INTRODUCTION
Parasites are ubiquitous in marine ecosystems and can have effects on individual hosts, host populations, communities and entire ecosystems (Mouritsen & Poulin, Reference Mouritsen and Poulin2002). For example, they can manipulate the behaviour of their host (Swennen, Reference Sweenen1969; Edelaar et al., Reference Edelaar, Drent and de Goeij2003; Thomas et al., Reference Thomas, Adamo and Moore2005; Bates et al., Reference Bates, Leiterer, Wiedeback and Poulin2011), reduce fecundity and reproduction (Lafferty, Reference Lafferty1993; Mouritsen & Poulin, Reference Mouritsen and Poulin2002; Fredensborg et al., Reference Fredensborg, Mouritsen and Poulin2005) and contribute to mortality (Jensen & Mouritsen, Reference Jensen and Mouritsen1992; Mouritsen & Jensen, Reference Mouritsen and Jensen1997), which can subsequently alter competition and predation interactions among hosts. Furthermore, parasites can play a vital role in marine ecosystems by significantly contributing to biomass (Kuris et al., Reference Kuris, Hechinger, Shaw, Whitney, Aguirre-Macedo, Boch, Dobson, Dunham, Fredensborg, Huspeni, Lorda, Mababal, Mancini, Mora, Pickering, Talhouk, Torchin and Lafferty2008), as well as affecting the topology and stability of food webs (Lafferty et al., Reference Lafferty, Allesina, Arim, Briggs, De Leo, Dobson, Dunne, Johnson, Kuris, Marcogliese, Martinez, Memmott, Marquet, McLaughlin, Mordecai, Pascual, Poulin and Thieltges2008; Dunne et al., Reference Dunne, Lafferty, Dobson, Hechinger, Kuris, Martinez, McLaughlin, Mouritsen, Poulin, Reise, Stouffer, Thieltges, Williams and Zander2013). However, more recently it has been shown that parasites can also act as a resource themselves; thus, local community composition of consumers of parasites can strongly influence parasite dynamics by interfering with transmission pathways (Johnson et al., Reference Johnson, Dobson, Lafferty, Marcogliese, Memmott, Orlofske, Poulin and Thieltges2010). This interference, which removes parasites from the system and prevents successful host infection, has been termed the ‘dilution effect’, and can lead to a reduction in disease risk (Keesing et al., Reference Keesing, Holt and Ostfeld2006; Johnson & Thieltges, Reference Johnson and Thieltges2010).
Dilution effects on macroparasite transmission are widespread and have been particularly well studied in trematode parasites from freshwater ecosystems (for review see Thieltges et al., Reference Thieltges, Bordalo, Hernández, Prinz and Jensen2008a). Trematodes have complex lifecycles, with vertebrates being used as definitive hosts. Miracidia, a free-living stage released from the definitive host, infect the first intermediate host (a mollusc) from which a second free-living stage (termed cercariae) is released. These cercariae have a short lifespan (usually <1 d) and infect a second intermediate host (invertebrates or fish, depending on the parasite species). When the second intermediate host is consumed by a definitive host the cycle is closed allowing the parasite to sexually reproduce and for the cycle to start again (Galaktionov & Dobrovolskij, Reference Galaktionov and Dobrovolskij2003). Recent studies have shown that the free-living stages of trematodes are also subjected to dilution effects in marine ecosystems (Mouritsen & Poulin, Reference Mouritsen and Poulin2003; Hopper et al., Reference Hopper, Poulin and Thieltges2008; Thieltges et al., Reference Thieltges, Jensen and Poulin2008b; Kaplan et al., Reference Kaplan, Rebhal, Lafferty and Kuris2009; Prinz et al., Reference Prinz, Kelly, O'Riordan and Culloty2009; Studer et al., Reference Studer, Kremer, Nelles, Poulin and Thieltges2013). A particularly strong factor seems to be predation by non-host species, either through active or passive predation. For example, it is postulated that some marine predators such as shrimps, may actively seek and consume free-living stages, whilst others, such as filter feeders, may not selectively but accidentally ingest cercariae. Another mechanism can be physical obstacles, e.g. algae, which may obstruct the cercariae from reaching the host. These examples illustrate how ambient fauna and flora may interfere with free-living trematode cercariae, thus removing infective stages from the system and reducing the disease risk for down-stream hosts in marine ecosystems.
