INTRODUCTION
Gnathiids have a polymorphic and biphasic life cycle (reproductive stage and ectoparasitic larval stage). There are three larval stages with each stage having two forms, namely praniza and zuphea. The praniza is usually a replete, haematophagous phase while the zuphea is an unfed benthic dweller phase (Smit & Davies, Reference Smit and Davies2004). Adults are free-living forms which do not feed and, as seen with Paragnathia formica (Hesse, 1864), can be found in groups with a single male and up to 43 females and immature specimens (Upton, Reference Upton1987).
Currently, the basic life cycle of gnathiid isopods has only been documented in representatives of four of the twelve gnathiid genera namely Elaphognathia Monod, 1926, Caecognathia Dollfus, 1901, Gnathia Leach, 1814 and Paragnathia Omer-Cooper & Omer-Cooper, 1916 (reviewed in Smit & Davies, Reference Smit and Davies2004). Furthermore, the gnathiid life cycle has only been studied in seven of approximately 180 known species (Smit & Davies, Reference Smit and Davies2004) with the most recent description being that of the South African intertidal gnathiid, G. africana Barnard, 1914. The recorded life cycles are all very similar and differ mainly in the length of the cycle as well as the different host species. Caecognathia calva (Van Hoffen, 1914) may take up to four or five years to complete the life cycle which may be due to the cold temperatures in the Antarctic where this species is found (Wägele, Reference Wägele1988). Elaphognathia cornigera (Nunomura, 1992) took 52 days to reach final stages (Tanaka, Reference Tanaka2003) and G. africana took ten more days with a final of 62 days to reach maturity (Smit et al., Reference Smit, Basson and Van As2003). Paragnathia formica females have a total life span of one year while the males may live to be more than two years old (Stoll, Reference Stoll1962; Charmantier, Reference Charmantier1980, Reference Charmantier1982; Upton, Reference Upton1987).
Gnathia pilosus Hadfield, Smit & Avenant-Oldewage, Reference Hadfield, Smit and Avenant-Oldewage2008, is the eighth gnathiid species to be described from South Africa, with five from the genus Gnathia, and one each from Caecognathia and Afrignathia Hadfield & Smit, Reference Hadfield and Smit2008 (Smit & Basson, Reference Smit and Basson2002; Hadfield & Smit, Reference Hadfield and Smit2008). It occurs on the warmer east coast compared to the other seven gnathiids which have been found on the colder west and south coasts of South Africa. This paper reports on the results of field and laboratory studies on the life cycle of G. pilosus.
MATERIALS AND METHODS
Fieldwork was carried out during March, June and September 2006 and February 2007, to collect sponges as well as intertidal and infratidal fish from the rocky shores of Tinley Manor (29°27′S 31°17′E) and Sheffield Beach (29°29′S 31°1′5E) on South Africa's east coast, Kwa-Zulu Natal. Sponges from the genus Hymeniacedon were examined under a dissection microscope for any gnathiids residing inside the fibrous material. Fish were collected using hand lines and nets, identified (Heemstra & Heemstra, Reference Heemstra and Heemstra2004; Branch et al., Reference Branch, Griffiths, Branch and Beckley2004), measured (total length), visually examined for the presence of gnathiids on the external body surfaces, and kept in aerated tanks (25 cm × 15 cm × 15 cm) until the gnathiids completed their feeding (Hadfield et al., Reference Hadfield, Smit and Avenant-Oldewage2008). Once fed, the gnathiids were removed, classified into larval stages, and kept alive in 50 ml sample bottles with fresh seawater until moulting occurred. Larvae observed with a swollen thorax or pereon were classified as pranizae (P) whereas those unfed larvae with distinct segmentation were classified as zuphea larvae (Z). These larvae were then further classified by size and feeding status into different larval instars with three stages of pranizae (P1, P2 or P3) and three stages of zuphea (Z1, Z2 or Z3). Live gnathiids and fish hosts were transported to a marine tank (150 cm × 60 cm × 60 cm) at the University of Johannesburg to study the life cycle under laboratory conditions. All surviving larvae were reared to adults to ensure they were the same species and were subsequently identified as Gnathia pilosus (Hadfield et al., Reference Hadfield, Smit and Avenant-Oldewage2008). The fish hosts used for the laboratory study included common intertidal fish found on the east coast namely the maned blenny, Scartella emarginata (Günther, 1861), and the horned rockskipper, Antennablennius bifilum (Günther, 1861), known to be common hosts for this species (see Hadfield et al., Reference Hadfield, Smit and Avenant-Oldewage2008). Live gnathiids were kept in a dark container at temperatures between 20°C and 25°C, cleaned daily with a fine artist brush (size 000), and the water was refreshed every second day with aerated seawater to provide better water quality for the survival of the gnathiids. It was not necessary to place a substrate with the gnathiids as they survived without any artificial resting place (see Smit et al., Reference Smit, Basson and Van As2003). The date of each moult was noted as well as the type of larval instar or adult it moulted into. Any gnathiids which did not survive were fixed in 70% ethanol.
