Introduction
Benthic marine animals have evolved a diversity of morphological and behavioural adaptations to overcome the need for dispersal, development and recruitment. Planktonic embryos and larvae (propagules) evolved as a means to offset some of these challenges. These propagules have the capacity to swim autonomously using cilia and/or muscular elements and often possess complex behavioural responses to environment cues. Autonomous locomotion allows propagules to gather food (for feeding), regulate vertical position, avoid predators/non-ideal conditions and select a specific settlement site (Chia et al., Reference Chia, Buckland and Young1984).
Swimming speeds are relatively easy to quantify and are a useful starting point for intra- and inter-specific comparisons. To date, the bulk of swimming speed studies have been conducted in phyla with multiple types of larval nutritional mode (e.g. Mollusca, Annelida, Echinodermata) but they have mainly focused on propagules with planktotrophic (feeding) development. Previously reported speed values for planktotrophic and lecithotrophic ciliated propagules range widely between 0.1 and 30.0 mm s−1 in species of Porifera (Maldonado, Reference Maldonado2006), Cnidaria (Mileikovsky, Reference Mileikovsky1973; Harii et al., Reference Harii, Kayanne, Takigawa, Hayashibara and Yamamoto2002), Mollusca (Chia et al., Reference Chia, Buckland and Young1984) and Echinodermata (Podolsky & Emlet, Reference Podolsky and Emlet1993). In contrast, propagules that use muscular elements or appendages to swim have speeds in the range of 30–300 mm s−1 (e.g. Arthropoda; Chia et al., Reference Chia, Buckland and Young1984).
While reviews have examined the swimming patterns of planktotrophic larvae (Mileikovsky, Reference Mileikovsky1973; Chia et al., Reference Chia, Buckland and Young1984; Koehl & Reidenbach, Reference Koehl and Reidenbach2007), and have compared the dispersal potential of propagules of different nutritional modes (Mercier et al., Reference Mercier, Sewell and Hamel2013), no study has ever explicitly explored the locomotory differences between ciliated planktotrophic and lecithotrophic propagules across multiple phyla. The term ‘propagules’ is used here when embryos and larvae are both being considered. Focusing on ciliated propagules (i.e. that use cilia for locomotion) is valuable from an evolutionary perspective since some form of ciliation is present across the propagules of diverse marine phyla and life stages. Similarly, the intersection of larval nutritional mode, swimming speed and size has not been examined across multiple phyla, leaving many questions unanswered including: do certain types of ciliated propagules swim faster? The relationship between larval swimming speeds and adult mobility also has not been explored, despite the potential for intriguing trade-offs between the need for dispersal and larval provisioning.
To address these uncertainties and tease out some of the potential drivers, we conducted a meta-analysis using propagule swimming speed data from the literature examined through the lens of propagule size, larval nutritional mode, and adult mobility.
Materials and methods
We gathered swimming speed data of pelagic ciliated propagules (embryonic and larval stages of species with larvae that use cilia for locomotion) across six of the main marine phyla (Porifera, Cnidaria, Annelida, Mollusca, Echinodermata, Bryozoa; N = 118 records, see Table A1 for raw data) from the scientific literature to compare phylum-based differences among larval nutritional modes. For example, taxa with appendaged or muscular larvae (e.g. Arthropoda, Urochordata) were not included. Poriferans (sponges) only possess lecithotrophic larvae (Ereskovsky, Reference Ereskovsky2010), and propagules of cnidarians (corals and sea anemones) are mainly lecithotrophic, with only few known planktotrophic representatives (e.g. Schwarz et al., Reference Schwarz, Weis and Potts2002). In contrast, Bryozoa, Annelida, Mollusca and Echinodermata are among the phyla comprised of a diverse mixture of species with planktotrophic and lecithotrophic larvae (Table A1).