For most marine parasite species and localities we lack an inventory of potential diluting organisms, making it difficult to evaluate the generality of dilution effects. This is also true for trematodes in the Wadden Sea, an extensive area of marine-to-estuarine intertidal mudflats and sub-tidal gullies along the Danish, German and Dutch coast, dominated by benthic molluscs and polychaetes (van der Graaf et al. Reference van der Graaf, de Vlas, Herlyn, Voss, Karin, Drent, Marencic and Vlas2009). The high production of invertebrates is used by a plethora of fish and birds, making it an ideal habitat for trematodes due to the presence of the necessary sequential hosts (Thieltges et al., Reference Thieltges, Engelsma, Wendling and Wegner2012). Dilution effects have only been studied in one of the dominant local trematodes, Himasthla elongata. This trematode uses the periwinkle Littorina littorea as first and mussels and cockles as second intermediate and birds as definitive hosts (Werding, Reference Werding1969). In a field experiment also conducted in the Wadden Sea, dilution effects by Pacific oysters (Crassostrea gigas) could be observed which reduced infection levels of H. elongata in blue mussels (Mytilus edulis) more than three-fold (Thieltges et al., Reference Thieltges, Reise, Prinz and Jensen2009). Laboratory experiments conducted at the Limfjord, a large brackish water system in the north of Denmark, suggest that other species also interfere with transmission of cercariae of H. elongata, namely the filter-feeding gastropod Crepidula fornicata, the crustacean predators Crangon crangon and Carcinus maenas and the bivalve Mya arenaria (Thieltges et al., Reference Thieltges, Jensen and Poulin2008b). However, it remains unclear if the experimental results from the study in the Limfjord can be transferred to different ecosystems, and what other species might cause dilution effects in this trematode species.
Here, our aim is to experimentally test the dilution potential of various common species from the Wadden Sea with regard to Himasthla elongata cercariae. In addition, from our results and other examples from the literature, we compile an extended inventory of diluters of H. elongata in different marine ecosystems.
MATERIALS AND METHODS
Selection of diluters
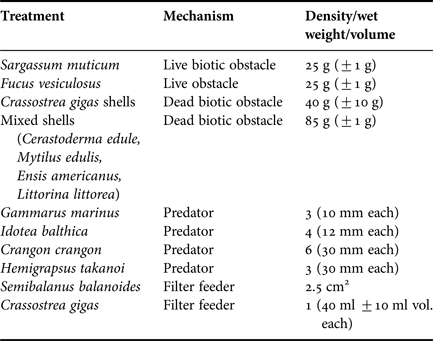
A range of potential diluters (predators, filter feeders, live biotic obstacles and dead biotic obstacles) were collected from the Wadden Sea, along the eastern coast of Texel, The Netherlands (Table 1). The organisms were chosen due to their observed presence and abundance in intertidal areas of the Wadden Sea where all Himasthla elongata hosts can be found (primarily on hard bottom structures like dykes, mussel and oyster beds). Densities used in the experiments were kept at levels observed in the field. Upon returning to the laboratory, all organisms and physical objects were cleaned and any epibionts carefully removed. The organisms were then stored in tanks (60 × 30 × 30 cm) filled with filtered and aerated seawater within a climate controlled room at 15°C (based on air temperatures at time of collection).
Table 1. Organisms used in the different treatments, their expected dilution mechanism and densities or fresh weights used in the experiments per 1.7 l tanks containing 1.5 l filtered seawater.

Source of cercariae
Periwinkles (Littorina littorea) collected from the field were screened for the presence of H. elongata infections by keeping them in an incubator at 25°C for several hours and checking for shed cercariae. Infected snails were then separated and kept in aquaria. To obtain cercariae for the experiments, 150–200 infected snails were incubated in 1.8 l of filtered seawater at 27°C and under light for 3 h to encourage the release of cercariae. The water and cercariae (hereafter termed ‘broth’) was drained into a 2 l beaker. The broth was then gently stirred (anticlockwise three times and then clockwise three times using a plastic spoon), and immediately after 50 ml of broth was scooped out of the beaker using a small measuring jug and added to experimental containers (1.7 l), resulting in a uniform infection dose. Eight samples of 50 ml were also taken to get an average of the number of parasites added to each container.
Experimental set-up
Five experiments were carried out, each testing two different diluters versus a control. For each experiment eight replicates were used for each of the three treatments. Each replicate consisted of a 1.7 l container with 1.5 l of filtered seawater randomly placed in a single climate controlled room at 18.5°C (±0.2°C), a typical water temperature which occurs during the summer transmission period.