After approximately a week post-moult, the zuphea larvae were allowed to feed on a single host fish placed in a smaller tank (150 cm × 60 cm × 60 cm). Feeding larvae were carefully observed to ensure the gnathiids were not eaten by the fish. The exact position of the gnathiid attachment during feeding was also recorded. The time taken to feed was noted as being from time of attachment to time of detachment, and the fed specimen was placed back in the sample bottle. The larval stages were also measured (total length) to ensure that the determination of their larval stage was accurate and presented as range (mean±standard deviation). Third stage praniza larvae which moulted into males or females were placed with the opposite sex into a bottle in order to stimulate reproduction and complete the life cycle. All the data were compiled to give an average estimation on time of moulting, time to feed and time for the entire cycle to occur. Photographs of the different stages were taken using a Zeiss Stemi 2000-CS dissection microscope with the AxioCam MRc camera and AxioVision AC computer software.
The different stages of praniza larvae found feeding on the host fish were recorded for each season and used to determine the seasonal percentage of the praniza population. The gnathiid attachments on thirty wild and thirty captive fish were also noted to determine the difference between wild and captive gnathiid feeding preferences. To ensure size of the host was not a factor, similar sized fish (60–70 mm) from the two host fish Scartella emarginata and the Antennablennius bifilum were used. A Chi-square goodness of fit test was used on the data collected to determine this significance.
The description of this life cycle follows work done by Wägele (Reference Wägele1988) and Klitgaard (Reference Klitgaard1991) for Caecognathia calva and C. abyssorum as well as the more recent work of Smit et al. (Reference Smit, Basson and Van As2003) on the life cycle of Gnathia africana.
RESULTS
During the four sampling trips, 316 fish were collected of which 223 were infested with gnathiids (Table 1). Fish were sampled between 7 am and 2 pm and all gnathiid larval stages were observed during this period. A total number of 1040 gnathiids were removed from the fish hosts of which 731 (70.3%) were P1 larvae, 186 (17.9%) were P2 larvae and 83 (8%) were P3 larvae (Table 1). The 40 zuphea larvae make up the remaining 3.8% collected throughout the year. Another 11 gnathiids were collected from sponges at the two sampling sites including two P1 larvae, one P3 larva and eight males. Of all these gnathiids (N = 1051), 53 moulted into males and 29 moulted into females, with 15 of these females being fertilized, but no larvae completed development in the female marsupium under laboratory conditions.
Table 1. Gnathia pilosus collections from March 2006 (autumn) to February 2007 (summer), including the host species, host size and number of fish collected and infested with gnathiids. Host size measured as total length and shown as range (mean).

In the data collected from the gnathiids feeding on the thirty wild fish hosts (N = 410), a distinct preference was observed for certain sites (Figure 2). Most of the larvae were found attached to the dorsal (21.1%), pectoral (19.5%), and the body surfaces (17.6%), as well as on the anal fins (16.8%). Gnathiid attachments to the head, pelvic fins, tail and belly of the fish, only ranged between 3% and 10% of total attachments and thus these sites seemed less preferable for feeding.