Metrics collected included: phylum, adult mobility level, larval nutritional mode, as well as size (length of longest axis) and swimming speed (mm s−1) for the given stage (Table A1). Life stages ranged from embryonic (blastula/gastrula) to competent larval forms (e.g. urchin pluteus), although the vast majority of the records involved larvae (>90%). Taxonomic classification was confirmed using the World Registry of Marine Species (WoRMS). Adult mobility level was defined as motile (exhibiting frequent movement or migration), sedentary (capable of movement but doing so less commonly) or sessile (permanently fixed for the majority of their life) modified from the standard terminology proposed by Costello et al. (Reference Costello, Claus, Dekeyzer, Vandepitte, Tuama, Lear and Tyler-Walters2015). The nutritional mode of larvae was defined as planktotrophic or lecithotrophic based on the classification presented by Poulin et al. (Reference Poulin, Boletzky and Féral2001) and Carrier et al. (Reference Carrier, Heyland and Reitzel2017). Propagule size (μm) was defined as the mean length of the longest axis; this value represents Feret diameter in spherical propagules. All speeds used in the analysis were absolute speeds and were not standardized to body size. Wherever possible, horizontal swimming speeds (mm s−1) were used, but these data were not always reported in the literature. When multiple swimming directions were reported, the fastest reported speed was used (e.g. among upward and downward swimming speeds).
Two-way ANOVA and Factorial Analysis of Mixed Data (FAMD) were used to test the relationship among all collected metrics. Statistical analyses were performed in Sigma Plot and R statistical software.
Results
Phylum is a greater driver of swimming speed than larval nutritional mode
