature at lengths greater than 170 mm L T. Females attained maturity at a larger size than males, with L 50 reaching 153.3 and 139.2 mm, respectively (Figure 8).

Fig. 8. Logistic curve based on the proportion of mature female and male Mullus surmuletus against total length (L T) in the south Aegean Sea.
Age estimation and validation
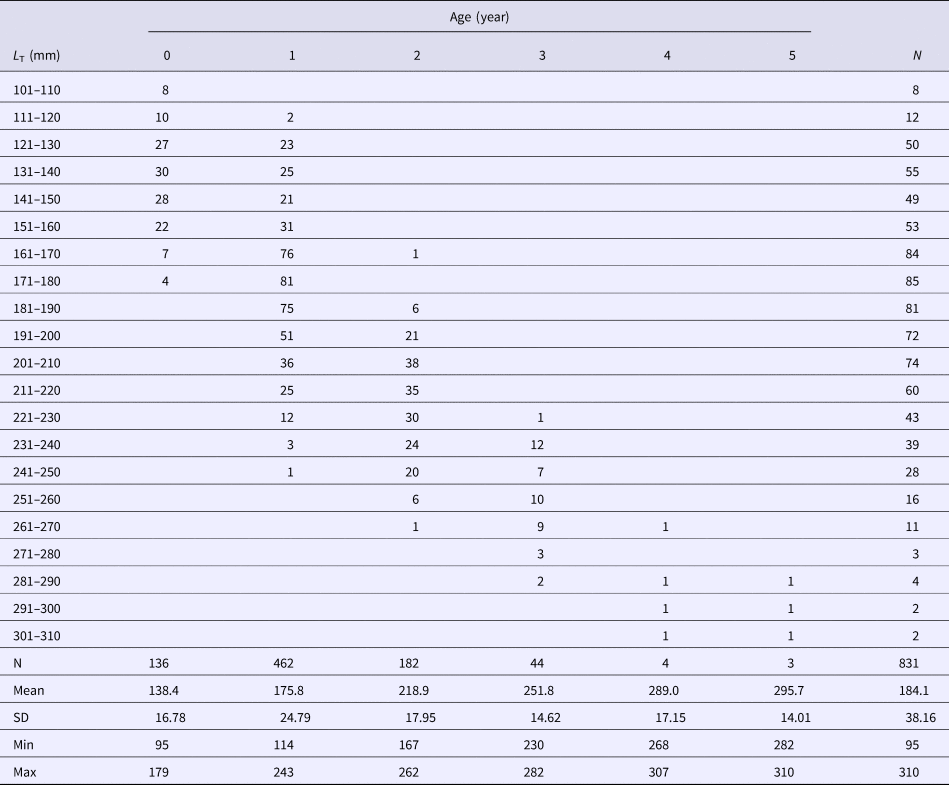
In total, up to 5 annuli were counted in 831 individuals. The age-length key for the examined sample of M. surmuletus is presented in Table 1 showing an overlap of lengths among age groups. A statistically significant difference was found among the mean L T values of the recorded age groups (ANOVA: F = 344.30, df = 830, P ANOVA < 0.001). Multiple range tests on the mean lengths of each age group showed statistically significant differences, except in the case of age groups 4 and 5 that belonged to a homogeneous group. The fish size–otolith radius relationship did not differ significantly between females and males (F = 0.82, P ANCOVA > 0.05) and was described by the equation L T = exp(4.54314 + 1.29599 × ln(R)) for combined sexes. The radius of annual rings differed significantly among age groups (KW = 479.988, P KW < 0.001).
Table 1. Age-total length (L T) key for Mullus surmuletus in the south Aegean Sea based on the macroscopically counted annuli
According to MIA, for individuals with one ring, the smallest marginal increment at the otolith edge was observed between March and April (spring) (Figure 9). The marginal increment was also examined for individuals with 2 and 3 rings, which also showed annual periodicity of growth increment formation, with the lowest values observed between February and April.

Fig. 9. Marginal increment analysis for the 1st, 2nd and 3rd age group in Mullus surmuletus in the south Aegean Sea.
The highest percentage of individuals with a translucent ring at the otolith edge was found in the period March–April, while a secondary period with lower percentage was observed between September and October (autumn) (Figure 10a). Considering the percentage of the five otolith edge types occurring per month (Figure 10b) it becomes obvious that edge Type A peaked in March and decreased afterwards, the highest percentage of edge Type B occurred between March and April, the highest percentage of edge Type C occurred in April, and both edge Types D and E increased from April to late June.

