INTRODUCTION
The Lacydoniidae Bergström, 1914 are free-living benthic polychaetes that inhabit sandy and muddy unconsolidated sediments mixed with shell debris and gravel. They can be found over a wide bathymetric range, from shallow subtidal to deeper continental shelf areas and even at abyssal zones (Rouse & Pleijel, Reference Rouse and Pleijel2001). There are no studies on the reproductive or feeding biology; however, lacydoniids have been considered carnivorous or omnivorous based on buccal morphology (Gathof, Reference Gathof, Uebelacker and Johnson1984; Pleijel & Fauchald, Reference Pleijel and Fauchald1993; Wilson, Reference Wilson, Beesley, Ross and Glasby2000). Observations on preserved specimens have led to the conclusion that lacydoniids are gonochoric (Rouse & Pleijel, Reference Rouse and Pleijel2001) with internal fertilization; for example, females of L. quadrioculata Magalhães et al. Reference Magalhães, Bailey–Brock and Rizzo2012 have been observed with large (0.2 mm in diameter), whitish intra-coelomic embryos.
Lacydoniids are small-sized polychaetes with few body segments. The prostomium is rounded and usually wider than long. A pair of latero-dorsal antennae and latero-ventral palps are present on the prostomium; a dorsal median antenna has been recorded in most species and might be present in all (Rouse & Pleijel, Reference Rouse and Pleijel2001). However, this is a fragile, minute structure, which can be easily lost or overlooked (Blake, Reference Blake, Blake and Hilbig1994). Eyes may be present, and have been used as important characters to separate groups of species. Ciliated nuchal organs are present laterally in the prostomial posterior margin and may be well developed. The pharynx is strongly muscular, eversible, and has a pair of lateral glands. The tentacular segment is achaetous with a pair of latero-ventral cirri. The first three parapodia are uniramous, with neuropodial lobes, dorsal and ventral cirri, and spinigerous neurochaetae. The subsequent parapodia are biramous, with notopodia and neuropodia, dorsal and ventral cirri, capillary notochaetae, and spinigerous neurochaetae. Notopodial and neuropodial aciculae support parapodial lobes. The pygidium has a terminal anus, two pygidial cirri and one or more papillae.
Lacydonia Marion & Bobretzky, Reference Marion and Bobretzky1875 includes 13 nominal species which in chronological order are: (1) L. miranda Marion & Bobretzky in Marion, Reference Marion1874; (2) L. mikrops Ehlers, Reference Ehlers1913; (3) L. papillata Uschakov, Reference Uschakov1958; (4) L. incognita Rullier, Reference Rullier1965; (5) L. oculata (Hartman, Reference Hartman1967); (6) L. cirrata (Hartman & Fauchald, Reference Hartman and Fauchald1971); (7) L. laureci Laubier, Reference Laubier1975; (8) L. antarctica Hartmann-Schröder & Rosenfeldt, Reference Hartmann-Schröder and Rosenfeldt1988; (9) L. elongata Hartmann-Schröder & Rosenfeldt, Reference Hartmann-Schröder and Rosenfeldt1992; (10) L. gordia Hartmann-Schröder, Reference Hartmann-Schröder1993; (11) L. hampsoni Blake, Reference Blake, Blake and Hilbig1994; (12) L. eliasoni Hartmann-Schröder, Reference Hartmann-Schröder1996; and (13) L. quadrioculata Magalhães, Bailey-Brock & Rizzo, Reference Magalhães, Bailey–Brock and Rizzo2012. Four out of these 13 species were synonymized with others or invalidated; L. antarctica was considered a junior synonym of L. oculata by Pleijel & Fauchald (Reference Pleijel and Fauchald1993), L. incognita is considered a questionable species because its description was based on an incomplete specimen and the parapodia did not correspond to lacydoniids (Blake, Reference Blake, Blake and Hilbig1994). This species was later considered to belong to a known species of Goniadidae (Böggemann, Reference Böggemann2005). Further, L. elongata was synonymized with L. papillata (Böggemann, Reference Böggemann2009) but see remarks herein for the material identified as L. cf. papillata. Lacydonia eliasoni may be considered a nomen nudum because it was named based on a previous description and with no deposition of type material (Magalhães et al., Reference Magalhães, Bailey–Brock and Rizzo2012). Therefore, only nine species of Lacydonia are valid. An identification key for all Lacydonia species was proposed by Uschakov (Reference Uschakov1972) including the four valid species described at that time. Blake (Reference Blake, Blake and Hilbig1994) reviewed the validity of the species and concluded that there were nine valid species.
Species of Lacydonia have been reported in the Mediterranean, North Atlantic, Gulf of Mexico, North-eastern Pacific and Antarctica (Blake, Reference Blake, Blake and Hilbig1994). Böggemann (Reference Böggemann2009) recently recorded two species from abyssal depths off the west coast of Africa.
In this study, we provide: (i) a review of the morphological features used in species descriptions in this family based on the literature and recently collected material off south-east Brazil; (ii) an updated key to all valid species; and (iii) complete descriptions and illustrations of three new species and five new records from the Campos Basin, southern Brazil.
MATERIALS AND METHODS
Material was collected during two sampling campaigns executed by Habitats/Petrobras – Campos Basin Environmental Heterogeneity Project, coordinated by CENPES/Petrobras. All necessary permits for the described field studies were obtained prior to the beginning of the sampling. Sampling was authorized under conditions specified by environmental licences (Prior Licences no. 284 2008 and Operation no. 782 2008) issued by the Brazilian Environmental Agency (IBAMA).
The first survey was performed from 2 May 2008 to 15 July 2008 and the second from 31 January 2009 to 17 March 2009 using the ‘Gyre’ and ‘Miss Emma MacCall’ research vessels, respectively. The stations were named according to sub-regions: continental shelf and slope: Hab 3–11, A-I (Figure 1A); 13; 16; Canyons: Cang 6–9 (Grussaí Canyon) and Canac 6–9 (Almirante Câmara Canyon) (Figure 1B). At each station, three replicates of sediment were collected, with three strata (0–2, 2–5 or 5–10 cm) in each. The sediment was collected using van Veen (total sampling area: 92 × 80 × 40; sieved sample area: 0.04 m2) and Box corer (50 × 50 cm) grabs. Polychaetes and other benthic organisms were removed from the sediment, fixed in 4% formalin solution, preserved in 70% alcohol, and identified. The lacydoniids were found from 25 to 2500 m depths. Line drawings were made with a camera lucida attached to a light microscope; measurements of length and width were taken with an ocular micrometer and photos taken under a videocamera Moticam 2000 coupled to a trinocular microscope Nikon E200-T. In order to improve visualization of some morphological structures, specimens were stained with Shirlastain A. The material was deposited in the Polychaeta Collection of the Invertebrate Zoology Laboratory, Rio de Janeiro State University (UERJ), in the Fluminense Federal University (UFF) and the holotypes at the Museu Nacional do Rio de Janeiro (MNRJ/P).

Fig. 1. Map of the study area: A, continental shelf and slope. B, canyons.
RESULTS
Morphology
All characters used in the taxonomy of Lacydonia are reviewed based on both published literature and analysis of specimens from the species recorded in this study.
BODY
Body dorso-ventrally flattened, widest in the anterior chaetigers (pharynx region), and slightly tapered in the posterior end. The body length can be less than 2 mm and usually with short parapodial lobes (i.e. L. mikrops and L. brasiliensis sp. nov., Figure 3C), or longer with more than 4 mm with longer parapodial lobes (i.e. L. gordia, L. jacki sp. nov., Figure 5B, C and L. oculata). The number of chaetigers may be proportional to the body length; species with longer bodies have about 40–45 chaetigers as adults, while shorter species have about 20 chaetigers. Transverse ciliated bands, better seen with SEM, are present dorsally and ventrally throughout the body. Segments are anteriorly as a single annulus, while posterior ones are indistinctly bi-annulated.
PROSTOMIUM
The prostomium is rounded with three dorsal antennae and two ventral palps; eyes can be present or absent. Prostomium can be as wide as long (e.g. L. cirrata, Figure 4A), or 1.5–3.0 times wider than long (as seen in L. anapaulae sp. nov., Figure 2A, B; L. brasiliensis sp. nov., Figures 3A, B & 10E, F; and L. jacki sp. nov., Figures 5A & 11C, D). Some variation can be observed: among 31 specimens, including the holotype of L. quadrioculata, for example, the prostomium width was usually twice the length, but three specimens presented a prostomium just 1.5 times wider than long, and one having the prostomium as long as wide. The anterior edge of the prostomium may have one or two latero-frontal incisions (as seen in L. anapaulae sp. nov. and L. jacki sp. nov., Figures 2B & 6A, respectively).

