INTRODUCTION
Species that co-occur and require similar feeding resources usually minimize the diet overlap by using different areas or capturing different prey, which is especially important when feeding resources are scarce (Pimm, Reference Pimm2002). In this sense, the niche differentiation among species is the process by which they evolve different forms of resource use, and has been used to explain their co-occurrence (Levins, Reference Levins1968; MacArthur, Reference MacArthur1972).
Considering the marine mammals, species using similar feeding resources may reduce niche overlap due to differences in their movement pattern and, consequently, their distributional range and feeding ground (Whitehead et al., Reference Whitehead, MacLeod and Rodhouse2003). For species that share similar distribution, size and morphology, the niche differentiation can be explained by differences in preferred habitat (e.g. coastal vs. offshore; pelagic vs. benthic) and behavioural budgets (e.g. diurnal vs. nocturnal activities) (Kiszka et al., Reference Kiszka, Sinom-Bouhet, Martinez, Pusineri, Richard and Ridoux2011).
In the last two to three decades, stable isotope ratios have provided complementary data on animals’ trophic ecology. This technique presents time-integrated information on food assimilation, and evaluates differences in trophic niche, trophic level and foraging sites (e.g. Hobson & Welch, Reference Hobson and Welch1992; Cherel et al., Reference Cherel, Hobson, Bailleu and Groscola2005; Di Beneditto et al., Reference Di Beneditto, Souza, Kehrig and Rezende2011, Reference Di Beneditto, Rezende, Camargo and Kehrig2013). Layman et al. (Reference Layman, Arrington, Montan and Post2007) introduced a number of metrics, borrowed from ecomorphological studies, to summarize quantitative information from stable isotope data sets, describing trophic structure at the species or community level. Additionally, Jackson et al. (Reference Jackson, Inger, Parnell and Bearhop2011) developed a Bayesian method for the comparison of these metrics among groups. This approach has recently been expanded, allowing a robust comparison of isotopic niches across species (e.g. Jackson & Britton, Reference Jackson and Britton2014; Knickle & Rose, Reference Knickle and Rose2014) and communities (e.g. Abrantes et al., Reference Abrantes, Barnett and Bouillon2014; Zapata-Hernández et al., Reference Zapata-Hernández, Sellanes, Thurber, Levin, Chazalon and Linke2014).
The target species of the present study are the coastal dolphins Pontoporia blainvillei (Gervais & D'Orbigny, 1844) and Sotalia guianensis (Van Bénedèn, 1864). These species are the most vulnerable dolphins along the South-western Atlantic Ocean due to interactions with fisheries (Secchi et al., Reference Secchi, Danilewicz and Ott2003; Barreto et al., Reference Barreto, Rocha Campos, Rosas, Silva Júnior, Dalla Rosa, Flores and da Silva2010; IUCN, 2015). The feeding habits of these dolphins were previously described and compared in a sympatric area by Di Beneditto & Ramos (Reference Di Beneditto and Ramos2001) and (Reference Di Beneditto, Ramos and Lima2004), and Di Beneditto et al. (Reference Di Beneditto, Souza, Kehrig and Rezende2011). Twenty-five prey species were recorded in the stomach contents of P. blainvillei, among which five (three pelagic and two demersal) are preferentially ingested; whereas 36 prey species were recorded for S. guianensis, and two (one pelagic and one demersal) are most ingested.
In the present study, we analysed for the first time the isotopic niche breadth of P. blainvillei and S. guianensis through quantitative niche metrics using a Bayesian framework. The isotopic data were compared with previous data on feeding habits to assess the feeding overlap between species and age classes, and to evaluate specific and age class strategies related with their co-occurrence. We hypothesize that (i) the niche breadth of P. blainvillei and S. guianensis is similar, considering that these dolphins have coastal habits and both pelagic aand demersal prey are consumed by them; and (ii) the niche breadth of mature specimens is larger than immatures for both dolphins, because in cetacean species mature individuals have more experience in selectivity and prey capture (Perrin et al., Reference Perrin, Würsig and Thewissen2009) and have larger body size which allow the ingestion of more diverse range of prey (Segura et al., Reference Segura, Franco-Trecu, Franco-Fraguas, Arim and Willian2015).