Fig. 1. Gnathia pilosus life forms. (A) The zuphea (Z2) after a completed moult and ready to feed; (B) the fully distended praniza (P2) larva after a blood meal on Scartella emarginata; (C) a yellow praniza (P3) after feeding on S. emarginata plasma; (D) a green praniza (P2) after feeding on Thalassoma purpureum plasma; (E) the anterior moult of the praniza (P1) larva into a zuphea (Z2); (F) the prominent mandibles and tubercles present on the male after the anterior moult; (G) the characteristic pigmentation of the adult male; (H) immature eggs present in the female larva; (I) the female larva's eggs fill the entire pereon before final moult; (J) the adult female thorax filled with unfertilized eggs. Scale bars 300 µm.

Fig. 2. Gnathia pilosus attachment preferences on their host fish. The percentage for each site was calculated using 378 captive (spots) and 410 wild (stripes) gnathiid attachments. The total number found feeding at each site is given above each bar.
In the experimental feeding on the thirty laboratory fish (Figure 2), preferences for body sites had the majority of the captive gnathiids (N = 378) attaching to anal (31%) and pectoral fins (29.6%) followed by dorsal fins (15%) and body surfaces (11%), with the head, pelvic fins, tail and belly only between (1% and 5%). This pattern is similar to the pattern observed on wild fish.
A Chi-square test was used to determine if captive gnathiids would attach in the same manner as the wild gnathiids or if there is random attachment. A value of χ2 = 61.79 revealed a P value below the significance value (0.05) thus the null hypothesis that captive and wild gnathiids will attach in the same manner cannot be accepted.
All three larval stages were present in each of the four months indicating a continuous life cycle. However, there was a definite dominance of certain larvae in the seasons which may suggest a life cycle with seasonal preference. As this study was carried out in South Africa, in the southern hemisphere, the seasons can be summarized as summer (December–February), autumn (March–May), winter (June–August) and spring (September–November). In autumn, a large number of P1 larvae were present as well as a low number of praniza 3 larvae. This could be the season where the Z1 larvae are released in large numbers and only a few P3 were present which had not yet moulted into adults. In winter, the P1 larvae had become P2 and thus there were fewer P1 larvae, a larger number of P2 larvae and more P3 larvae. Spring showed a higher number of P3 larvae as the number of P2 larvae decreased. Thus, the majority of adults would start appearing in summer and reproducing to form the larvae which would be released in autumn again. This was only a general life cycle however, as the average life cycle for this species is between 125 and 140 days in the laboratory.
The seasonal percentage of the praniza population was calculated after recording all the life stages observed feeding on the host fish during each season (Figure 3). There were a total of 104 gnathiids collected during autumn, of which 96 (92.3%) were P1 larvae, 6 (5.8%) were P2 larvae and 2 (1.9%) were P3 larvae. In winter 288 larvae were collected with 201 (69.8%) being P1 larvae, 59 (20.5%) P2 larvae and 28 (9.7%) P3 larvae. There were 135 gnathiid larvae collected in spring including 96 (71.1%) P1 larvae, 18 (13.3%) P2 larvae and 21 (15.6%) P3 larvae. A total number of 513 larvae were collected in summer, of which 353 (68.8%) were P1 larvae, 116 (22.6%) were P2 larvae and 44 (8.6%) were P3 larvae.

Fig. 3. Gnathia pilosus praniza stages (P1 spots, P2 horizontal stripes, P3 diagonal stripes) collected during each seasonal period, as well as the total number for the year. Seasons in the southern hemisphere: summer (December–February), autumn (March–May), winter (June–August) and spring (September–November).
In this study, larval growth rate (the growth in millimetres between each larval stage) decreased during the winter months and increased from spring onwards (Table 2).
Table 2. Gnathia pilosus larval details indicating the average size, average moult or rest period (in days) and average feeding times (in hours) for each larval stage for each season. Standard deviation provided in parentheses. Seasons in the southern hemisphere: summer (December–February), autumn (March–May), winter (June–August) and spring (September–November).

N/A, not applicable.