Fig. 2. Lacydonia anapaulae sp. nov. (MNRJ P455). A, Anterior region, dorsal view, showing prostomium, antennae and anterior segments; chaetae omitted. B, Anterior region, ventral view showing pair of palps. C, Compound neurochaeta. D, Capillary notochaetae. E, Pygidium, ventral view.

Fig. 3. Lacydonia brasiliensis sp. nov. (MNRJ P456). A, Complete specimen, dorsal view showing pattern of pigmentation on very posterior end. B, Anterior end, dorsal view. C, Parapodium 8, chaetal tips omitted. D, Notochaeta. E, Neurochaeta.

Fig. 4. Lacydonia cirrata (UFF 06). A, Anterior region, dorsal view, showing shape of prostomium and anterior segments. B, Posterior region including pygidium, ventral view. C, Long and short spinigerous notochaetae. D, Detail of shaft on the right. E, Long capillary notochaeta. F, Short capillary notochaetae.

Fig. 5. Lacydonia jacki sp. nov. (MNRJ P457). A, Anterior region, dorsal view. B. Parapodium. C, Capillary notochaeta. D, Compound neurochaetae. (A–D, holotype MNRJ P457.)

Fig. 6. Lacydonia laureci (UFF 04). A, Anterior region, dorsal view, chaetae and median antenna omitted. B, Parapodium. C, Capillary notochaeta, detail of median region. D, Compound neurochaeta.
The prostomium can also have two lateral lobes on the posterior edge, as in L. laureci (Figure 6A). Eyes, when present, are large, black or brownish (occupying up to almost one third of prostomial length, as in L. miranda, Figure 7A, L. oculata, Figure 8A, B, and L. jacki sp. nov., Figure 5A), or are small, punctiform brownish (as in L. mikrops, L. quadrioculata and L. brasiliensis sp. nov., Figure 3A, B). When large, eyes are positioned laterally, sometimes at the same level as the median antenna, or slightly in front of or behind it (L. oculata, Figure 8A and L. miranda, Figure 7A, respectively). On the other hand, one or two pairs of punctiform eyes may be present on the lateral and central areas of the prostomium, sometimes with only one of the two pairs well-defined and the other present as scattered dark brown spots. Lacydonia mikrops has only one pair of lateral eyes, near the prostomial margin, while L. quadrioculata and L. brasiliensis sp. nov. (Figures 3B & 10E) have two pairs in a trapezoidal arrangement.

Fig. 7. Lacydonia miranda (UERJ 1140). A, Anterior region, dorsal view. B, Parapodium 4, cirri omitted. C, Parapodium 22. D, Capillary notochaeta. E, Compound neurochaeta. F, Pygidium.

Fig. 8. Lacydonia oculata. (UERJ 1459). A, Anterior end, dorsal view. B, Complete specimen, dorsal view. C, Pygidium. D, Capillary notochaeta. E, Compound neurochaeta.
A pair of antennae and a pair of palps, all short, are inserted latero-dorsally and latero-ventrally (lined up with the oral aperture, which is ventral) respectively, on the prostomium. Latero-dorsal antennae about twice the length of palps; antennae may be digitiform (Figures 4A, 7A & 9A), filiform (Figures 2A, 3A, B & 6A), papilliform (Figure 4A), fusiform, oval or conical (Figures 7A & 8B). The median antenna is difficult to see because it is very fragile and can be easily broken; however, if stained, an insertion scar is visible medially at the prostomium. The prostomium can be distinctly separated from the tentacular segment. A pair of nuchal organs forming a ciliated collar between the prostomium and the tentacular segment, can be seen latero-dorsally or ventrally in some species, as seen in L. hampsoni (Blake, Reference Blake, Blake, Hilbig and Scott1997, p. 183).

Fig. 9. Lacydonia cf. papillata (UFF 13). A, Anterior region, dorsal view, median antenna omitted. B, Parapodium (cirri lost). C, Capillary notochaeta. D, Compound neurochaeta.
PHARYNX APPARATUS
The pharynx is a muscular bulb, jaws may be present. The pharynx can be seen through the body wall; it is a muscular axial proboscis that is rarely seen everted (Rouse & Pleijel, Reference Rouse and Pleijel2001, Figure 26.2c). A terminal papillose ring may be present (Marion & Bobretzky, Reference Marion and Bobretzky1875, p. 60). The pharynx lies between a pair of large lateral glands, extends through chaetigers 1–6, and can be used as a diagnostic specific feature. The lateral glands can be roughly cylindrical or tapered anteriorly, with median and posterior regions of about the same diameter. A tangle of sclerotized channels is reported in the anterior part of the pharynx in some Lacydonia species, such as L. miranda and L. brasiliensis sp. nov. (Figure 11A). These channels are situated between the first and second chaetigers when the pharynx is introverted. They arise from each lateral gland, divide into two slightly thicker channels, and finish in a pair of small pectinate blade-shaped structures (Figure 7A, see also Marion & Bobretzky, Reference Marion and Bobretzky1875, Figure 17D, Plate 8). Brownish granulate patches associated with the posterior end of the lateral glands are present in L. miranda (see Marion & Bobretzky, Reference Marion and Bobretzky1875, Figure 17 and 17D, Plate 8). The nature and function of the small pectinate blade-shaped structures and lateral glands are still unknown; they may aid food capture and digestion. The jaw apparatus in Lacydonia shares some similarities with the Lopadorhynchidae Claparède, Reference Claparède1868 (Marion & Bobretzky, Reference Marion and Bobretzky1875). The gut is a straight tube that ends in a termino-ventral anus.
TENTACULAR SEGMENT
The first segment is achaetous and herein defined as the tentacular segment. This segment is shorter than the following chaetigers in all known species. It has one pair of latero-ventral conical, digitiform or papilliform tentacular cirri (Figures 2A, B, 3A, B, 4A, 6A, 7A, 8A & 9A).
PARAPODIA
Three pairs of anterior uniramous parapodia (segments 2−4) have neuropodial lobes and dorsal and ventral cirri, globular to conical; dorsal cirrus inserted near to parapodial base, and ventral cirrus near the middle of the parapodial lobe. Parapodia of remaining segments are biramous with notopodial and neuropodial rami separated; notopodia are shorter than neuropodia. In species having a body length of less than 2 mm, parapodial lobes are usually truncate or blunt, short, and sometimes, distally bilobed (e.g. L. jacki sp. nov., Figure 5B), while in those with long bodies, lobes are elongated (e.g. L. cf. papillata, Figure 8B). Conical and thickened notopodial lobes usually have two lamellae, prechaetal and post-chaetal, both of the same length. Neuropodial lobes are usually 1.5 times longer than notopodial ones and may be conical, narrow, or sometimes distally pointed (e.g. L. oculata, Figure 8A, B). Dorsal and ventral cirri are short, inserted near to the middle of the noto- and neuropodial lobe, respectively; it might be easily detached in some species (as in L. jacki sp. nov. for example, Figure 5B) or non-deciduous. Dorsal cirri have been described as oval, globular, conical (Figures 3C, 5B & 7C), ovoid or acute. Ventral cirri may present similar length to the dorsal ones or may be slightly shorter; they can be slender, elongated, ovoid or tapered. Noto- and neuropodial lobes are supported by a straight to slightly curved acicula, not projecting beyond the corresponding lobes.
CHAETAE
Lacydoniids present only two main types of chaetae: notochaetae are capillaries (Figure 7D) and neurochaetae are compound spinigers (Figure 7E). The capillaries may be smooth (as in L. oculata, Figure 8D) or serrated; in the latter, they can be thicker basally, tapered distally with a coarsely serrated margin (as L. jacki sp. nov., Figure 5C, and L. miranda, Figure 7D); however, in some species, they are distally enlarged and finely serrated (L. cirrata, Figure 4F). Capillaries can be diagonally striated (when viewed under oil immersion), as in L. cirrata (Figure 4F). The compound spinigers may have blades of slightly different lengths. Shafts have one large apical tooth on one side and one or two smaller additional teeth on the other side, at a ratio of 2:1 (i.e. L. cirrata, Figure 4D). It is possible that the presence of the two small teeth has been overlooked in most species and future SEM imaging is recommended. Blades are finely serrated.