Fig. 10. Percentage (%) of translucent ring occurrence (Y) or absence (N) at the edge of the otolith by month (a); Percentage (%) of the 5 otolith edge types by month; beginning of formation of a non-continuous translucent zone at the edge of the otolith (Type A); continuous and thin (narrow) translucent zone at the edge of the otolith (Type B); continuous and thick (wide) translucent zone at the edge of the otolith (Type C); continuous and thick translucent zone followed by a non-continuous thin and weak opaque zone at the edge (Type D); continuous and thick translucent zone surrounded by a continuous and fully formed opaque zone (Type E) (b).
The length–frequency distribution of the species' population during the main months of annulus formation (March, April and May) is presented in Figure 11. The first mode identified using Bhattacharya's method (Reference Bhattacharya1967) in this length–frequency distribution was found at 167.3 mm.

Fig. 11. Length–frequency distribution of Mullus surmuletus population in the Aegean Sea for spring 2016.
The average fish length at which the first translucent annulus is deposited (mean L T = 155.2 mm; range: 113.9–205.6 mm), which was back-calculated following Campana's method (Reference Campana1990), was smaller than the average fish length of the mode of the YOY identified by Bhattacharya's method (L T = 167.3 mm), but within the range of the back-calculated lengths.
Finally, the mean value of the first annulus radius (R 1; mean ± SD: 1.45 ± 0.14 mm, range: 1.04–1.86 mm) was lower than the otolith radius (1.56 mm) of the young of the year (YOY), as estimated by the L T–R equation using the average length of the mode of the YOY identified by Bhattacharya's method (L T = 167.3 mm) in Figure 11. However, the value of otolith radius in this case was within the range of the first annulus radius.
Length–weight relationship
Females ranged from 103 to 320 mm L T (mean ± SD = 193.4 ± 37.2 mm L T) and from 10 to 381 g W T (mean ± SD = 96.7 ± 57.0 g W T). Males ranged from 121 to 262 mm L T (mean ± SD = 172.2 ± 30.9 mm L T) and from 22 to 231 g W T (mean ± SD = 66.0 ± 37.4 g W T). The median values of L T and W T were significantly higher in females compared with males (W = 53,001.5, P W < 0.05; W = 51,196.5, P W < 0.05, respectively).
The length–weight relationships were described by the following equations: W T = 0.0155L T2.915 (R 2 = 0.96) in females, W T = 0.0032L T2.976 (R 2 = 0.98) in males and W T = 0.0013L T2.943 (R 2 = 0.96) overall (Figure 12). Examination of the length–weight relationship revealed a positive relationship between these parameters and isometric growth, regardless of sex (P t > 0.05). Considering only the common size range of female and male individuals, both the slope and the intercept were significantly higher in females compared with males (F = 62.15, P ANCOVA < 0.01).

Fig. 12. Total length (L T)–total weight (W T) relationship of female and male Mullus surmuletus in the south Aegean Sea.
Growth modelling
The parameters of the von Bertalanffy growth function were L inf = 373.2 mm, k = 0.255 year−1 and t 0 = −0.999 years for sexes combined and L inf = 346.1 mm, k = 0.299 year−1 and t 0 = −0.984 years for females (Figure 13). Longevity (t max) reached 11.75 years for sexes combined and 10.02 years for females.

Fig. 13. Growth curve fitted to the observed length-at-age data for Mullus surmuletus in the south Aegean Sea.
Discussion
This paper fills a significant scientific gap concerning the life-history traits of Mullus surmuletus in the Aegean Sea, where information on age, growth and reproduction has not been reported since the 1990s (Vassilopoulou & Papaconstantinou, Reference Vassilopoulou and Papaconstantinou1992).