Zuphea 1 (Z1)
This is the first larval form expected to be released from the female marsupium (Figure 1A). Although no Z1 larvae were seen leaving the female in the laboratory observations, 14 were collected during field sampling before they could feed on their host. In the laboratory, this active stage swam to a suitable host, attached with the hooked gnathopods and ingested the fish's blood. The larvae were almost uniform in size, ranging from 0.9–1.1 mm (1.03±0.07 mm) in total length. The time taken to feed on the host was between 3 and 3.5 hours, where the anterior hindgut filled, the pereon swelled and the intersegmental membrane became fully distended. The colour of the larva depended on the contents of the meal and the species of the fish. The larva would be red after having fed on the blood of a blenny (Figure 1B), yellow after having fed on the plasma of a blenny (Figure 1C) and green after having fed on the plasma of a wrasse (Figure 1D). Upon completion of the feed, the larvae which appeared to have lost all apparent segmentation between somites five and seven during feeding were now referred to as praniza 1 larvae.
Praniza 1 (P1)
This larva detached from the host and swam to find a suitable substrate where it remained until it had moulted into the following zuphea stage. The larvae survived on the previously ingested blood which was evident through the thin cuticle and some died if the blood meal was not sufficient to keep it alive for the period it required to moult. This was evident as all the other influencing factors on the gnathiids were kept constant and only the undernourished larvae died. The surviving P1 larvae ranged from 1–1.5 mm (1.19±0.11 mm) which was, on average, a 0.16 mm increase in length due to the feed. The P1 started to moult into the next zuphea approximately 35 days post feeding. The old cuticle splits radially between the third and fourth pereopods with the posterior portion being cast off first (Figure 1E), followed a day later by the anterior moult. In the laboratory, 442 P1 larvae survived to become P2 larvae.
Zuphea 2 (Z2)
After moulting into the second zuphea, the Z2 once again attached to a host on which it could feed. The remaining blood or lymph meal was still visible in the gut and was slowly digested until the next feed. These larvae ranged from 1–1.5 mm (1.13±0.09 mm) and took approximately one to two weeks before the gnathiid was ready to feed again. When placed near a host before the larva was ready (meal still present in the gut), it merely attached and would not feed on the host. The larvae could survive for a few weeks without feeding but eventually became too weak to swim and attach to a host if a suitable host was not found. However, when ready (empty gut, normally around 13 days post-moult), the gnathiid fed for up to 3.8 hours (similar to the previous feed) and left the host as P2 larvae. A total number of 125 Z2 larvae were able to successfully feed and become P2 larvae in the laboratory.
Praniza 2 (P2)
The detached P2 larvae remained in a suitable resting place until the next moult could occur. It ranged from 1.3–1.9 mm (1.57±0.13 mm) in length and this was on average 0.38 mm longer (33.6%) then the previous (zuphea) stage and indicated the rapid increase in size from the one feed. They moulted into the Z3 forms between 35 days post-feeding, following a similar pattern as the P1 larvae with a day between posterior and anterior moults. The resulting Z3 larvae then awaited the final feeding stage. There were 138 P2 larvae which moulted into Z3 larvae in the laboratory.
Zuphea 3 (Z3)
Once moulted, the Z3 larvae were ready to feed within 12 days. These larvae seemed to be more active when compared to the other zuphea larvae and swam much quicker to attach to a suitable host. This zuphea was the largest stage and thus caused the most amount of discomfort to the fish host parasitized possibly due to the larger mouthparts. Despite attempts by the host fish to avoid being fed upon, the Z3 larvae were found to be aggressively persistent and eventually fed on the hosts. The length of these zuphea larvae ranged from 1.5–2.1 mm (1.75±0.13 mm) and were approximately 0.18 mm longer (11.5%) than the previous stage (P2). The P3 larvae fed for an average of 4.2 hours and became the final larval stage, P3. In the laboratory, 108 Z3 larvae were able to feed and become P3 larvae.
Praniza 3 (P3)
This large and fully fed praniza detached from its host after the longest and largest feed. The last ingested meal was used to maintain the P3 larvae as well as the adult stages which follow. These larvae are generally larger than the other larval stages, ranging from 1.8–3 mm (2.19±0.2 mm) in length and increasing by an average 0.44 mm (25.1%) from the last meal. The P3 became very lethargic towards the final moult and remained sedentary for days before it became an adult male or female. There were 112 P3 larvae which survived to become adults in the laboratory. The P3 larvae could easily be separated into male and female larvae before their final moult and will thus be discussed individually below.