All organisms were starved and kept in the experimental containers for 24 h prior to adding the cercariae for acclimation. At the start of the experiment, 50 ml of cercariae broth was added to each container (see above). After 3 h large dilutors were quickly removed by forceps and discarded; smaller dilutors were fixed along with the cercariae. Experiments were run for 3 h as survival of cercariae usually starts to decrease after about 10 h (Thieltges & Rick, Reference Thieltges and Rick2006), to avoid the decrease in cercarial survival confounding the effects of diluters (maximum age of cercariae in our experiments was 6 h, including the 3 h incubation period). At the end of the experiment, all water from the containers was filtered through a 25 µm sieve to retain any remaining cercariae. Containers were then rinsed and filtered twice. Subsequently, cercariae were washed from the sieve with 50–100 ml filtered seawater and fixed using 10 ml 96% ethanol and stained using rose Bengal. Cercariae were then counted in Petri dishes under a light microscope.
Statistical analysis
The effect of a potential diluter versus the respective control was tested using an ANOVA followed by Dunnett's family error post-hoc test. All ANOVAs were conducted on raw, untransformed data as all experiments proved to be normally distributed after checking for normality and homoscedasticity. Finally, the percentage of cercariae removed by each potential diluter (versus the mean of the respective control) was calculated. All results shown are means ±standard error.
RESULTS
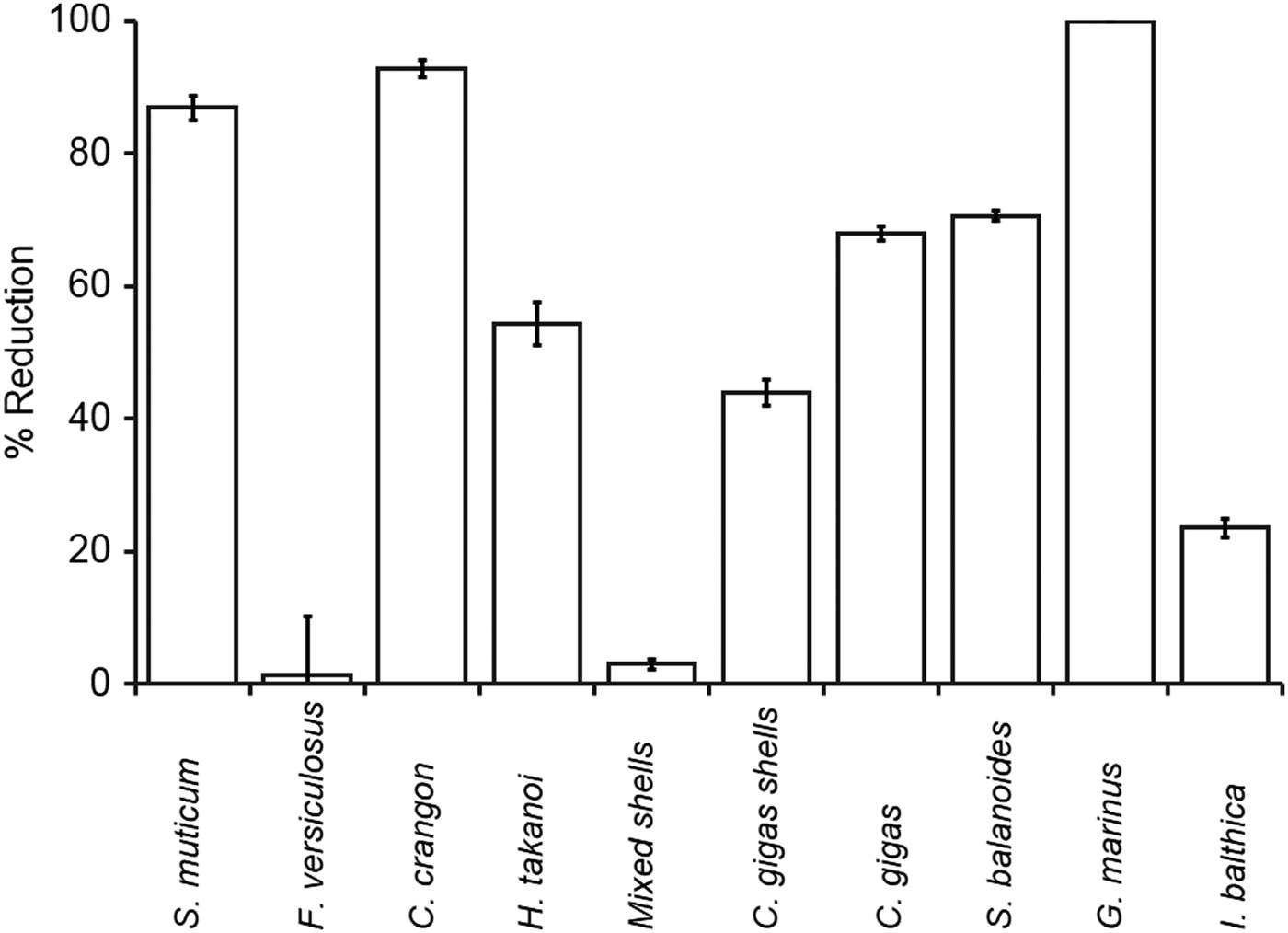
Overall, most species tested resulted in a reduction of cercariae of more than 40% (Figure 1; Table 2). The grazer G. marinus, the predator C. crangon and the live biotic obstacles S. muticum removed the most amount of cercariae (87–100%). Other species, H. takanoi, C. gigas shells, C. gigas, S. balanoides and I. balthica, removed between 24% and 71% (Figure 1; Table 2). However, F. vesiculosus and mixed bivalve shells did not show a significant reduction (Figure 1; Table 2).