PYGIDIUM
The pygidium of lacydoniids has a terminal anus with a termino-lateral pair of cirri, and usually one mid-ventral papilla or cirrus located between them. Specimens of L. miranda were described and illustrated having one or two ventral papillae (Marion & Bobretzky, Reference Marion and Bobretzky1875). The termino-lateral cirri are usually filiform and long (reaching the last 3 chaetigers; i.e. L. laureci), or can be oval to globular (not extending beyond the last chaetiger; i.e. L. miranda, Figure 7F and L. oculata, Figure 8C). The mid-ventral papilla can be as long as the termino-lateral pair of cirri and, sometimes, more similar to a cirrus than to a papilla (i.e. L. laureci, L. papillata). Lacydonia miranda and L. oculata have a typical papilla; L. mikrops have the termino-lateral cirri short and a medio-ventral one resembling a papilla. Lacydonia quadrioculata has a typical mid-ventral papilla and SEM images show sensorial cilia along the distal part, and paired cirri lacking such structure (Magalhães et al., Reference Magalhães, Bailey–Brock and Rizzo2012, Figure 3C). Some species can have two pigmented spots, one on each cirrus, sub-distally or termino-laterally on the pair of cirri (L. anapaulae, Figure 2E), in addition to a pair of sub-dermal pigmented spots on the pygidium (i.e. L. brasiliensis sp. nov., Figure 3A, and L. cirrata, Figure 4B).
PIGMENTATION
Preserved specimens are usually shades of white, yellow or green, however, some species are brownish (Figure 11C). Red-brown pigment spots are usually present, mainly on the prostomium, first chaetiger, parapodial base (usually one or two spots, Figure 11B) and dorso-laterally (Figure 10B), dorsal cirri and pygidium. Lateral pygidial cirri can have one pair of red-brownish pigment spots distally as mentioned above. Sometimes, glands are arranged as two or three longitudinal series, in dorso-lateral and central position (as seen in L. cirrata, Figure 10A and L. brasiliensis, Figure 10F). Lacydonia papillata seems to be the only species with four large circular dark spots of unknown function on the dorsal side of the second chaetiger.

Fig. 10. Species of Lacydonia in dorsal view. A., L. cirrata. B, Lacydonia sp. (Tree of life; http://tolweb.org/images/Phyllodocida/2508). C, L. mikrops (type material). D, L. miranda. E–F, L. brasiliensis sp. nov. (B–C: no scale).
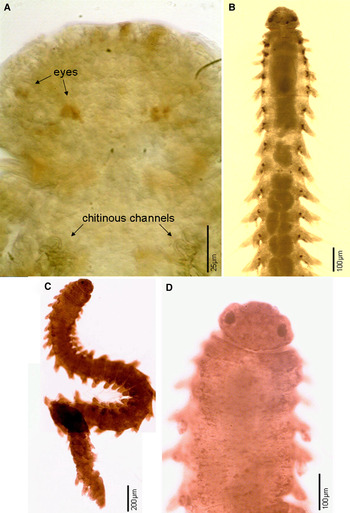
Fig. 11. Species of Lacydonia in dorsal view. A, Prostomium and tentacular segment of L. brasiliensis sp. nov. B, L. oculata. C–D, L. jacki sp. nov.
REMARKS
Lacydonids are rare organisms usually found in low numbers (<5 individuals) (Hartman & Fauchald, Reference Hartman and Fauchald1971; Sardá, Reference Sardá1982; Gathof, Reference Gathof, Uebelacker and Johnson1984; Hartmann-Schröder & Rosenfeldt, Reference Hartmann-Schröder and Rosenfeldt1992; Pleijel & Fauchald, Reference Pleijel and Fauchald1993). Therefore, in this family, it is common to find a species description based upon a single specimen, or holotype. This is the case of L. oculata (Hartman, Reference Hartman1967) initially described as belonging to the genus Scalispinigera within the family Scalibregmatidae, and likewise for L. laureci, L. elongata, L. antarctica (= L. oculata), and one of the three new species described in this study, L. anapaulae sp. nov. Thus, it is remarkable that from 10 valid Lacydonia species, five have been found in the Campos Basin in addition to three undescribed species. The currently valid species, not found in this region were: L. mikrops from Antarctic waters in 380 m depth, L. hampsoni Blake Reference Blake, Blake and Hilbig1994 described from Californian continental slope in 985–1990 m depth, L. gordia Hartmann-Schröder, Reference Hartmann-Schröder1993 from Antarctic in 164–177 m depth, and L. quadrioculata Magalhães, Bailey-Brock & Rizzo, Reference Magalhães, Bailey–Brock and Rizzo2012 from Hawaii in 27–65 m depth.
Most species in this family are found at depths between 200 and 5500 m, whereas L. miranda, L. antarctica, L. mikrops, L. oculata, L. gordia, L. quadrioculata and including L. jacki sp. nov., are found in shallow waters (<200 m). The same pattern of bathymetric distribution was found in the Campos Basin for L. miranda and L. oculata recorded in the continental shelf, while all others were collected on the continental slope and submarine canyons (Table 1). The record of this family in the South Atlantic Ocean (Campos Basin, Brazil) expands its distributional range and adds to the diversity of lacydoniids in this region.
Table 1. Bathymetric distribution of Lacydonia species from the Campos Basin.

The main morphological characters used to separate Lacydonia species have been the presence of eyes and its relative size, the proportions of the prostomium (width:length), and length of pygidial cirri. Although the proportion of the prostomium had been widely used some species may present variation. Therefore this characteristic should be used carefully together with the other characteristics. Beyond that, additional features were observed and are proposed herein as diagnostic. However, although sometimes difficult for preserved specimens, the pigmentation pattern can be a useful feature to distinguish Lacydonia species. For instance, there are species such as L. miranda that are colourless, and others with defined pigment patterns; for example, basally and dorso-laterally on parapodial lobes, on the prostomium, or pygidium. The presence of gland masses has been observed in most species and this could be an additional character.
A pair of lateral glands and a striated pharynx between these glands as part of the pharyngeal apparatus have been observed for some species and possibly overlooked in others. In addition, L. miranda and L. brasiliensis sp. nov. have chitinous channels with a distal, small pectinate structure associated with the lateral glands usually in the first chaetiger. We cannot confirm that these channels and pectinate structures are present in other species because they are difficult to see with light microscopy. The internal anatomy of the anterior portion of the digestive tract was well-described by Marion & Bobretzky (Reference Marion and Bobretzky1875) for L. miranda. Since then, no other study has addressed the structure of the digestive tract or assessed relevance to understanding phylogenetic relationships. This omission can be explained by the scarcity of specimens that discourages dissection and histological studies.
The length of parapodia might be related to species size; thus, species with long bodies apparently have longer parapodia, while species with short bodies tend to have shorter parapodia. We have also noticed that the dorsal and ventral cirri are apparently different among species, not only in shape or size. Cirri in lacydoniids seem to be of two types, those similar to phyllodocids, and defined here as deciduous, and those that seem to be an extension of the parapodial lobes and cannot be easily lost (not deciduous). Species with large body size seem to have cirri from the second type such as L. gordia and L. papillata. Similarly, the chaetae of lacydoniids have received little attention as useful characters for distinguishing species. The capillary notochaetae can be marginally smooth or serrated; when serrated, they can be finely or coarsely serrated along the entire margin, or only along the distal region. The same seems to occur with the neurochaetae, which have two unequal teeth on the shaft's distal region. The proportion of these teeth might be used as a criterion to distinguish closely related species.
The species could be separated into two groups or genera by the types of chaetal surface; i.e. finely vs coarsely spinulose. If notochaetae could be used to separate species groups, then those with finely spinulose capillaries are L. anapaulae sp. nov., L. brasiliensis sp. nov., L. cirrata, L. oculata and L. papillata, and with coarsely spinulose or denticulate notochaetae are L. jacki sp. nov., L. laureci and L. miranda. If neurochaetae are to be used, then those with finely spinulose neurochaetal blades are L. brasiliensis sp. nov., L. miranda, L. papillata and with coarsely spinulose blades L. cirrata, L. jacki, L. laureci and L. oculata. An evaluation of type material for all described species and an evaluation of other phylogenetically informative characters must be done for this potential taxonomic change.
In conclusion, many taxonomically informative characters have been overlooked in previous studies. Exploring the potential use of these characters could significantly contribute to the assessment of the phylogenetic relationships within Lacydonia based upon morphological characters.