MATERIALS AND METHODS
The specimens of P. blainvillei and S. guianensis analysed in this study were incidentally captured from 2001 to 2005 through commercial gillnet fisheries practiced in south-eastern Brazil, from 21°35′S to 22°25′S, in waters from 0.4 to 47 km from the coastline, and in depths varying from 6 to 30 m. Each dolphin specimen was grouped into immature or mature according to age, calculated by the number of teeth growth layers (Ramos et al., Reference Ramos, Di Beneditto and Lima2000). For P. blainvillei and S. guianensis, specimens ≤2 years old and ≤4 years old, respectively, were considered as immature. Specimens that were still suckling were excluded from this study due to the great influence of the mother's milk in their isotopic ratios. Thus, our sample was composed of 13 specimens of P. blainvillei (seven immatures and six matures), and 18 of S. guianensis (six immatures and 12 matures).
A sub-sample from the back dorso-lateral muscle was removed from the dolphins. The tissue samples were freeze-dried and homogenized with a mortar and pestle for stable isotope analyses (δ 15N and δ 13C). The isotopic analyses were performed with a ThermoQuest Finnigan Delta Plus (Finnigan MAT) mass spectrometer coupled to an elemental analyser. Pee Dee Belemnite carbonate and atmospheric nitrogen were standard values for carbon and nitrogen analyses, respectively:
 $$\eqalign{{\delta ^{\,j/i}}X{\rm} = & {\left( {^j X{/^i}X} \right)_{{\rm sample}}}-{\rm} 1 \cr & {\left( {^j X{/^i}X} \right)_{{\rm standard}}}} $$
$$\eqalign{{\delta ^{\,j/i}}X{\rm} = & {\left( {^j X{/^i}X} \right)_{{\rm sample}}}-{\rm} 1 \cr & {\left( {^j X{/^i}X} \right)_{{\rm standard}}}} $$where jX is the heavier isotope (15N or 13C), and iX is the lighter isotope (14N or 12C). The analytical precision was ±0.3‰ for δ 15N and ±0.2‰ for δ 13C, determined in triplicate at each of five samplings. Lipids were not extracted from the muscle samples prior to analysis; however, the C/N ratios were lower than 3.5, indicating low levels of lipids. Therefore, the interpretation of the results of δ 13C was not compromised (Post et al., Reference Post, Layman, Arrington, Takimoto, Quattrochi and Montaña2007).
Quantitative metrics based on the position of individuals in the niche space formed by δ 13C and δ 15N were applied to estimate isotopic niche breadth (Layman et al., Reference Layman, Arrington, Montan and Post2007; Jackson et al., Reference Jackson, Inger, Parnell and Bearhop2011). Metrics were calculated using the functions for Stable Isotope Bayesian Ellipses in R (SIBER – Jackson et al., Reference Jackson, Inger, Parnell and Bearhop2011), available within the package Stable Isotope Analysis in R (SIAR) (Parnell et al., Reference Parnell, Inger, Bearhop and Jackson2010; R Development Core Team, 2011). Six metrics were calculated as described below. The first four reflect trophic diversity in the δ 13C-δ 15N scatterplot, and the other two represent trophic redundancy (how closely positioned individuals in species are to each other within their respective niches).
The metrics are described as: (1) δ 15N range (NR): distance between maximum and minimum δ 15N values for a given species (P. blainvillei and S. guianensis) or age class within species (immature and mature) which indicates the difference between trophic levels. A large NR implies higher trophic levels within species or age class; (2) δ 13C range (CR): distance between maximum and minimum δ 13C values which indicates the variability of food sources consumed. A large CR implies difference in basal resources within trophic levels (e.g. benthic vs. pelagic; coastal vs. oceanic); (3) Standard ellipse area (SEA): trophic niche breadth for a given species or age class. The standard ellipse is centred on the group centroid and scaled to encompass a 40% chance of including a subsequently sampled datum; (4) Mean distance to centroid (CD): average Euclidean distance of each individual to the group centroid (mean δ 15N and δ 13C value), which provides the average degree of trophic diversity within a species or age class; (5) Mean nearest neighbour distance (MNND): average Euclidean distances to the individual's nearest neighbour in scatterplot space which provides a measure of the overall species or age class packing. A set of many individuals with similar trophic ecologies would show a smaller MNND than a set in which individuals are more varied in terms of diet; and (6) Standard deviation of nearest neighbour distance (SDNND): a measure of evenness of species or age class packing in the scatterplot, in which low values indicate a more even distribution of individuals in the trophic niche space.