The sex ratio found in this study favoured female M. surmuletus, as has been recorded in previous studies in both the Mediterranean (Andaloro, Reference Andaloro1982; Morales-Nin, Reference Morales-Nin1991; Reñones et al., Reference Reñones, Massutí and Morales-Nin1995) and the Atlantic (Pajuelo et al., Reference Pajuelo, Lorenzo, Ramos and Villamil-Mata1997; Mahé et al., Reference Mahé, Coppin, Vaz and Carpentier2013). The dominance of females may be attributed to the differences in the spatial distribution between females and males, as suggested for this species (Lozano-Cabo, Reference Lozano-Cabo1983). A different pattern with the sex ratio favouring males was recorded in the central Aegean Sea and was attributed to small sample size (Vassilopoulou & Papaconstantinou, Reference Vassilopoulou and Papaconstantinou1992). In relation to size, no males were recorded in larger size groups, and specifically >270 mm L T. Similar results were found in previous studies reporting female dominance at sizes >230 mm L T in the central Aegean Sea (Vassilopoulou & Papaconstantinou, Reference Vassilopoulou and Papaconstantinou1992), >280 mm L T off Balearics (Reñones et al., Reference Reñones, Massutí and Morales-Nin1995) and >260 mm L T off the Canary Islands (Pajuelo et al., Reference Pajuelo, Lorenzo, Ramos and Villamil-Mata1997). Furthermore, the decreasing trend of males reaching larger size groups has also been observed in other studies (Reñones et al., Reference Reñones, Massutí and Morales-Nin1995; Pajuelo et al., Reference Pajuelo, Lorenzo, Ramos and Villamil-Mata1997).
The examination of maturity indicated that M. surmuletus exhibits sexual dimorphism in the studied area, with females reaching maturity at a larger size compared with males. Specifically, L 50 equalled 153.3 and 139.2 mm in females and males, respectively, implying that males reach maximum gonadal growth earlier than females and reflecting the presence of growth dimorphism. A similar pattern was found by Vassilopoulou & Papaconstantinou (Reference Vassilopoulou and Papaconstantinou1992) in the central Aegean Sea (138.4 and 115.5 mm L 50 for females and males, respectively) and by Hashem (Reference Hashem1973) in Tunisian waters (150 and 130 mm L 50 for females and males, respectively). Moreover, the species seems to mature at a smaller size in the eastern compared to the western Mediterranean (168 and 155 mm L 50 for females and males, respectively (Reñones et al., Reference Reñones, Massutí and Morales-Nin1995), 177 mm L 50 for combined sexes (Kherraz et al., Reference Kherraz, Benghali, Mouffok and Boutiba2014)) and the Atlantic Ocean (169 and 163 L 50 mm for females and males, respectively (Pajuelo et al., Reference Pajuelo, Lorenzo, Ramos and Villamil-Mata1997), 169 and 162 mm L 50 for females and males, respectively (Mahé et al., Reference Mahé, Coppin, Vaz and Carpentier2013)). The observed geographic differentiation in L 50 values may mirror differences in local environmental conditions (Nikolsky, Reference Nikolsky1963) and productivity among areas (Azov, Reference Azov1991), as well as genetic drivers (Matić-Skoko et al., Reference Matić-Skoko, Šegvić-Bubić, Mandić, Izquierdo-Gomez, Arneri, Carbonara, Grati, Ikica, Kolitari, Milone, Sartor, Scarcella, Tokaç and Tzanatos2018), differences in the sampling scheme (e.g. number of samples per length class) and the ageing methodology applied (Kousteni & Megalofonou, Reference Kousteni and Megalofonou2015).
The monthly variation of the gonadosomatic index (I G) and the sexual maturity stages of female M. surmuletus suggested that the reproductive period of the species in the south Aegean Sea extended during several months with a spawning peak from March to April. This suggestion is supported by the extended period of post-spawning individuals (stage VI), from April to December, although spawning individuals occurred in few months in our samples. In the central Aegean Sea, over 40% of females were in spent condition in summer, while a few spent individuals were also recorded in autumn (Vassilopoulou & Papaconstantinou, Reference Vassilopoulou and Papaconstantinou1992). Previous studies suggested that the reproductive period of the species may range from February (Canary Islands: Pajuelo et al., Reference Pajuelo, Lorenzo, Ramos and Villamil-Mata1997) to September (Edremit Bay: Torcu-Koç et al., Reference Torcu-Koç, Erdoğan, Üstün and Joksimović2015). In general, an extended reproductive period has been recorded for the species in the Mediterranean Sea (from April to September in Edremit Bay: Torcu-Koç et al., Reference Torcu-Koç, Erdoğan, Üstün and Joksimović2015; from April to May in Saros Bay: Arslan & İşmen, Reference Arslan and İşmen2013; from March to June off the Balearics: Reñones et al., Reference Reñones, Massutí and Morales-Nin1995) compared with the eastern Atlantic (from May to June in the Bay of Biscay: N'Da, Reference N'Da1992; from May to July in the North Sea: Mahé et al., Reference Mahé, Coppin, Vaz and Carpentier2013). The variation in maturation progress could be attributed to different ecological and climatic conditions (Nikolsky, Reference Nikolsky1963), and changes in the temperature regime (Wootton, Reference Wootton1998). Gamete dispensing seems to affect the condition factor of the species, although no statistical differences were found among months. Arslan & İşmen (Reference Arslan and İşmen2013) did not observe seasonal variation in K, which showed the lowest value in July and a peak in September. In this study, the lowest value of K was also found in July after the spawning peak. An effect of body size on the progress of reproductive maturation was also found in this study, with individuals <180 mm L T maturing later than those >180 mm L T.