SYSTEMATICS
Order phyllodocida Dales, Reference Dales1962
Family lacydoniidae Bergström, Reference Bergström1914
Genus Lacydonia Marion and Bobretsky in Marion, Reference Marion1874
Scalispinigera Hartman, Reference Hartman1967:134
TYPE-SPECIES. Lacydonia miranda Marion and Bobretsky in Marion, Reference Marion1874, by monotypy.
DIAGNOSIS (after Magalhães et al., Reference Magalhães, Bailey–Brock and Rizzo2012)
Prostomium rounded, palps ventral, one median and two lateral antennae, all short; eyes present or absent; nuchal organs sometimes present. Tentacular segment (=achaetous segment 1) reduced, bearing a single pair of short cirri. First three segments uniramous, all subsequent biramous. Parapodia well-developed, with dorsal and ventral cirri. Notochaetae capillaries; neurochaetae compound spinigers. Pygidium with two lateral cirri and one median, ventral papilla or cirrus.
KEY TO SPECIES OF LACYDONIA
-
1. Eyes present (Figures 2A, B, 4A, 6A & 7A, B)2
-
— Eyes absent (Figures 1A, 3A, 5A & 8A)7
-
-
2(1) Eyes small, punctiform (Figure 2A, B)3
-
— Eyes large (Figures 4A, 6A & 7A, B)5
-
-
3(2) One pair of eyes, laterally positioned; prostomium as wide as long L. mikrops
-
— Two pairs of eyes, in trapezoidal arrangement; prostomium variable4
-
-
4(3) Prostomium usually as wide as long; antennae and pygidial cirri short; notochaetae smoothL. quadrioculata
-
— Prostomium usually twice wider than long; antennae and pygidial cirri long; notochaetae finely serrated L. brasiliensis sp. nov.
-
-
5(2) Prostomium usually as wide as long; body without pigmented spotsL. miranda
-
— Prostomium usually 1.5–2.0 times wider than long; body with pigmented spots6
-
-
6. Prostomial anterior margin without incisions; notochaetae finely serratedL. oculata
-
— Prostomial anterior margin with two lateral incisions; notochaetae coarsely serratedL. jacki sp. nov.
-
-
7(1) Prostomium usually as wide as long8
-
— Prostomium usually twice wider than long11
-
-
8(7) Prostomial posterior edge with two lateral lobes L. laureci
-
— Prostomial posterior edge without lobes9
-
-
9(8) Prostomium not widely separated from the tentacular segment; parapodia short .L. cirrata
-
— Prostomium widely separated from the tentacular segment by ciliated nuchal organ; parapodia elongated 10
-
-
10(9) Prostomium 1.5 times wider than long, with two dorso-lateral depressions; second chaetiger with four large dorsal dark spots; dorsal and ventral cirri rounded, rudimentary, not deciduousL. papillata
-
— Prostomium about twice wider than long, without dorso-lateral depressions; second chaetiger without dorsal dark spots; dorsal and ventral cirri globular to conical, deciduous?L. elongata
-
-
11(7) Prostomium with a slit on the median anterior edge; without lobes in the posterior edge L. anapaulae sp. nov.
-
— Prostomium without slit on the median anterior edge, lobes in the posterior edge12
-
-
12(11) Prostomium 1.5 times wider than long; antennae digitateL. hampsoni
-
— Prostomium 3 times wider than long; antennae oval L. gordia
-
Lacydonia anapaulae sp. nov.
(Figure 2A–E)
TYPE MATERIAL
Holotype: MNRJ/P455 (complete, female), 23.i.2009, Station HAB8E9R1, 1272 m, 22°39′7″S, 40°5′53″W, Box corer.
DIAGNOSIS
Prostomium with anterior slit, twice wider than long; antennae and palps filiform; eyes absent; small rounded glandular agglomerates near base of noto- and neuropodia, present throughout; dorsal and ventral cirri conical; lateral pair of filiform pigidial cirri distinctly longer than median one. Pigidial cirri with distal pigmentation.
DESCRIPTION
Holotype 2 mm in length for 22 chaetigers; dorso-ventrally slightly flattened, anteriorly stout. Uniannulate anterior segments. Preserved specimen yellowish, sometimes with scattered small red-brown pigmented spots. Small rounded glandular agglomerates near parapodial bases. Prostomium with anterior slit, slightly pigmented, twice wider than long (Figure 2A, B); three antennae and two palps, all similar in shape, slender, filiform; lateral antennae longer than palps; median antennae as long as lateral ones. Prostomium separated from peristomium. Eyes absent. Pharynx not everted, not observed through body wall. Pigment patches internal, probably inside lateral glands (Figure 2A). Tentacular segment, slightly shorter than following segments; with one pair of conical cirri, inserted latero-ventrally. Anterior three pairs of parapodia uniramous with conical neuropodial lobes; short dorsal and ventral cirri; both inserted near parapodial bases. Subsequent parapodia biramous; notopodial lobes slightly shorter than neuropodial lobes. All parapodial lobes with one straight aciculum, and numerous chaetae in both rami. Notopodial capillaries (Figure 2D) finely serrated distally. Neuropodial spinigers with blades of slightly different lengths and fine serration along the entire length (Figure 2C). Pygidium with anus and a pair of termino-lateral slender, filiform cirri and a distinctly shorter mid-ventral papilla. Lateral pygidial cirri with distal pigmentation (Figure 2E).
REMARKS
Lacydonia anapaulae sp. nov. is the only species known to present a slit on the median anterior region of the prostomium. When compared with known species without eyes and with wide prostomia, such as L. gordia, L. anapaulae differs by the prostomial slit and the glandular agglomerates near the base of the noto- and neuropodia. Among other species without eyes, L. cirrata shows a prostomium only slightly wider than long and shaft of compound neurochaetae with a distally bidentate tip, both characters not present in L. anapaulae sp. nov. Even though only a single specimen was collected, we believe that the differences described are significant to support the description of a new taxon.
ETYMOLOGY
This species is named to honour Ana Paula Falcão, the biologist and environmental manager in charge of the Habitats/Petrobras project, for her respectful and supportive attitude concerning taxonomy and taxonomists.
DISTRIBUTION
Only known from Campos Basin, Brazil, in 1272 m depth.
Lacydonia brasiliensis sp. nov.
(Figures 3A–E, 10E–F & 11A)
TYPE MATERIAL
Holotype: MNRJ/P456 (complete) 26.vi.2008, Station Hab6 Canac-9 R1, 1379 m, 21o43′40″S 39o55′23″W, Box corer.
Paratype: UFF 09 (complete), 08.ii.09, Station Hab9G7R1, 680 m, 22°7′35″S 39°54′21″W, Box corer.
DIAGNOSIS
Prostomium twice wider than long. Eyes punctiform, two pairs. Prostomium distinctly separated from tentacular segment. Pharynx reaching chaetigers 3–4. Dorsal and ventral cirri globular to conical. Capillaries slightly enlarged, finely serrated distally. Spinigers with unequal blades and with finely serrated edges.
DESCRIPTION
Holotype 1.4 mm long, 17 chaetigers (paratype: 2 mm long, 22 chaetigers). Body slightly dorso-ventrally flattened; anteriorly more robust, tapered posteriorly (Figure 3A). Preserved specimens white to yellow in colour, pale dorsal pigmentation with reddish-brown punctiform pigments mainly on anterior region of prostomium, parapodial lobes, pre-anal segments and pygidium (Figure 10E). Gland masses arranged in two dorso-lateral longitudinal series (Figure 10F). Uni-annulated anterior segments (1–3) with the following ones being indistinctly bi-annulated. Prostomium rounded anteriorly, twice wider than long with three antennae and two palps (Figure 3B). Two small pairs of eyes in trapezoidal arrangement; innermost pair reniform, lateral pair circular; one pair of antennae and one pair of palps latero-dorsally and latero-ventrally inserted, respectively; latero-frontal antennae twice longer than palps (or half as long as prostomium); median antenna lost, insertion scar present on posterior half of prostomium. Prostomium distinctly separated from tentacular segment. Nuchal organs present, forming ciliated collar between prostomium and tentacular segment, and laterally on prostomium near eyes. Pharynx retracted, anterior region of digestive tract strongly muscular located between paired lateral glands and viewed by transparency reaching chaetigers 3–4; sclerotized channels present on anterior part (Figures 3B & 11A). Tentacular segment, slightly shorter than following segments, with a pair of conical latero-ventrally inserted cirri. Anterior three pairs of parapodia uniramous with neuropodial lobes and dorsal and ventral globular to conical cirri; dorsal cirrus inserted near parapodial base and ventral cirrus near middle of parapodial lobe. Following parapodia biramous with notopodial and neuropodial rami separated; notopodial lobe triangular with tapering posterior half (Figure 3C); inferior region of the notopodial prechaetal lobe slightly elongated. Notopodial prechaetal and postchaetal lamellae of same length. Dorsal and ventral cirri conical; non-deciduous, both inserted near middle of noto- and neuropodial lobes, respectively (Figure 3C). Noto- and neuropodial lobes with straight acicula. Numerous capillary notochaetae, each slightly enlarged and finely serrated distally (Figure 3D). Unequal spinigerous compound neurochaetae (Figure 3E), with blades of slightly different lengths; blades apparently finely serrated along margin, difficult to observe under low magnification. Pygidium with a pair of digitiform lateral cirri (Figure 3A) as long as last two chaetigers and a ventral median papilla.