The differences between species and age classes regarding δ 15N and δ 13C values were assessed via two-way ANOVA. The SEAs of species (P. blainvillei and S. guianensis) and age classes (immature and mature) were compared probabilistically with the posterior Bayesian distributions, calculating the proportion of ellipses for group 1 that was larger than ellipses for group 2 in the simulated draws (Jackson et al., Reference Jackson, Inger, Parnell and Bearhop2011). The per cent of overlapping SEA between species and age classes was the measure of isotopic niche overlap. Two-way ANOVAs were also used to assess differences and interactions between species and age classes considering CD and MNND. The statistic SDNND, being a standard deviation, was compared between groups by an F-ratio test. The P values were interpreted as strengths of evidence toward null hypotheses, rather than on the dichotomic scale of significance testing (Hurlbert & Lombardi, Reference Hurlbert and Lombardi2009).
RESULTS
The isotopic values for P. blainvillei and S. guianensis are presented in Figure 1. For P. blainvillei, the mean values for δ 15N and δ 13C were +13.8 ± 0.3‰ and −16.0 ± 0.3‰ (immature specimens) and +13.2 ± 1.1‰ and −15.7 ± 0.6‰ (mature specimens), respectively. Considering S. guianensis, the mean values for δ 15N and δ 13C were +14.0 ± 0.5‰ and −15.4 ± 0.3‰ (immature specimens) and +14.1 ± 1.1‰ and −15.8 ± 0.8‰ (mature specimens), respectively. The two-way ANOVAs were unable to detect differences in the means of the isotopic values between species (F = 2.6, df = 1, 27, P = 0.12 for δ 15N, F = 1.14, df = 1, 27, P = 0.29 for δ 13C,) and maturity (F = 0.6, df = 1, 27, P = 0.44 for δ 15N, F = 0.05, df = 1, 27, P = 0.82 for δ 13C). No interactions were detected between species and age class factors (F = 1.2, df = 1, 27, P = 0.28 for δ 15N, F = 1.9, df = 1, 27, P = 0.18 for δ 13C). This result was expected given that within-group variation is considerably large compared with the differences among groups (see Figure 1 for details). The diagnostic plots of residuals and fitted values (not shown) did not indicate noticeable departures from assumptions of normality and homoscedasticity.

Fig. 1. Stable isotopes values of the dolphin species Pontoporia blainvillei and Sotalia guianensis for (A) the entire sample, and (B) separating age classes. Lines depict the standard ellipse (the 40% confidence interval) for the isotopic niches with solid lines for P. blainvillei and dashed lines for S. guianensis. Pb, Pontoporia blainvillei; Sg, Sotalia guianensis; Im, immatures; Mat, matures.
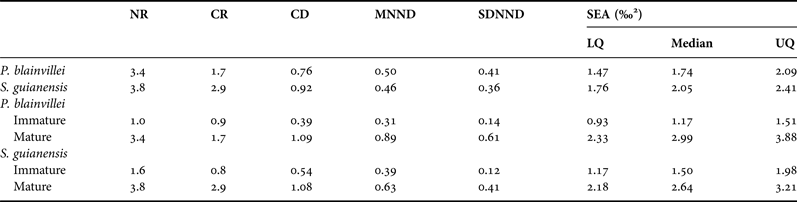
The SEAs based on species were similar in size and position in the δ 13C–δ 15N scatterplot. However, the SEAs based on age class showed that the mature ellipses are larger than the immatures’ for both dolphins (Figure 1 and Table 1). A probabilistic comparison between the ellipses areas based on the posterior distribution of simulated ellipses indicated that specific differences (P = 0.32) are negligible compared with age class differences (P = 0.04 for P. blainvillei, P = 0.13 for S. guianensis).
Table 1. Quantitative niche metrics by species and age classes.

NR, δ15N range; CR, δ13C; SEA, standard ellipse area; CD, distance to centroid; MNND, mean nearest neighbour distance; SDNND, standard deviation of nearest neighbour distances; LQ, lower quartile; and UQ, upper quartile.