The MIA is one of the most commonly applied methods for validating the periodicity of growth increment formation in the skeletal ageing structures of fish (otoliths, vertebrae etc.), given its modest sampling requirements and low cost (Campana, Reference Campana2001). In this study, MIA confirmed the annual periodicity of increment formation in M. surmuletus otoliths that showed the lowest values between February and April, thus confirming previous results in both the Mediterranean (Bakali et al., Reference Bakali, Talbaoui and Bendriss2016) and the Atlantic (Mahé et al., Reference Mahé, Coppin, Vaz and Carpentier2013). The annual formation of a single growth increment was further supported using otolith edge analysis, i.e. recording of the presence of either an opaque or translucent zone at the otolith edge, indicating the translucent zone formation mainly from February to May, whereas that of the opaque zone from May to December. The secondary peak of translucent ring occurrence recorded in autumn was not considered as an annulus but rather as a false ring, probably attributed to the reproductive activity of the species, since all of these otoliths belonged to individuals in either the V or VI maturity stage. Nevertheless, more samples are needed to make safe assumptions, since the increase of translucent margin in autumn corresponded to less than 30% of the otoliths examined during that season.
Furthermore, the length–frequency distribution revealed a mode that was higher but within the range of lengths estimated by Campana's method. Finally, the otolith radius of the YOY estimated using the identified mode in the length distribution in the period of the annulus formation was higher, but within the range of the first annulus radius. These differences can be explained by the fact that the identified mode in the length–frequency was based on the observed lengths, while the back-calculated lengths concern the exact length of the annulus formation. No relevant information regarding M. surmuletus exists in the published literature.
In this study, M. surmuletus otoliths examined revealed six age groups (from 0+ to 5+ years) based on the macroscopically counted annuli. Similar findings have been reported in other locations in the eastern Mediterranean (Machias et al., Reference Machias, Somarakis and Tsimenides1998; Mehanna, Reference Mehanna2009) and in the central Mediterranean (Gharbi & Ktari, Reference Gharbi and Ktari1981), while seven age groups have been reported in the central Aegean (Vassilopoulou & Papaconstantinou, Reference Vassilopoulou and Papaconstantinou1992). The maximum recorded age of the species for combined sexes varies from 4 years off the Balearics (Morales-Nin, Reference Morales-Nin1986) to 10 years in Moroccan waters (Bakali et al., Reference Bakali, Talbaoui and Bendriss2016), quite a wide range that reflects the various size classes included in these studies (Table S1 in Supplementary Material).
Consistent with the results of previous studies (Reñones et al., Reference Reñones, Massutí and Morales-Nin1995; Bakali et al., Reference Bakali, Talbaoui and Bendriss2016), a significant overlap of lengths among age groups was observed for the species in the south Aegean Sea, although the mean length differed significantly among age groups. This finding, along with the extended reproductive activity of the species in the studied area, may indicate that the species is a batch spawner, as has been found for the co-generic red mullet Mullus barbatus (Linnaeus 1975) (Carbonara et al., Reference Carbonara, Intin, Modugn, Maradonn, Spedicato, Lembo, Zupa and Carnevali2015). Further histological studies are needed to verify this assumption. Moreover, the difference in the reproductive period between small and large individuals revealed in this study could support the difference in size among individuals of the same age group, which may result in size overlap among different age groups. The aforementioned overlap could also be explained by the fact that the age-length key was constructed based on samples distributed all-year round and not only from the period of annulus formation.