Lecithotrophic and planktotrophic propagules across the whole dataset had mean absolute swimming speeds of 3.72 ± 0.65 mm s−1 and 1.22 ± 0.18 mm s−1, respectively (Table 1, Figure 1; ANOVA P = 0.009). Lecithotrophic propagules generally swam faster than planktotrophic counterparts in those phyla with multiple nutritional modes, including Annelida (2.84 ± 0.64 vs 1.44 ± 0.21 mm s−1), Bryozoa (4.60 ± 0.61 vs 1.9 mm s−1), Echinodermata (0.49 ± 0.15 vs 0.40 ± 0.07 mm s−1) and Mollusca (1.71 ± 0.25 vs 1.50 ± 0.43; Figure 1). Phyla that rely exclusively or predominantly on lecithotrophy (Porifera, Cnidaria) had faster average propagule swimming speeds (11.04 ± 3.77 and 2.83 ± 0.57 mm s−1, respectively) than the other phyla under study (Annelida, Mollusca, Echinodermata, Bryozoa; overall mean = 1.4 mm s−1).
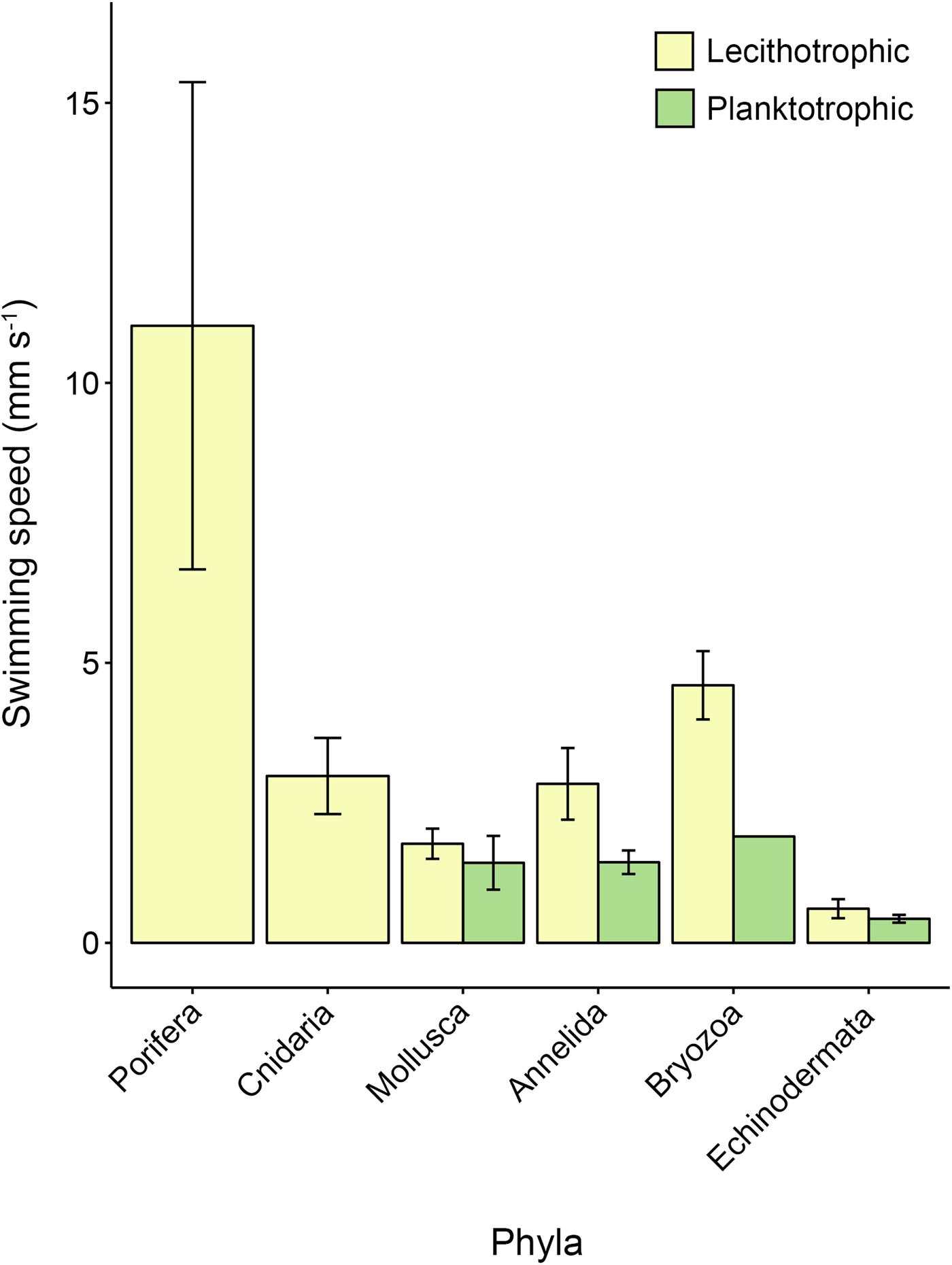
Fig. 1. Mean propagule swimming speed (mm s−1 ± SE) varies among phyla and larval nutritional modes. Phylum Cnidaria and Porifera only have one bar as these taxa only have one larval nutritional mode. Error bars are present where more than one record per phylum and category were available. See Table A1 for raw data.
Table 1. Mean length/diameter and swimming speed summarized across the two larval nutritional modes and five phyla featured in the dataset (see Table A2 for raw data)

a Phylum Porifera is entirely lecithotrophic, Phylum Cnidaria is mainly lecithotrophic with a few planktotrophic representatives (Schwarz et al., Reference Schwarz, Weis and Potts2002).
b Only one swimming speed record of planktotrophic Bryozoa could be located.
Porifera and Echinodermata stood out at opposite ends of the propagule-swimming-speed spectrum when a follow-up FAMD analysis was performed (Table 2). In particular, planktotrophic propagules of echinoderms swam slower on average than all other propagules (P < 0.001; Table 2); whereas poriferan propagules (all lecithotrophic) swam faster than other tested propagules (FAMD P < 0.001; Table 2). Poriferan propagules were also much larger than planktotrophic echinoderm propagules (mean size 571 ± 68 vs 267 ± 35 µm). Lecithotrophic echinoderm larvae are also large (mean size 705 ± 66 µm), however, they do not even come close to reaching the speeds displayed by poriferan larvae (0.49 ± 0.15 vs 11.04 ± 3.77 mm s−1).