ETYMOLOGY
This species was named based on its occurrence in Brazilian waters.
REMARKS
Lacydonia brasiliensis sp. nov. belongs to a group of species with small eyes, together with L. mikrops and L. quadrioculata; however, L. mikrops has only one pair of eyes situated in the lateral prostomial margins, while the other two species have two pairs in a trapezoidal arrangement. In L. quadrioculata, the two pairs of eyes are similar to each other, i.e. circular, while in L. brasiliensis sp. nov., the innermost pair is reniform and the lateral pair is circular, sometimes dimmed dorsally and being best observed in a ventral view. Another difference from L. mikrops is that its prostomium is almost as wide as long and indistinctly separated from the tentacular segment, while in L. brasiliensis sp. nov. and L. quadrioculata, it is about twice wider than long and distinctly separated from the tentacular segment. Lacydonia mikrops seems to lack pigmentation, at least it was not mentioned in the original description, except for a pair of pigmented spots in the pygidium; such pigmentation could not be observed on the type material (Figure 10C). Lacydonia brasiliensis sp. nov. and L. quadrioculata present some pigmentation in the prostomium, dorsum, parapodia and cirri. Finally, L. brasiliensis sp. nov. differs from L. quadrioculata by having lateral antennae that are twice longer than palps. In addition, in L. brasiliensis sp. nov., the lateral pair of pygidial cirri is as long as two chaetigers, while the median papilla is shorter, not surpassing the last chaetiger; whereas the pygidial cirri is as long as the ventral papilla in L. quadrioculata. Regarding the presence of dorsal gland masses, L. brasiliensis sp. nov. has two longitudinal series in dorso-lateral position, while L. cirrata has three, a dorso-lateral pair and another in the median position that is present throughout (Figure 10A).
DISTRIBUTION
Only known from Campos Basin, Brazil in 680–1379 m depth.
Lacydonia cirrata (Hartman & Fauchald, Reference Hartman and Fauchald1971)
(Figures 4A–F & 10A)
Scalispinigera cirrata Hartman & Fauchald, Reference Hartman and Fauchald1971: 128, pl. 20, Figure 20 b–d.
Lacydonia cirrata: Blake, Reference Blake, Blake, Hilbig and Scott1997: 181.
MATERIAL EXAMINED
Five specimens: UFF 06 (1, incomplete; mature female with oocytes in coelom), 14.v.08, Station Hab3I8R1, 984 m, 21°10′59″S 40°9′16″W, Box corer; UFF 07 (1, incomplete), 11.ii.09, Station HAB9G9R2, 1296 m, 22°7′4″S 39°49′3″ W, Box corer; UFF 10 (1, complete), 27.vi.08, Station Hab6Canac9R2, 1335 m, 21°43′40″S 39°55′,23″W, Box corer; UFF 11 (1, complete), 06.ii.09, Station Hab9H8R1, 1005 m, 21°40′12″S 39°58′12″W, Box corer; UFF 12 (1, complete), 06.ix.09, Station Hab9Canac8R3, 1030 m, 21°45′50″S 39°59′33″W, Box corer.
TYPE-LOCALITY
Atlantic Ocean – Western North Atlantic, off New England, USA.
DIAGNOSIS
Prostomium as wide as long, slightly rounded. Eyes absent. Prostomium and peristomium indistinctly separated. Pharynx reaching chaetiger 4. Protruding notopodial aciculae. Capillary notochaetae with sub-distal expansion and marginally serrated. Spinigerous neurochaetae with unequal articulation and bidentate distal tip.
DESCRIPTION
Complete specimen 2.8 mm long, 29 chaetigers (UFF 12). Body slightly dorso-ventrally flattened, anteriorly wider (pharynx region) (Figure 4A). Preserved specimens white to yellow in colour, pale dorsal pigmentation with reddish-brown dots on prostomium and sub-distally on dorsal and ventral cirri. Gland masses in three longitudinal rows dorso-laterally and centrally disposed (Figure 10A). Anterior segments uni-annulated. Prostomium rounded, slightly wider than long; a pair of latero-dorsal antennae, and a pair of latero-ventral palps present. Antennae and palps short, papilliform, similar in shape and size; median antenna not observed, probably lost. Eyes absent. Nuchal organs laterally on prostomium near lateral antennae as ciliated collar on dorso-posterior side of prostomium. Prostomium indistinctly separated from tentacular segment (Figure 4A). Pharynx not everted; anterior region of digestive tract strongly muscular located between a pair of lateral glands (seen by transparency), reaching chaetigers 3–4 (Figure 4A). Tentacular segment slightly shorter than following segments, with a pair of conical cirri, latero-ventrally inserted. Anterior three pairs of parapodia uniramous with neuropodial lobes and dorsal and ventral cirri, globular to conical; dorsal cirri inserted near parapodial base, ventral cirri near middle parapodial lobe. Following parapodia biramous, with noto- and neuropodia rami separated; triangular notopodial lobe half as long as neuropodial lobe, tapering distally. Notopodial pre- and postchaetal lamellae of same length, as long as neuropodial lamellae. Dorsal and ventral cirri globular with a small papilla; both inserted basally of noto- and neuropodium, respectively. Noto- and neuropodial lobes with straight acicula, distally slender, projecting beyond notopodial lobe. Capillaries of two types: serrated sub-distally with expansion numbering about five (Figure 4F), and elongated and apparently smooth, numbering 1–3 (Figure 4E). Spinigers numbering less than 10 per fascicle, with blades of different lengths (Figure 4C); with distally bidentate tips (Figure 4D) and blade with finely serrated margin. Pygidium with short dorsal lateral cirri, distally pigmented, and a mid-ventral papilla (Figure 4B).
REMARKS
This species was formally described by Hartman & Fauchald (Reference Hartman and Fauchald1971) as the type species of Scalispinigera within Scalibregmatidae. These authors described and illustrated dorsal and ventral papillae in the tentacular segment, which might have been a mistake because all described lacydoniids present only a pair of latero-ventrally cirri inserted on the tentacular segment. They also described palps as ventro-lateral antennae, and did not mention the latero-dorsal pair. Some differences were observed when compared with the original description such as: (a) a sub-distal enlargement in the capillary notochaetae that are short and marginally serrated, (b) presence of long, smooth capillaries; (c) presence of spinigers with different blade lengths; and (d) bidentate articulate tip on spinigers. The gland masses have never been reported in members of this family; this species, in particular, present three longitudinal rows, two of them dorso-lateral, and one mid-dorsal.
DISTRIBUTION
Atlantic Ocean – Western North Atlantic, in deep slope depths (2022 m depth). This species had only been previously known from the type locality off New England (USA), and now it is newly recorded for the southern Atlantic in the Campos Basin, Brazil in 700–1335 m.
Lacydonia jacki sp. nov.
(Figures 5A−D & 11C, D)
TYPE MATERIAL
Holotype: MNRJ/P457 (complete) 08.vii.2009, Station Hab16 H03 R2, 72 m, 21o43′5″S 40o11′37″W, van Veen.
Paratype: UERJ 1822 (1, complete) 04.vii.2009, Station Hab16 E04 R3, 103 m, 22o17′37″S 40o27′05 W, van Veen.
DIAGNOSIS
Prostomium wider than long with 2 latero-frontal incisions. A pair of large eyes present. Capillary notochaetae basally smooth, distally serrated; spinigerous neurochaetae shaft with a wide apical tooth and two small teeth.