Fig. 1. Percentage (±standard error) of cercariae of Himathla elongata removed from the experimental containers by the different potential diluters compared to the relevant control.
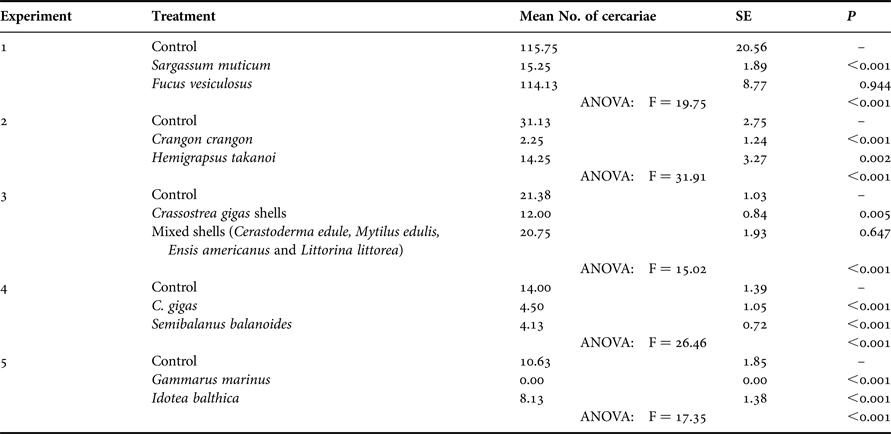
Table 2. Mean number of cercariae (±standard error) recovered from tanks containing different potential diluting organisms in five different experiments. Controls contained no diluting organism. Also shown are the P values from Dunnett's family error post-hoc tests, which compared controls with the respective treatments and the overall ANOVA results for each experiment; N = 8 replicates per treatment. Degrees of freedom for all F values = 23. SE, standard error; P, significance level of test.

The average number of cercariae recovered from controls decreased with each experiment (Table 2). Whilst an average of 116 cercariae were recovered in the controls of the first experiment (testing S. muticum and F. versiculosus), an average of only 11 was observed in the final experiment (which tested G. marinus and I. balthica). This reduction was also shown in the sub-samples (decreasing from 128 ± 6 to 38 ± 4, 23 ± 2, 17 ± 2 and 14 ± 1, respectively) and was presumably due to depletion of the cercarial production by the pool of snails during the repeated shedding procedures. However, as we only compare the respective controls with the treatments within each experiment, such a reduction in production does not affect the overall results.
DISCUSSION
The experiments showed that most of the potential diluters tested significantly reduced the number of cercariae, with all significant diluters resulting in a >24% reduction. Only two of the 10 tested organisms did not show any dilution effect. This suggests that dilution effects on the trematode H. elongata are widespread, with the potential to cause strong effects on the parasite's population dynamics.