Table 2. Summary of results from an FAMD testing the relationship among phylum, propagule size, adult activity level, larval nutritional mode and propagule swimming speed in the dataset

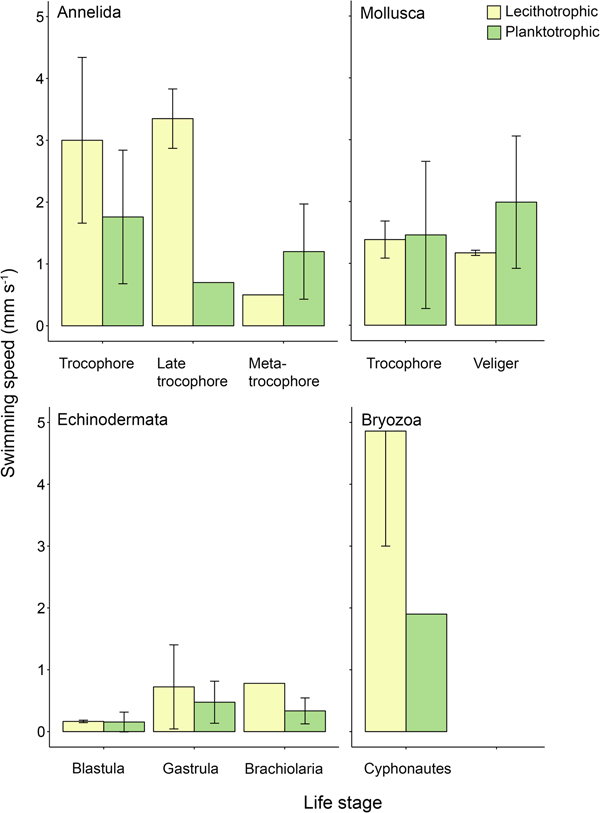
Patterns of swimming speed between lecithotrophic and planktotrophic propagules were not consistent across phyla when differences within life stages were considered. Lecithotrophic annelid trocophores and late trocophores, echinoderm brachiolariae, and bryozoan cyphonautes larvae swam faster on average than their planktotrophic counterparts (Figure 2). Lecithotrophic mollusc trocophores, and echinoderm embryos swam at similar speeds to planktotrophic equivalents (Figure 2). Lastly, lecithotrophic mollusc veligers and annelid metatrocophores swam slower than planktotrophic equivalents (Figure 2).
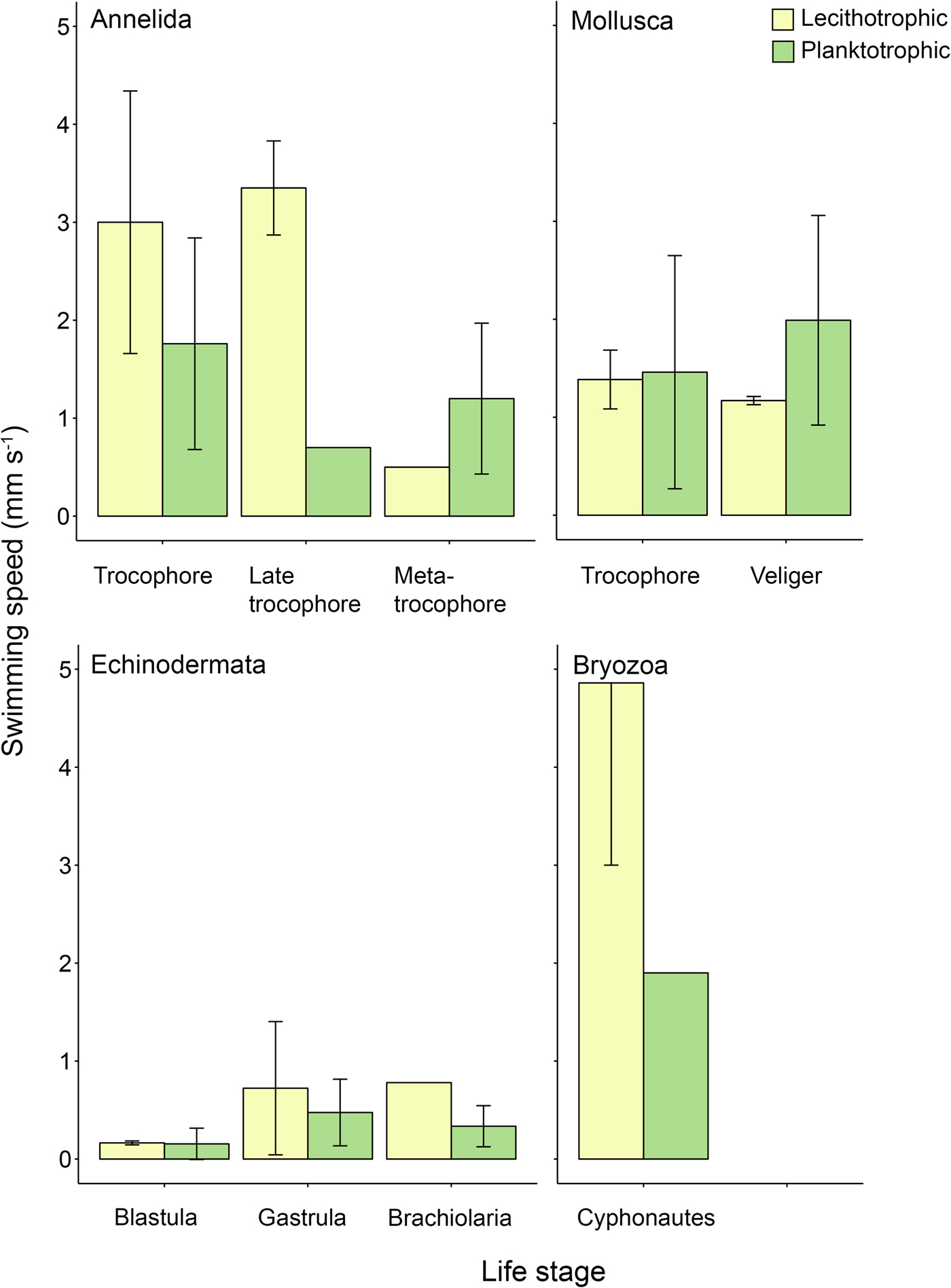
Fig. 2. Mean propagule swimming speed (mm s−1 ± SD) of specific life stages varies with nutritional mode. Taxa and life stages presented here had both planktotrophic and lecithotrophic representatives in the dataset. Error bars are present where more than one record per phylum and category were available. See Table A1 for raw data.