The quantitative metrics to estimate the isotopic niche breadth are in Table 1. Trophic level measures (δ 15N range) indicate that the dolphin species are comparable as predators, and that immature specimens have a lower range of trophic levels than mature ones. In terms of variability of food sources (δ 13C range), S. guianensis showed a larger value than P. blainvillei. For both species, mature specimens have larger δ 13C range than immatures.
Neither CD, MNND or SDNND presented a clear difference between species (CD P = 0.39, MNND P = 0.84, SDNND P = 0.31), indicating similarities in the average degree of trophic diversity, redundancy and evenness. Both CD and MNND differed between age classes regardless of species (CD P = 0.01, MNND P = 0.01) as no interaction of factors was detected (CD P = 0.72, MNND P = 0.23). The average degree of trophic diversity (CD) in mature specimens is two times larger than that of the immatures. Comparing SDNND between age classes within species, we observed that immatures presented smaller values than matures in both species (P. blainvillei P < 0.01; S guianensis P = 0.01). The distribution of immatures in trophic space is tighter and more even (based on MNND and SDNND) than the distribution of mature specimens (Table 1). The diagnostic plots of residuals and fitted values for the two-way ANOVAs, as well as the SDNND comparison, indicated normal but heteroscedastic residuals for both metrics (CD and MNND). Logarithmic transformations corrected the deviation from model assumptions but maintained the conclusions unchanged.
The percentages of SEA overlap between P. blainvillei and S. guianensis, and S. guianensis and P. blainvillei were 52.1 and 39.7%, respectively. For both dolphins, there is a high isotopic niche overlap between immature and mature specimens (Table 2), because the immature ellipses are almost completely contained by the mature ellipses. Comparing age classes between species, it was noted that immatures of P. blainvillei and S. guianensis have a minimal niche overlap (<3%), whereas the percentage for mature specimens was greater (~45–55%) (Table 2).
Table 2. Overlapping SEA (%) between age classes for Pontoporia blainvillei and Sotalia guianensis (isotopic niche overlap).

DISCUSSION
This study compared the isotopic niche breadth of the coastal dolphins P. blainvillei and S. guianensis, showing to what extent they are sharing or segregating feeding resources in a sympatric area. The comparison of quantitative niche metrics indicated that specific differences (P. blainvillei vs. S. guianensis) are negligible compared with age class differences (immature vs. mature specimens). For both species, the niche breadth of immature specimens is smaller than mature ones.
The δ 15N values are generally applied as measures of trophic level (predators have higher values than their prey), and δ 13C values may indicate the origin of feeding resources (e.g. coastal vs. oceanic sources; pelagic vs. benthic sources) (Hobson & Welch, Reference Hobson and Welch1992; Cherel et al., Reference Cherel, Hobson, Bailleu and Groscola2005). In the study area (~21–22°S), previous studies using stable isotopes (δ 15N and δ 13C) as diet proxies showed that P. blainvillei and S. guianensis are top predators whose main feeding resources are associated with coastal waters (Di Beneditto et al., Reference Di Beneditto, Souza, Kehrig and Rezende2011, Reference Di Beneditto, Rezende, Camargo and Kehrig2013; Kehrig et al., Reference Kehrig, Seixas, Malm, Di Beneditto and Rezende2013). The isotopic values of the present study corroborate these findings, but the large δ 13C range in S. guianensis might indicate a wider movement along the continental shelf and, consequently, a mixture of coastal and oceanic influences in the set of prey. This is corroborated by the record of Ariosoma opistophthalmum in its stomach contents, which is a fish species typically found more distant from the coastline (Di Beneditto & Ramos, Reference Di Beneditto, Ramos and Lima2004; Froese & Pauly, Reference Froese and Pauly2015).