Male development
Two distinct structures, identified as testes, could be seen in the pereon of the male larvae approximately two weeks before moulting into an adult male. These testes were situated in the middle of the pereon and close to the dorsal surface. The posterior moult occurred first, where the last three pereonites became greatly distended and wide, followed by the anterior moult where the cephalosome became large and square, the maxillae disappeared, and the mandibles enlarged (Figure 1F). This happened between 19 and 71 days (42±15.35) after the final feed. There was very clear distinction between the larvae and the male after the posterior moult had been completed. The posterior half of the moulting male gnathiid was considerably wider than the anterior larval body. The new posterior male pereopods were also not functional for up to three hours after the posterior moult. There was a day between posterior and anterior moults, and the male seemed to be very vulnerable at the stage directly after the final moult as the new exoskeleton was still soft with the cuticle being very transparent and not yet hardened enough to protect the gnathiid. This cuticle hardened in a few days and became a rather dark exoskeleton with white patches randomly scattered over the dorsal surface (Figure 1G). The anterior moult resulted in a completely different and larger cephalosome than that of the P3 larva, lacking pigmentation and sometimes deformation or malformation were observed when complications occurred during the moulting process. A deformed male might have under-developed mandibles, eyes and mouthparts. During this study, ten male adults were not able to complete their anterior moult and six had cephalosome features which appeared distorted or different from the holotype. The male's size ranged from 1.6–2.4 mm (2.03±0.15 mm) depending on the nutrition and length of the final feeding. Those males which were able to feed for longer periods throughout their larval phases grew larger than those which only had a short feed time. It is uncertain as to whether the type of meal affects their growth (i.e. blood or plasma). In captivity the males were able to survive for at least two to three months.
Female development
Female P3 larvae were characterized by two thin lines of ovaries filled with eggs in the centre of the dorsal pereon. These were seen approximately two to three weeks before moulting into a female (from 10 days post-feed onwards). The eggs and ovaries grew to cover the digestive caecae (Figure 1H) and eventually filled the entire pereon before the final moult (Figure 1I). This moult occurred between 14 and 75 days (48±14.82) after the final feed by means of two separate moults (posterior and anterior). One day separated the two moults with the posterior moult of the female being less obvious visually as compared to that of the male. Once the moulting process began, the last few pereonites became very large and round, with the anterior moult producing a small head with modified and reduced mouthparts. The large, round females ranged from 1.8–2.5 mm (2.1±0.17 mm) in length and were fertilized within 24 hours after the final moult. At this stage, the dorsally situated ovaries were filled with eggs (Figure 1J). The two adults copulated and within a week after fertilization the eggs began to move from the dorsally situated ovaries to the ventrally situated marsupium.
Eggs progressed from the ovaries to the marsupium regardless of whether they were fertilized, with unfertilized eggs appearing irregular in shape. Those females whose eggs were not fertilized did not survive long. Their eggs disintegrated inside the body leaving the body cavity empty and dishevelled, and after a few days, the females died. Those females with fertilized eggs were kept separate to ensure other gnathiids did not interfere with the development. In one incident where the male was left with the female, the male clawed out the eggs from the female marsupium with his pereopods, pulling the oostegites open and thus releasing the eggs. In other specimens (N = 40), fertilized eggs were round and cell division occurred within days. Unfortunately none of this species' females survived longer than this phase.
DISCUSSION
Feeding
The distribution of the Gnathia pilosus gnathiid larvae over the fish bodies were compared between those gnathiids feeding on wild caught fish and those allowed to feed on fish in captive controlled conditions. The fins and body surfaces (specifically the side flanks) may be preferred as they are the larger surface areas of the fish and thus are more accessible for easy attachment. The four less popular sites may show fewer attachments due to the smaller fins and body surfaces being less accessible for attachment, being less protected and more likely to be scraped off by body scouring. The lack of attachment to the dorsal fin and body in the experimental feeding may also be due to the manner in which the gnathiid was released into the feeding tank or how the fish reacted to the presence of the gnathiid. Captive fish were more sensitive and reacted more violently to the gnathiids attempting to feed which resulted in many gnathiid mortalities, as the fish ate all gnathiids it could reach (i.e. those on the tail and posterior body surfaces), as well as difficulty in trying to attach as the fish flicked off many of the gnathiids. Gnathiids which did manage to feed were sometimes eaten by the fish once it detached from the body and was swimming for shelter.