DESCRIPTION
Holotype slightly dorso-ventrally flattened, anteriorly more robust, tapered posteriorly; 4.1 mm long, 39 chaetigers. Holotype and paratype brownish in colour with pigmented spots on prostomium and dorsal region including parapodia and dorsal cirri (Figure 11C). Uni-annulated anterior segments, followed by segments indistinctly bi-annulated. Prostomium twice wider than long with two latero-frontal incisions (Figures 5A & 11D). Two latero-dorsal antennae and two latero-ventral palps; median antenna or insertion scar not seen, probably lost. Antennae and digitiform, same-sized. Eyes large, black, one pair, near prostomial margins; diameter of each about 1/5 prostomial width (Figures 5A & 11D). Nuchal organs forming a ciliated deep pit, lateral, ventral between prostomium and first achaetous segment. Pharynx not everted; striated and with a pair of lateral glands reaching chaetiger 5, seen by transparency.
Tentacular segment shorter than following segments; a pair of digitiform cirri latero-ventrally inserted. Anterior three pairs of parapodia uniramous with neuropodial lobes and dorsal and ventral cirri, globular to conical; dorsal cirri inserted near parapodial base and ventral cirri near middle of parapodial lobe; anterior parapodia shorter than posterior ones. Subsequent parapodia biramous with noto- and neuropodial lobes, both widely separated and with notopodial lobes slightly shorter than neuropodial ones (Figure 5B). Dorsal and ventral cirri deciduous, oval to conical with pointed tips; dorsal cirri slightly larger than ventral ones, both deciduous and inserted between middle and distal point in noto- and neuropodial lobes, respectively (Figure 5B). All parapodial lobes with straight acicula. Capillaries numbering about 10, smooth basally, coarsely serrated distally (Figure 5C). Spinigers with slightly different blade lengths and marginally coarsely serrated; shaft with one wider apical tooth, another tooth smaller (proportion 1:4; Figure 5D). Pygidium with two dorso-lateral digitiform cirri, a distal reddish spot, and a mid-ventral papilla, slender and as long as, or slightly shorter than dorso-lateral cirri.
REMARKS
Lacydonia jacki sp. nov. is the third described large-eyed Lacydonia species; the other two are L. miranda and L. oculata (Blake, Reference Blake, Blake, Hilbig and Scott1997). Lacydonia jacki sp. nov. differs from L. miranda because its prostomium is wider than long, and from L. oculata for having the prostomium laterally incised, with papiliform and short antennae and eyes comprising one fifth of the prostomial width. In L. miranda, the eyes cover approximately one third of the prostomial width, are darker and placed on the posterior half of prostomium. In L. oculata, the eyes are slightly smaller, covering about one quarter of the prostomial width, brownish, and placed on anterior half of prostomium. In addition, the presence of an incised prostomium is a unique feature of this new species.
ETYMOLOGY
The name of this species is in honour of Jack, a male Siberian husky dog that belonged to the first author (AER).
DISTRIBUTION
Only known from Campos Basin, Brazil, in shallow waters (72–103 m).
Lacydonia laureci Laubier, Reference Laubier1975
(Figure 6A−D)
Lacydonia laureci Laubier, Reference Laubier1975: 76, Figure A–E; Böggemann (Reference Böggemann2009): 333–334, Figures 65, 66, 69.
MATERIAL EXAMINED
Three specimens: UFF 03 (1, incomplete), 27.vi.2008, Station Hab6 CANAC 9R3, 1395 m, 21°43′40″S 39°55′23″W, Box corer; UFF 04 (1, incomplete), 12.ii.2009, Hab10 H10R1, 1900 m, 21°37′10″S 39°35′51″W, Box corer; UFF 05 (1, incomplete), 16.i.2009, Station Hab8 B8R1, 1001 m, 23°13′44″S 40°55′59″W, Box corer.
TYPE-LOCALITY
Matapan trench, eastern Mediterranean.
DIAGNOSIS
Prostomium with two conspicuous lateral lobes on posterior margin; filiform antennae and palps; eyes absent; dorsal and ventral conical cirri; lateral pair of filiform pigidial cirri distinctly longer than median papilla.
DESCRIPTION
Specimens with up to 3.5 mm long, 26 chaetigers; dorso-ventrally slightly flattened, anteriorly stout. Uni-annulated anterior segments followed by indistinctly biannulate ones. Preserved specimens yellow in colour, sometimes with diffusely distributed small reddish-brown pigmented spots, especially on prostomium and first segments. Prostomium rectangular, twice wider than long, with two conspicuous lateral lobes on posterior margin (Figure 6A). Three antennae, and two ventral palps of similar shape, all slender and filiform; frontal antennae slightly longer than palps, inserted latero-dorsally; palps latero-ventrally inserted; median antenna about as long as latero-frontal ones, inserted near posterior margin of prostomium. Eyes absent. Prostomium distinctly separated from tentacular segment by presence of nuchal organs, forming laterally ciliated slits. Pharynx retracted in all specimens. Tentacular segment slightly shorter than following segments; a pair of conical cirri present, inserted latero-ventrally. Anterior three pairs of parapodia uniramous 1–3 uniramous with neuropodial lobes and dorsal and ventral cirri short, conical; both inserted near parapodial bases. Subsequent parapodia birramous, with notopodia slightly shorter than neuropodia, both robust; neuropodial lobes distally pointed. Dorsal and ventral cirri same-sized, rounded; non-deciduous, both inserted near base of noto- or neuropodial lobes on anterior region; ventral cirri shifting to a median position on posterior neuropodial lobes (Figure 6B). Parapodial lobes with one straight or slightly curved aciculum and numerous notopodial capillaries and neuropodial spinigerous compound chaetae.
Notopodial capillaries internally with diagonally striated structures, under immersion, and with coarsely serrated distal edges (Figure 6C). Neuropodial spinigers with blades of slightly different lengths; sub-distally blades with coarsely serrated margins over entire length (Figure 6D); shafts with one large apical tooth and apparently an additional one on both sides. Posterior end features not observed.
DISTRIBUTION
Mediterranean Sea, Matapan trench (Laubier, Reference Laubier1975) (4690 m depth); Atlantic Ocean: Angola, Cape and Guinea Basin (Böggemann, Reference Böggemann2009; 5052–5497 m depth). Lacydonia laureci was found between 1001 and 1900 m depth in the Campos Basin, Southern Brazil.
REMARKS
Lacydonia laureci is the only known species with two lateral lobes in the posterior margin of the prostomium. Even though Böggemann (Reference Böggemann2009) stated that this is only well-developed and easily seen in larger specimens, these lateral lobes were clearly seen in all specimens collected from the Campos Basin. However, the pigmentation patterns on the prostomium and along the body were not very evident in small specimens as seen in the larger ones. In addition, specimens from the Campos Basin differed from the material illustrated by Böggemann (Reference Böggemann2009) in having the dorsal and ventral cirri more robust and rounded. Böggemann (Reference Böggemann2009) described the margin of the notopodial capillaries as presenting fine serrations along the entire length, however, observing his figures, the capillaries can be described as having coarse serrations present only distally, similar to what we observed. It was not possible to observe the pygidium and pygidial cirri/papilla because the collected specimens were incomplete; however, Laubier (Reference Laubier1975) describes a pygidium with a pair of termino-lateral slender and filiform cirri and a distinctly shorter medio-ventral papilla, differing from those specimens identified by Böggemann (Reference Böggemann2009: 334), which had a medio-ventral cirrus, not papillae, in addition to the dorso-lateral cirri.
Lacydonia miranda Marion & Bobretzky in Marion, Reference Marion1874
(Figures 7A−E & 10D)
Lacydonia miranda Marion & Bobretzky in Marion, Reference Marion1874; Fauvel, Reference Fauvel1923: 198, Figure 74A−D; ?Gathof, Reference Gathof, Uebelacker and Johnson1984: 34−3; Uschakov, Reference Uschakov1972: 227; Sardá, Reference Sardá1982: 25, Figure 1.
MATERIAL EXAMINED
Four specimens: UERJ 1140 (1, complete) 21.ii.2009, Station Hab11 B04 R3, 105 m, 23o09′59″S 41o03′12″W, van Veen; UERJ 1141 (1, complete) 21.ii.2009, Station Hab11 B04 R1, 107 m, 23o10′00″S 41o03′12″W, van Veen; UERJ 1143 (1, complete) 05.iii.2009, Station Hab13 I02 R3, 53 m, 21°22′54″S 40°19′47″W, van Veen; UERJ 1460 (1, complete) 03.vii.2009, Station Hab11 D03 R2, 90 m, 22o51′57″S 40o57′34″W, van Veen.