In general, P. blainvillei and S. guianensis were similar in the niche metrics, with SEA overlap, which confirms the first hypothesis of this study. Similarities in niche metrics can reflect prey's isotopic values, as previous recorded in Di Beneditto et al. (Reference Di Beneditto, Souza, Kehrig and Rezende2011), and variations in food intake. Pontoporia blainvillei ingests prey with minor body size, but in greater amounts than S. guianensis. The first species feed mainly on juveniles or small-sized fish and cephalopod species, up to 15.0 cm length (Di Beneditto & Ramos, Reference Di Beneditto and Ramos2001; Danilewicz et al., Reference Danilewicz, Rosas, Bastida, Marijo, Muelbert, Rodríguez, Lainson-Brito, Ruopollo, Ramos, Bassoi, Ott, Caon, Rocha, Catão-Dias and Secchi2002), while the second species is a primarily piscivorous predator that has plasticity in relation to its prey size, varying from <10.0 to 100.0 cm length (Di Beneditto & Ramos, Reference Di Beneditto, Ramos and Lima2004; Di Beneditto & Siciliano, Reference Di Beneditto and Siciliano2007). At least for some prey species the total amount (biomass) ingested in each meal is equivalent between the dolphins (Di Beneditto et al., Reference Di Beneditto and Ramos2001). Variations in prey size and quantity of each prey ingested can lead to similar isotopic values in consumers (Lassalle et al., Reference Lassalle, Chouvelon, Bustamante and Niquil2014).
For both dolphins, mature specimens have greater trophic diversity (CD) and minor trophic redundancy (MNND) than immatures. Different age classes can differ in many ecological ways, including resources use (Bolnick et al., Reference Bolnick, Svanbäck, Fordyce, Yang, Davis, Hulsey and Forister2003). In a population, groups with more generalized diet (as the mature specimens of both dolphins) tend to be more ecologically heterogeneous, exhibiting greater niche breadth (Bolnick et al., Reference Bolnick, Svänback, Araujo and Persson2007). The niche breadth of mature specimens enlarges the niche breadth of these dolphins’ population as a whole, reducing the intraspecific competition (Bolnick et al., Reference Bolnick, Amarasekare, Araujo, Bürger, Levine, Novak, Rudolf, Schreiber, Urban and Vasseur2011).
Differences between age classes regarding the niche breadth support our second hypothesis. This reveals the greater experience of matures in utilization of available feeding resources and capturing prey, expanding their niche breadth. It is expected in cetaceans due to their social behaviour, which includes long-term parental care and developed feeding strategies (e.g. Rendell & Whitehead, Reference Rendell and Whitehead2001; Perrin et al., Reference Perrin, Würsig and Thewissen2009; Tardin et al., Reference Tardin, Espede, Nery, D'Azeredo and Simão2011; Oliveira et al., Reference Oliveira, Tardin, Poletto and Simão2013). In addition, animals with larger body size are able to ingest larger prey than smaller ones (Segura et al., Reference Segura, Franco-Trecu, Franco-Fraguas, Arim and Willian2015). This condition may also explain the differences between the mature and immature dolphins, since small prey of a given species are in general less δ 15N enriched than large ones (Jennings et al., Reference Jennings, Pinnegar, Nicholas and Warr2002), which influences the isotopic value of predator.
The immatures’ isotopic niche has high overlap with mature ones. This indicates an intraspecific sharing of food resources and feeding grounds, typical in cetacean species that live in groups (Tardin et al., Reference Tardin, Espede, Nery, D'Azeredo and Simão2011). The immature specimens in turn, show reduced isotopic niche overlap between species. This niche divergence might facilitate the species' co-existence during the beginning of solid food ingestion.
In conclusion, P. blainvillei and S. guianensis specimens have similar isotopic niches, but pronounced differences between immature and mature specimens. Isotopic niche and quantitative metrics associated together with previous data on stomach contents provide a strong representation of species niche and their relationships, both intraspecific and interspecific. Moreover, the utilization of multiple methods to analyse feeding preferences reduces bias in data interpretation and may elucidate much more about species’ behaviour and movement pattern (Di Beneditto et al., Reference Di Beneditto, Souza, Kehrig and Rezende2011, Reference Di Beneditto, Santos, Rosa and Siciliano2015; Kehrig et al., Reference Kehrig, Seixas, Malm, Di Beneditto and Rezende2013).
ACKNOWLEDGEMENTS
We thank the fishermen from Atafona harbour and technician Silvana Ribeiro Gomes, who provided us with the dolphin specimens that were incidental capture in the study area.
FINANCIAL SUPPORT
This study was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq (grants no 301.405/13-1); Fundação de Amparo a Pesquisa do Estado do Rio de Janeiro – FAPERJ (grant no E-26/201.161/2014); and CNPq INCT Material Transference from the Continent to the Ocean (grant no 573.601/08-9).