There are very few accounts of the preference for attachment sites for gnathiid isopods. Gnathiid isopods parasitizing epaulette sharks, Hemiscyllium ocellatum, were found to have a preferred attachment around the cloacae for both sexes of hosts (Heupel & Bennett, Reference Heupel and Bennett1999). In a more recent study by Munday et al. (Reference Munday, Schubert, Baggio, Jones, Caley and Grutter2003) on coral-dwelling gobies, gnathiids were found to attach mostly to the fins of each Gobiodon species. However, on Paragobiodon xanthosomus, the gnathiids attached more frequently to the body. This supports our conclusion that G. pilosus larvae do not have a preferred site of attachment.
Seasonal distribution
Gnathia pilosus is capable of breeding throughout the year, but with majority of the gnathiids breeding in summer and releasing their young in autumn. Tanaka & Nishi (Reference Tanaka and Nishi2008) examined the habitat pattern of Elaphognathia discolor using bimonthly samples and found all life forms in terebellid polychaete tubes throughout the year. Their prevalence however, varied from 57.1% to 80% with the highest prevalences being in June and lowest in February.
Previous work on the effects temperature has on gnathiid growth shows similar results, however these studies were all done in the northern rather than the southern hemisphere. Stoll (Reference Stoll1962) discussed the effects temperature variations may have on the gnathiid life cycle using Paragnathia formica from Penzé, near Roscoff (Finistère) in the northern hemisphere. The temperature was found to have an influence on the development of the pranizas during each intermoult. Paragnathia formica was found to have blocked digestion and therefore also blocked moulting at low temperatures. The praniza can remain in this state for four months and will continue digestion when returned to normal temperature. High temperatures also have negative effects, with temperatures over 25°C being fatal for these gnathiids. The female P3 larvae seem to be more sensitive to these higher temperatures and the majority die when the mud temperatures reach 20°C. Thus, temperature has an influence on the development of P. formica pranizae during each intermoult, but it seems that the particular sensitivity of P3 for temperature variations could be one of the factors which determines the differences between the male and female cycles (Stoll, Reference Stoll1962).
Seasonal population changes were also observed by Upton (Reference Upton1987) for Paragnathia formica, which revealed breeding in the summer months (northern hemisphere) and the release of annual generation of Z1 larvae in autumn. This study recorded a halt in development of the larvae in winter where the mud temperatures decreased, but which resumed development again in warmer conditions. Likewise, Tanaka (Reference Tanaka2003) found that the natural gnathiid population of Elaphognathia cornigera in winter (northern hemisphere) was composed exclusively of overwintered P2 larvae and adult males. The overwintered larvae would develop into adults in the following spring once growth resumed.
The explanation behind most of the above recorded results is attributed to cold environments (below 7°C) influencing the growth rate of the Paragnathia formica and Elaphognathia cornigera. Gnathia pilosus larvae also showed a decrease in growth over the colder months but the temperatures were never extreme enough to cause a complete halt in development or overwintering during the colder seasons. These data sets (seen in Table 2) indicate slower moulting in the winter months presumably caused by a decrease in water temperature and increased again in the warmer summer months.
Many of the gnathiids collected in the present study did not survive to adult forms, thus leading to a gradual decrease in gnathiid numbers as they progressed to the adult stages. In the wild the majority of the collected gnathiids were P1 larvae. However, as they grow and moult, many die for a number of reasons such as: the gnathiid not feeding long enough and not having enough food to last to the next moult; the gnathiid having complications during moulting where the shed cuticle hooks on their legs so they cannot swim or on their head so they are unable to feed; being eaten by the host when attaching; or being preyed on by other predators. Using the collection data for all four seasons, an average of only 13 (9–20±9) gnathiids out of a brood size of 50 (26%) will survive to become adults. Unfortunately, no information on the survival rate of other isopods is available for comparison with what was found for Gnathia pilosus.
Life cycle
Using all the data collected the life cycle of Gnathia pilosus can thus be summarized as follows (Figure 4): the Z1 appears to be released from the female marsupium in the autumn months and subsequently attaches to their host fish. During feeding, the Z1 transform into the P1. The P1 detaches from the host and rests on the bottom substrate, utilizing the blood meal to sustain itself until moulting into the Z2 after 35 days. The Z2 feeds on a host to become the P2 and moults into a Z3 which will also feed to become the very swollen P3.