Adult motility level correlates with larval nutritional mode and swimming speed
When all phyla were considered together, species with sessile and sedentary adults produce faster swimming larvae on average than species with motile adults (4.63 ± 1.17 and 2.73 ± 0.87 vs 1.14 ± 0.22 mm s−1; ANOVA, P < 0.001; Table 1). This pattern was particularly noticeable among sessile species like sponges, corals and bryozoans that produced fairly large, fast swimming larvae, relative to other phyla in the dataset like Echinodermata, that have motile adults (Figure 3). Propagules from sessile and sedentary adults also swam faster within those phyla containing a mix of adult mobility types, such as Annelida, Cnidaria and Mollusca, although speed data for cnidarians with motile adults (e.g. hydro- and scyphozoan medusae) are scarce in the literature (Figure 3), and insufficient to generate a solid statistical comparison.

Fig. 3. Mean propagule swimming speed (mm s−1 ± SE) varies with taxa and level of adult mobility. Sessile adults are incapable of movement, sedentary adults have the capacity to move but do so rarely and motile adults move readily and often. Error bars are present where more than one record per category were available. See Table A1 for raw data.
Swimming speed scales with size in planktotrophic, but not in lecithotrophic propagules
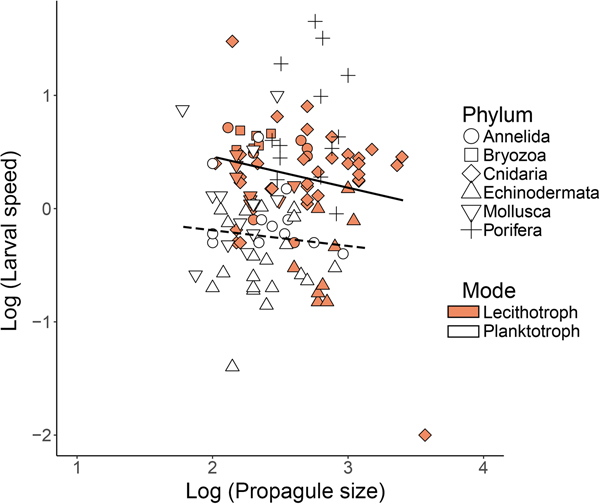
When all phyla were considered together, planktotrophic propagules were smaller than lecithotrophic propagules (263 ± 25 vs 597 ± 61 µm); although this difference was mainly driven by lecithotrophic poriferans and echinoderms that were larger on average than all other types of propagules (Table 1). No definitive relationship between propagule size and swimming speed was seen among planktotrophic and lecithotrophic propagules (Figure 4). However, lecithotrophic propagules appeared to have a greater capacity for faster swimming speeds, as the majority of the speed values for lecithotrophic propagules were higher than for planktotrophic counterparts of the same size (Figure 4, ANCOVA, P < 0.001).