TYPE-LOCALITY
Gulf of Marseille (France, Mediterranean Sea).
DIAGNOSIS
Prostomium approximately wider than long. A pair of large eyes present; three antennae and two palps of same size, short, and conical to digitiform. Notochaetae capillary of two types, long and short; long ones marginally smooth to finely serrated and short ones coarsely serrated; neurochaetae compound spinigerous, coarsely serrated. Pygidium with a pair of termino-lateral cirri and a medio-ventral short papilla.
DESCRIPTION
Body slightly flattened dorso-ventrally, more robust on first 5–6 segments, tapered posteriorly (Figures 7A & 10D). Complete specimens up to 2.8 mm long, 28 chaetigers. Body whitish to yellowish, dorsal pigmentation when present, red-brownish spots diffusely distributed on prostomium, anterior parapodial lobes, dorsal cirri and pygidium. Uni-annulated anterior segments, followed by indistinctly bi-annulated segments. Prostomium anteriorly rounded, approximately wider than long (Figures 7A & 10D); two antennae and two palps of same size, short, conical, inserted latero-dorsally and latero-ventrally, respectively; median antenna not seen. Eyes black, large, one pair (each about 1/3 prostomial width; Figure 7A). Nuchal organs forming a ciliated collar between prostomium and tentacular segment. Pharynx retracted, situated between a pair of large lateral glands viewed by transparency, reaching chaetiger 6. Lateral glands with tangle of chitinous channels situated between chaetigers 1–2, and a distal pectinate structure. Tentacular segment slightly shorter than following segments, with a pair of conical cirri latero-ventrally inserted. Anterior three pairs of parapodia uniramous with neuropodial lobes and dorsal and ventral cirri, globular to conical; dorsal cirri inserted near parapodial base and ventral cirri near middle of parapodial lobe. Subsequent parapodia biramous with noto- and neuropodial lobes conical and widely separated; lobes of similar length with neuropodia slightly shorter and more rounded than notopodia. Anterior biramous parapodia of same length as posterior ones (Figure 7B, C). Dorsal and ventral cirri of similar shape, globular to conical, non-deciduous both inserted near middle of noto- and neuropodial lobes, respectively. All parapodial lobes with a straight aciculum. Capillaries of two types: long, marginally smooth to finely serrated, or short, coarsely serrated (Figure 7D). Spinigers with blades finely serrated (Figure 7E). Pygidium with a pair of short, digitiform, termino-lateral cirri; medio-ventral papilla short, all of similar length (Figure 7F).
REMARKS
Gathof (Reference Gathof, Uebelacker and Johnson1984) had specimens with 8 mm long and 26 chaetigers, much longer than our specimens (2.8 mm long, 28 chaetigers). We have pointed out that species with short bodies have fewer chaetigers when compared with those with longer bodies. However, the parapodia illustrated by Gathof (Reference Gathof, Uebelacker and Johnson1984) appear to be different, with foliose dorsal and ventral cirri, as Blake (Reference Blake, Blake, Hilbig and Scott1997: 180) indicated and it might not belong to Lacydonidae. Despite the lack of significant differences between L. miranda and L. oculata, Pleijel & Fauchald (Reference Pleijel and Fauchald1993) did not synonymize these species considering their unlikely Mediterranean and Antarctic geographic distribution. Nevertheless, some differences can be observed between L. miranda and L. oculata; in L. miranda, the prostomium is slightly wider than long, instead of being clearly wider than long (1.5–2.0 times); the eyes are larger (occupying approximately one third of the prostomial length), darker and placed on posterior half of prostomium of L. miranda, instead of a slightly smaller (occupying about one quarter of the prostomial length), brownish, lenticulated and centrally positioned on the prostomium of L. oculata. Another difference is that L. miranda presents two types of capillary notochaetae, long and short, with the long ones marginally smooth to finely serrated and the short ones with coarsely serrated margins, while in L. oculata, the capillary notochaetae are almost entirely smooth (Pleijel & Fauchald, Reference Pleijel and Fauchald1993) except for one or two that are shorter, distally curved, sub-distally expanded with serrated margin located in the uppermost side of the notopodial bundle. This species had been reported in the Mediterranean (type-locality) with incursions to the North Atlantic and Gulf of Mexico. This is the southernmost record of this species.
DISTRIBUTION
Mediterranean – France (Gulf of Marseille, type locality), Denmark, Sweden, Ireland, Italy (Sicily), Iberian Peninsula (Cádiz), Gulf of Mexico and Brazil (Southern); from 38–43 m depth. Lacydonia miranda was found between 53–107 m depth, in the Campos Basin.
Lacydonia oculata (Hartman, Reference Hartman1967)
(Figures 8A−E & 11B)
Scalispinigera oculata Hartman, Reference Hartman1967: 134, pl. 41A–C; Kudenov & Blake, Reference Kudenov and Blake1978: 428, 441; Blake, Reference Blake1981: 1131, 1157.
Lacydonia antarctica Hartmann-Schröder & Rosenfeldt, Reference Hartmann-Schröder and Rosenfeldt1988: 36, Figure 11–13.
Lacydonia oculata: Pleijel & Fauchald, Reference Pleijel and Fauchald1993: 673, Figure 1.
MATERIAL EXAMINED
Eight specimens: UERJ 1377 (1, complete) 03.vii.2009, Station Hab16 C04 R3, 91 m, 22o51′57″S 40o57′35″W, van Veen; UERJ 1457 (1, complete) 03.vii.2009, Station Hab16 C04 R1, 90 m, 22o51′57″ 40o57′34″W, van Veen; UERJ 1459 (1 female with eggs, complete), 03.vii.2009, Station Hab11 D03 R2, 90 m, 22o51′57″S 40o57′34″W, van Veen; UERJ 1461 (1, complete) 22.ii.2009, Station Hab11 C04 R1, 92 m, 22o51′57″S 40o57′35″W, van Veen; UERJ 1458 (1, incomplete) 22.ii.2009, Station Hab11 C04 R3, 92 m, 22o51′57″S 40o57′35″W, van Veen; UERJ 1851 (1, complete) 03.vii.2009, Station Hab16 C04 R1, 90 m, 22o51′57″S 40o57′34″W, van Veen; UFF 01 (1, incomplete), 04.vii.2009, Station Hab16E05R1, 149 m, 22°23′34″S 40°20′48″W, Van Veen; UFF 02 (1, incomplete), 07.vii.2009, Station Hab16G05R2, 149 m, 22°6′6″S 40°3′12″W, Van Veen.
TYPE-LOCALITY
Antarctic Peninsula.
DIAGNOSIS
Prostomium about 1.5 times wider than long, anteriorly rounded, distinctly separated from tentacular segment; three antennae and two palps, antennae slightly longer than palps, conical to digitiform. A pair of large eyes present. Ciliated nuchal organs between prostomium and achaetous segment. Notochaetae usually smooth to finely serrated capillary, not sub-distally expanded.
DESCRIPTION
Body dorso-ventrally slightly depressed, anteriorly more robust, tapered posteriorly (Figure 8B). Complete specimens up to 4.1 mm long, 33 chaetigers. Body whitish to brownish, reddish-brownish dots dorsally concentrated or diffusely distributed on prostomium and parapodial lobes (Figures 8A & 11B); parapodia with two pigmented spots, latero-dorsally in parapodial bases reaching base of dorsal cirri. Anterior and posterior segments uni-annulated. Prostomium anteriorly rounded, almost 1.5 times wider than long. Two antennae and two palps short, conical to digitiform, latero-dorsally and latero-ventrally inserted, respectively; lateral antennae as long as palps; median antenna or incision scar not observed, probably lost (Figure 8A). Eyes present in one pair, brownish, large (about one quarter of prostomial length), lenticulated. Prostomium distinctly separated from tentacular segment (Figure 8A). Nuchal organs forming a ciliated collar between prostomium and tentacular segment. Pharynx retracted in all specimens; anterior region of digestive tract strongly muscular and located between a pair of large lateral glands, viewed by transparency and reaching chaetiger 5 (Figure 8A, B). Tentacular segment slightly shorter than following segments, with a pair of conical cirri latero-ventrally inserted. Anterior three pairs of parapodia uniramous with neuropodial lobe and dorsal and ventral cirri, globular to conical; dorsal cirri inserted near parapodial base and ventral cirri near middle of parapodial lobe. Subsequent parapodia biramous, with noto- and neuropodium widely separated; notopodia two thirds as long as neuropodia. Dorsal and ventral cirri, globular to conical, of similar size, inserted near middle of the noto- and neuropodial lobes, respectively. All parapodial lobes with straight aciculae not surpassing parapodial lobe. Capillaries of two types: smooth to finely serrated, numbering 10–13 per bundle, tapered, not expanded sub-distally (Figure 8D), and sub-distally expanded, serrated, only one per bundle, in uppermost region. Spinigers numbering 10−20, blades finely serrated; heterogomph shaft (Figure 8E). Pygidium with a pair of short lateral, conical to digitiform cirri (not reaching the last chaetiger; Figure 8C), one median papilla also short, all of same length. Pygidium with a pair of pigmented spots (Figure 8C).