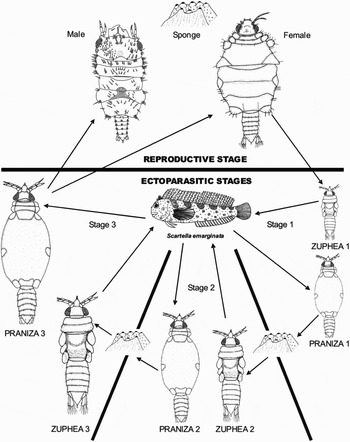
Fig. 4. Gnathia pilosus life cycle represented schematically. The host fish, Scartella emarginata, is fed on three times by the three zuphea larval stages and after the final praniza stage an adult male or female will moult and reproduce to continue the lifecycle. Not drawn to scale.
Within a few weeks, the sex of the P3 can be determined by the presence of either the testes in the male larva or thin ovary strands in the female larva. The male P3 moulted (42 days post-feeding) before the female P3 (52 days post-feeding) in order to be ready for reproduction as soon as the females mature. This is important as the female requires fertilization immediately after completing its moult. The larvae increase in length and width with each moult, obtaining their average adult size of 2.1 mm for a female and 2 mm for a male. Embryos develop in the female marsupium until released from the oostegite openings as Z1 larvae. The life cycle from Z1 to adult female fertilization ranges between 125 and 140 days (Figure 4).
This life cycle may be influenced by the seasons with the breeding period increasing in summer as compared to the rest of the year but future research is required for absolute certainty. This is very similar to another South African gnathiid, Gnathia africana, which is also present throughout the year (Smit et al., Reference Smit, Basson and Van As2003) as well as Elaphognathia cornigera which has three or four generations in a year (Tanaka & Aoki, Reference Tanaka and Aoki2000). This latter species' life cycle was attributed to the warm Japan waters increasing the rate of reproduction and this could also be the case in G. pilosus.
Another resting place for the inactive gnathiid stages may need to be sought as only eleven gnathiids were found in the sponges, which is unusual as intertidal gnathiids are commonly found inhabiting the surrounding sponges. No females were found in the sponges which may also indicate that females do not reside in the sponges but have another hiding place. This was also the case with the Gnathia africana females which were absent from sponges and tunicates collected along the south coast of South Africa (Smit et al., Reference Smit, Basson and Van As2003). Barnard (Reference Barnard1914a, Reference Barnardb) also never collected G. africana females from sponges but did manage to find some in the serpulid worms. Thus, sponges may not be the ideal location for the females to rest and reproduce and they may be found hiding in small crevices or other worm tubules.
Gnathia pilosus can be considered a varied feeder as it is not host family specific, not host species specific and not even site specific. Grutter & Poulin (Reference Grutter and Poulin1998) also found gnathiids on a range of families (35 genera from 20 families). However, they found that certain species from the families Chaetodontidae, Synodontidae and Pomacentridae, had few or no gnathiids present even with large sample numbers (3 to 84). They suggested several factors for the distribution on reef fish including the variation in the distribution of the fish hosts, the feeding time preference for both the gnathiid and the fish, differences in the external surfaces of the fish, etc (Grutter & Poulin, Reference Grutter and Poulin1998).
Gnathia pilosus seems to portray a number of similarities with the other intertidal South African species, G. africana, which may be due to their geographically close proximity. Its distribution currently seems to be confined to the east coast of South Africa but future research will need to be carried out to determine if this restriction is accurate or if it has a larger distribution and even a larger host selection, depth preference and if its distribution overlaps with areas where other African gnathiids have previously been found.
ACKNOWLEDGEMENTS
The financial assistance of the National Research Foundation (NRF) towards this research is hereby acknowledged. Opinions expressed and conclusions arrived at, are those of the authors and are not necessarily to be attributed to the NRF. The help provided by Dr Andy Cockcroft, Marine and Coastal Management (MCM) in obtaining collection permits is also acknowledged.