Fig. 4. Larval swimming speed (mm s−1) vs propagule body size (μm) in lecithotrophic and planktotrophic larvae of various phyla (Porifera, Cnidaria, Mollusca, Annelida, Echinodermata, Bryozoa) on log10 scales. Log scales were used to examine scaling relationships across a wide range of propagule sizes and speeds. Points represent mean values for individual species. Symbols depicting the various phyla are either solid/open to indicate the lecithotrophic/planktotrophic larval feeding mode (except for Porifera, which is fully lecithotrophic and identified with +). N = 66 total. The solid lines show regression results. Planktotrophs: y = −0.017x −0.25, R 2 = 0.01; Lecithotrophs: y = −0.26x + 0.97, R 2 = 0.02.
Discussion
Swimming speeds of ciliated marine propagules clearly varied with phylogeny, though differences between larval nutritional modes emerged when life stages were considered individually. Differences also emerged among propagules produced by adults with sessile/sedentary lifestyles vs those with more mobile ones.
On a phylogenetic level, the biggest differences in mean swimming speeds in the dataset was found between poriferan and echinoderm propagules. On average, poriferan propagules swam 10–12× faster than planktotrophic and lecithotrophic echinoderm propagules. This difference may be a result of propagule size and chemical composition differences (e.g. relative amounts of protein, lipid and calcified elements) between these two taxa. Poriferan propagules are a simple prolate spheroid shape and therefore may experience less drag or fluid interactions than other propagule shapes. Planktotrophic echinoderm larvae (and some late-stage lecithotrophic echinoderm larvae) use a completely different morphological strategy than poriferans; they often possess calcified elements and appendages that modulate their density and interaction with fluid and may constrain their swimming abilities (Grünbaum & Strathmann, Reference Grünbaum and Strathmann2003; Strathmann & Grünbaum, Reference Strathmann and Grünbaum2006). Interestingly, the gastrula of many planktotrophic and lecithotrophic species (e.g. Annelida, Mollusca, Echinodermata) are similar in shape to poriferan propagules (spheroid) and are also uniformly ciliated with cilia of similar length (~20–25 µm; Strathmann, Reference Strathmann1971; Chia et al., Reference Chia, Buckland and Young1984; Maldonado, Reference Maldonado2006). However, these gastrulae swim at much slower speeds, which suggests that variability in other features such as ciliary beating rates, buoyancy and energy usage could be at play. Some larval types can also supplement their ciliary movements with muscular contractions (e.g. some segmented annelid larvae; Chia et al., Reference Chia, Buckland and Young1984) but this addition is usually seen in older, more complex larval forms. Clearly there is something unique about the swimming mechanics of sponge larvae that deserves further attention.
Swimming speed differences between planktotrophic and lecithotrophic propagules were not consistent when life stages were considered individually. Most lecithotrophic versions of the considered life stages swam similarly, or faster than their planktotrophic equivalents (e.g. annelid and molluscan trocophores among others). Only planktotrophic late-stage annelid and mollusc larvae swam faster than the lecithotrophic counterparts, possibly due to the presence of complex ciliation patterns that enhance swimming capacity, or the precursors to muscular swimming in late-stage annelid larvae. Taken together, these data challenge the sometimes-held belief that lecithotrophic larvae experience constraints to their locomotion from their large size and generally positive buoyancy (Emlet, Reference Emlet1991, Reference Emlet1994) and are in line with recent studies that test planktotrophic and lecithotrophic propagules together under similar conditions (Krug & Zimmer, Reference Krug and Zimmer2004; Mercier et al., Reference Mercier, Sewell and Hamel2013; Montgomery et al., Reference Montgomery, Hamel and Mercier2017, Reference Montgomery, Hamel and Mercier2018). In fact, lecithotrophic propagules swim as fast as planktotrophic propagules even in species that display poecilogony (mixed modes of development) like the nudibranch Alderia modesta (Krug & Zimmer, Reference Krug and Zimmer2000; Krug & Zimmer, Reference Krug and Zimmer2004). Species with mixed modes of development provide an interesting system to further examine nutritional mode differences without potential phylogenetic constraints; they can be found in Annelida, Mollusca, and Echinodermata, with notable examples including the gastropod Alderia modesta (Krug & Zimmer, Reference Krug and Zimmer2000), the polychaete Capitella capitata (Butman et al., Reference Butman, Grassle and Buskey1988) and the sea star Henricia lisa (Mercier & Hamel, Reference Mercier and Hamel2008).