The length–weight relationships supported isometric growth regardless of sex. Similar results have been reported in Egyptian waters (Mehanna, Reference Mehanna2009), while the species has shown positive allometry in several locations, such as the central Aegean Sea (Vassilopoulou & Papaconstantinou, Reference Vassilopoulou and Papaconstantinou1992), Moroccan waters (Bakali et al., Reference Bakali, Talbaoui and Bendriss2016), Algerian waters (Kherraz et al., Reference Kherraz, Benghali, Mouffok and Boutiba2014), the eastern Aegean Sea (Arslan & İşmen, Reference Arslan and İşmen2013) and the eastern Atlantic (Mahé et al., Reference Mahé, Coppin, Vaz and Carpentier2013) (Table S1 in Supplementary Material). The geographic variation of growth type may be attributed to the combined effect of environmental conditions and genotypes (Conover & Schultz, Reference Conover and Schultz1995; Garvey et al., Reference Garvey, Devries, Wright and Miner2003). The length–weight relationships also indicated sexual dimorphism with females being significantly heavier than males of the same length, thus confirming the results of previous studies (Reñones et al., Reference Reñones, Massutí and Morales-Nin1995; Arslan & İşmen, Reference Arslan and İşmen2013; Kherraz et al., Reference Kherraz, Benghali, Mouffok and Boutiba2014).
According to the estimated VBGF parameters, the asymptotic length for M. surmuletus was greater in the south Aegean Sea compared with that reported by other studies in the Mediterranean Sea (Figure 14, Table S1 in Supplementary Material), but smaller than that reported in the Atlantic Ocean (N'Da, Reference N'Da1992; Mahé et al., Reference Mahé, Destombes, Coppin, Koubbi, Vaz, Le Roy and Carpentier2005). This could be attributed to the different age interpretation methodology used each time and to differences in localized environmental conditions, sampling methods or different growth rates between different stocks. For example, the size range of the sample examined in some of the previous studies was very limited (Table S1 in Supplementary Material). It is worth noting the fact that the estimated L inf was slightly higher than the maximum observed length (320 mm L T), which means that the L inf estimate is quite reasonable and could be used for assessing the stock of M. surmuletus for the purposes of sustainable fisheries management and the avoidance of overexploitation of the species.

Fig. 14. Von Bertalanffy growth curves for Mullus surmuletus in different locations; Eastern Mediterranean Sea: 1 (present study), 2 (Machias et al., Reference Machias, Somarakis and Tsimenides1998), 3 (Moldur, Reference Moldur1999), 4 (Arslan & İşmen, Reference Arslan and İşmen2013), 5 (Mukadder & İşmen, Reference Mukadder and İşmen2013), 6 (Üstün, Reference Üstün2010), 7 (Ilhan et al., Reference Ilhan, Akalin, Özaydin, Tosunoğlu and Gurbet2009), 8 (Mehanna, Reference Mehanna2009); Central Mediterranean Sea: 9 (Andaloro, Reference Andaloro1982), 10 (Gharbi & Ktari, Reference Gharbi and Ktari1981), 11 (Jabeur, Reference Jabeur1999); Western Mediterranean Sea: 12 (Bakali et al., Reference Bakali, Talbaoui and Bendriss2016), 13 (Morales-Nin, Reference Morales-Nin1991), 14 (Morales-Nin, Reference Morales-Nin1992), 15 (Reñones et al., Reference Reñones, Massutí and Morales-Nin1995), 16 (Sánchez et al., Reference Sánchez, Morales-Nin and Martin1983), 17 (Morales-Nin, Reference Morales-Nin1986); Eastern Atlantic Ocean: 18 (N'Da, Reference N'Da1992), 19 (Mahé et al., Reference Mahé, Destombes, Coppin, Koubbi, Vaz, Le Roy and Carpentier2005) and 20 (Pajuelo et al., Reference Pajuelo, Lorenzo, Ramos and Villamil-Mata1997). The VBGF parameters are presented in Table S1 (Supplementary Material). In the black & white version of the Figure, the curves correspond to the following references downwards: 19, 18, 1, 20, 2, 12, 8, 3, 9, 15, 13, 14, 4, 5, 16, 7, 17, 11, 10 and 6, respectively.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0025315419000353
Author ORCID
Vasiliki Kousteni, 0000-0003-3455-5357.
Acknowledgements
We would like to thank the reviewers for their time and effort in improving the quality of the manuscript by providing constructive comments. The authors would also like to thank Mr Stefanos Kavadas for length–frequency distributions drawing. English language editing was provided by Litterae® Language Services Ltd (http://www.litterae.gr/).

