REMARKS
Lacydonia oculata was described by Hartman (Reference Hartman1967) from a single specimen, as belonging to Scalispinigera within Scalibregmatidae. Hartman (Reference Hartman1967) described a preocular area with a row of transverse papilla on the prostomium that was not confirmed by Pleijel & Fauchald (Reference Pleijel and Fauchald1993). Most Brazilian specimens presented lenticulated eyes not observed in previous studies (two exceptions: UFF01 and UFF02). Pleijel & Fauchald (Reference Pleijel and Fauchald1993) transferred S. oculata to Lacydonia and although the authors did not find enough characteristics to distinguish between L. oculata and L. miranda, they preferred to maintain them as distinct species because of the geographically distant type localities; L. oculata occurs in Antarctica and L. miranda, in the Mediterranean and surrounding areas. Both L. oculata and L. miranda were recorded in this study at a location (Campos Basin) roughly situated between Antarctica and the Mediterranean. The differences between both species are pointed out in the remarks for L. miranda (see above).
DISTRIBUTION
Antarctic Peninsula from 10–63 m depth, on shore; L. oculata was found between 90–149 m depth in the Campos Basin, Southern Brazil.
Lacydonia cf. papillata Uschakov, Reference Uschakov1958
(Figure 9 A–D)
Lacydonia papillata Uschakov, Reference Uschakov1958: 206, Figure D–F; 1974: 213, Pl. 34, Figs 1–3.
MATERIAL EXAMINED
Six specimens: UERJ 1142 (1, complete) 13.ii.2009, Station Hab9 H09 R3 E0-2, 1302 m, 21°39′15″S 39°54′3″W, Box corer; UERJ 1790 (1, complete) 15.ii.2009, Station Hab10 E11 R3, 2520 m, 22°47′0S 39°55′28″W, Box corer; UFF 13 (1, incomplete), 07.vii.2008, Station Hab7F7R1, 707 m, 22°19′53″S 40°2′5″W, Box corer; UFF 14 (1, incomplete), 06.ii.2009, Station Hab9Canac7R1, 780 m, 21°47′22″ S 40°2′1″W, Box corer; UFF 15 (1, incomplete), 14.ii.2009, Station Hab9I9R2, 1300 m, 21°11′2″S 40°3′12″W, Box corer; UFF 08 (1, complete), 10.ii.09, Station Hab9E7R2, 700 m, 22°26′56″S 40°10′0″W, Box corer.
TYPE-LOCALITY
Pacific Ocean, Kuril-Kamchatka Trench.
DIAGNOSIS
Prostomium anteriorly rounded; conical to digitiform antennae and palps. Eyes absent. Dorsal and ventral cirri globular to conical, both rudimentary; noto- and neuropodial lobes widely apart. Notopodium with capillary chaetae, neuropodium with spinigerous chaetae. A pair of termino-lateral pygidial cirri digitiform to conical of same length as median pygidial papilla or slightly longer.
DESCRIPTION
Body slightly dorso-ventrally flattened, anteriorly more robust, tapered posteriorly; specimens up to 4.1 mm long, 32 chaetigers. Body white to yellow, with scarce reddish-brown spots on prostomium and distal region of dorsal cirri and pygidium. Anterior segments uni-annulated and subsequent segments indistinctly bi-annulated. Prostomium almost twice wider than long; anterior margin rounded, posterior margin straight (Figure 9A). Three antennae and two palps present; all short, conical to digitiform, dorso-laterally and latero-ventrally inserted, respectively; antennae slightly longer than palps; median antenna almost as long as palps and inserted on posterior half of prostomium. Eyes absent. Prostomium distinctly separated from tentacular segment. Nuchal organs forming a ciliated groove between prostomium and tentacular segment (Figure 9A). Pharynx retracted in all specimens, strongly muscular, with a pair of lateral glands reaching chaetiger 5 viewed by transparency (Figure 9A). Tentacular segment slightly shorter than following segments; a pair of digitiform cirri present, laterally inserted. Anterior three pairs of parapodia uniramous with neuropodial lobes and dorsal and ventral cirri globular to conical; dorsal cirri inserted near parapodial base and ventral cirri near middle parapodial lobe. Subsequent parapodia biramous with noto- and neuropodial lobes, same-sized and widely separated (Figure 9B); inferior part of notopodial prechaetal lobe and superior part of neuropodial prechaetal lobe slightly elongated. Dorsal and ventral cirri not observed in any specimen (Figure 9B). All parapodial lobes with straight aciculae. Numerous apparently smooth capillary notochaetae (Figure 9C). Spinigers (Figure 9D) with blades of slightly different lengths, serrated. Pygidium, pygidial cirri and papilla not observed.
REMARKS
According to Ushakov (Reference Uschakov1958), Lacydonia papillata differs from other species lacking eyes, except L. elongata, by the presence of elongated parapodial lobes; further, L. papillata differs from L. elongata by presenting a prostomium about 1.5 times wider than long, presence of dorso-lateral depressions on the prostomium, four dark spots on the second chaetiger, and rudimentary, dorsal and ventral cirri. Lacydonia cirrata, another species without eyes, has proportionally minute antennae and palps, and its prostomium is almost as wide as long, indistinctly separated from the tentacular segment. In addition, the prostomium in L. cirrata is more quadrangular than rectangular, and the capillary notochaetae have a distal enlargement, which is not observed in L. papillata. The type material of Lacydonia elongata was examined by Böggemann (Reference Böggemann2009) who considered it synonymous with L. papillata without examining the type material of the latter. Nonetheless, he mentioned that there were differences but they were considered few and insignificant without pointing them out. Böggemann's specimens apparently differed from the original description by the absence of depressions on the prostomium, presence of dorsal and ventral cirri that were globular to conical, deciduous, and capillary notochaetae being finely serrated instead of smooth. Boggemann's material might be more similar to L. elongata than L. papillata. We believe that the common Pacific and Atlantic (eastern and western) occurrence is unlikely for this species and such a conclusion would have to be based on comparisons with the type material of both species. Moreover, we think that the differences mentioned above are relevant to separate and maintain these species as distinct. Therefore, we consider L. elongata as a valid species. Based on the original description our specimens differ from L. papillata by the absence of lateral depressions on the prostomium and four patches of dark spots on the second chaetiger, however would be necessary to examine type-material to make a definitive statement about this differences. Nevertheless, we maintained our specimens as L. cf. papillata, until comparison to type-material is possible.
DISTRIBUTION
Pacific Ocean – Kuril-Kamchatka Trench (Russia) and off Japan (Uschakov, Reference Uschakov1958, Reference Uschakov1972); Lacydonia cf. papillata was found between 1302–2520 m depth in the Campos Basin.
ACKNOWLEDGEMENTS
Our thanks to CENPES/PETROBRAS through the Habitats Project – Campos Basin Environmental Heterogeneity, for donating the material for this study. Thanks also to Kathrin Philipps-Bussau from the Zoologisches Institut und Zoologisches Museum der Universität Hamburg for sending images of the type material for Lacydonia mikrops Ehlers, Reference Ehlers1913 (ZMH 8549). We appreciate the opportunity to use the facilities and assistance from the Department of Zoology in the Institute of Biology at the State University of Rio de Janeiro (UERJ), and the Department of Marine Biology at the Fluminense Federal University (UFF). We also thank Nice Shindo for assistance in translating and editing the manuscript and Vinícius Mitchell for his assistance with drawings. Drs Sérgio Salazar-Vallejo, Julie Bailey-Brock, Markus Böggemann and Robin Wilson provided suggestions that greatly improved an earlier version of this manuscript.