It emerged that the majority of benthic species with fully sessile adults (in phyla Porifera, Cnidaria and Bryozoa) that have ciliated pelagic propagules are lecithotrophic. Exceptions to this include some bryozoan taxa (e.g. order Cheilostomata, Hayward, Reference Hayward, Kermack and Barnes1985) that produce long-lived planktotrophic larvae, and some anthozoan planulae (Cnidaria) that ingest symbiotic zooxanthellae while swimming in the plankton (e.g. Schwarz et al., Reference Schwarz, Weis and Potts2002). The predominance of lecithotrophy among sessile marine invertebrates raises many questions, including how larval nutritional mode, adult mobility and larval behaviour might be linked. If we compare across phyla (e.g. Porifera to Echinodermata), propagules from sessile adults clearly swim faster. This pattern also appears to be conserved among sessile adults within Bryozoa using the limited data available in the literature; planktotrophic propagules swam with average speeds of 19 mm s−1 vs 34–49 mm s−1 among lecithotrophic propagules (data from Chia et al., Reference Chia, Buckland and Young1984; Wendt, Reference Wendt2000). Interestingly, the pattern also seems to hold in phyla with sedentary (rather than fully sessile) adults, where propagules produced by sedentary adults tend to swim faster than propagules produced by motile adults (e.g. Mollusca and Annelida), though this was not supported statistically.
Maternal reserves likely facilitate radical shape changes during metamorphosis and the construction of morphological features prior to feeding at the juvenile stage (e.g. external skeleton, polyps, choanocytes), which may explain the predominance of lecithotrophy among species with sessile adults. Sessile species also have high specificity and sensitivity to certain settlement cues at the advanced larval stage (Mundy & Babcock, Reference Mundy and Babcock1998; Leys et al., Reference Leys, Cronin, Degnan and Marshall2002). The consequences of deviating from standard patterns of larval behaviour and settlement are also likely higher for sessile organisms given the fact that they have limited opportunities to get it right (Raimondi & Morse, Reference Raimondi and Morse2000). In contrast, species with motile adults probably do not experience the same pressure to settle in ideal conditions as they can migrate and relocate post settlement. Thus, the production of faster moving, lecithotrophic propagules by sessile adults, relative to motile adults, could enable sessile adults to overcome some of the constraints associated with a sessile lifestyle, such as the need for dispersal, settlement specificity and the energetic costs of metamorphosis.
The present study suggests that the swimming capacity of planktotrophic propagules may be more constrained by size than that of lecithotrophic propagules, since swimming speeds scaled with size in planktotrophic but not lecithotrophic propagules in the dataset. This may be explained by the fact that planktotrophs generally experience greater change in size during their development than lecithotrophs. For instance, planktotrophic propagules of the green sea urchin, Strongylocentrotus droebachiensis, range in size from 150 µm as embryos to 1000 µm as fully competent larvae (Meidel et al., Reference Meidel, Scheibling and Metaxas1999). In contrast, lecithotrophic propagules of the Australian sea urchin, Heliocidaris erythrogramma, range in size from 300 µm as embryos to 600 µm as competent larvae (Emlet & Hoegh-Guldberg, Reference Emlet and Hoegh-Guldberg1997). This size change of nearly 7 times in planktotrophs vs 2 times in lecithotrophs may place additional size-based locomotory constraints on the former, as cilia in feeding larvae are thought to be arranged to maximize feeding, not swimming (Strathmann & Grünbaum, Reference Strathmann and Grünbaum2006).
Swimming speeds may be affected by something other than size in lecithotrophic propagules, which do not require external nutrition to complete metamorphosis, typically do not undergo daily vertical migration during development and often are positively buoyant, which causes them to float to the top of the water column following spawning. To this effect, propagule form and behaviour could therefore influence swimming in conjunction with nutritional mode along with the need to disperse more effectively (e.g. dispersal constraints of sessile adults). Refining our understanding of the morphology and swimming speeds of different types of propagules will help improve the reliability of dispersal models and other predictive means for population management (e.g. marine protected area planning) by highlighting the importance of considering different larval features and ultimately identifying new model parameters.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0025315418001091.
Acknowledgements
The authors wish to thank the Field Services Unit of Memorial University for assistance with animal collections and the two anonymous reviewers for their helpful suggestions on the manuscript. The authors have no conflict of interest to declare. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Financial support
This study was funded by a Natural Sciences and Engineering Research Council Canadian Graduate Scholarship Doctoral-2 to E.M.M. and a Natural Sciences and Engineering Research Council Discovery Grant to A.M.