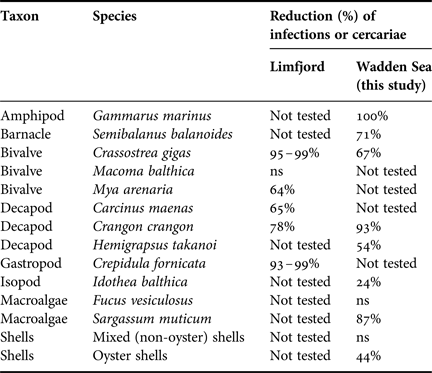
The results shown here are in line with previous findings from a study conducted in the brackish Limfjord in Denmark, where the shrimp Crangon crangon and the bivalve Crassotrea gigas also strongly reduced the numbers of cercariae of H. elongata (Thieltges et al., Reference Thieltges, Jensen and Poulin2008b; Table 3). This suggests that dilution effects on this trematode species may be more widespread than shown previously and are not confined to specific habitats or ecosystems. The Limfjord study also found additional diluters not tested in this study (Table 3). Our study now adds several new diluting species, including filter feeders and dead and live biotic obstacles, to the list of known diluters of H. elongata. While filter feeder ingest cercariae via their filtration current, the effect of biotic obstacles is different. The debris from organisms such as shells probably act as a physical barrier: cercariae become entangled in the structure preventing the free-living parasite stages from getting to their hosts within their short lifespan. The convoluted structure of oyster shells may explain why dilution effects were observed in the oysters shell (44%) but not in the mixed shell treatment (consisting of species with much smoother shell surfaces). Similarly, differences in structural complexity probably also explain the observed differences between the two algae species (see detailed discussion below). However, other mechanisms such as olfactorial cues or other exudates cannot completely be ruled out and deserve further study.
Table 3. Organisms shown to reduce free living Himasthla elongata cercariae in the Limfjord, Denmark (Thieltges et al., Reference Thieltges, Jensen and Poulin2008b, Reference Thieltges, Reise, Prinz and Jensen2009) and in the Wadden Sea (this study); ns, no significant effect.

The resulting inventory of diluters shows that a wide range of organisms interferes with the transmission of H. elongata cercariae (Table 3). In general, we observed relatively high dilution rates and it can be questioned whether this reflects actual dilution rates under natural conditions in the field. In addition to the biotic dilution effect there are also various abiotic factors (e.g. temperature and salinity) known to affect the transmission stages of trematodes (Pietrock & Marcogliese, Reference Pietrock and Marcogliese2003; Thieltges & Rick, Reference Thieltges and Rick2006; Studer & Poulin, Reference Studer and Poulin2013). This suggests that the observed dilution effects may actually be simply compensatory rather than additive, since mortality of cercariae is very high in the field anyway. However, there is evidence from a field experiment that dilution effects can be additive and comparably high in more natural settings: treatments with Pacific oysters showed a more than three-fold reduction in infection levels of target hosts with H. elongata compared to controls without oysters, despite the presumed high natural background mortalities of cercariae (Thieltges et al., Reference Thieltges, Reise, Prinz and Jensen2009).
The interference caused by diluters on cercarial transmission may potentially have strong effects on trematode population dynamics, and thus, have consequences for the hosts. Metacercarial infections (resulting from penetrating cercariae) of trematodes have been shown to have a range of detrimental effects on their invertebrate intermediate hosts, including reducing survival and growth (Jensen et al., Reference Jensen, Jensen and Mouritsen1998; Desclaux et al., Reference Desclaux, de Montaudouin and Bachelet2004; Fredensborg et al., Reference Fredensborg, Mouritsen and Poulin2005; Thieltges, Reference Thieltges2006). Since such effects are usually density-dependent (Fredensborg et al., Reference Fredensborg, Mouritsen and Poulin2005; Thieltges, Reference Thieltges2006), any reductions of parasite loads will relieve the hosts from the negative effects of infections. Here we looked at single species effects on dilution rates. However, in natural settings, cercariae will encounter many different identities and densities of diluters with the potential for a multitude of compensatory, additive or synergistic effects on dilution rates resulting in an interesting avenue for further research.
In general, the strength of the dilution effect seems to depend more on the diluter identity than on the respective dilution mechanism. Direct comparison among different diluters and mechanisms is difficult because the strength of the dilution effect is related to the density of diluters (Thieltges et al., Reference Thieltges, Reise, Prinz and Jensen2009). However, for two diluters a direct comparison is possible, since the same weight was used for the two algae species, S. muticum and F. vesiculosus. While S. muticum significantly reduced cercariae by 87%, F. vesiculosus did not. The difference between the two species probably results from the fact that S. muticum has a very fine branching habitus, leading to the entanglement of cercariae, while F. vesiculosus has broad blades which probably do not trap cercariae. Interestingly, S. muticum is classed as an invasive species in the Wadden Sea, and this draws attention to potential ‘positive’ impacts that non-native organisms may have on their new habitat (Buschbaum et al., Reference Buschbaum, Chapman and Saier2006; Thieltges et al., Reference Thieltges, Strasser and Reise2006). Here, it was shown that S. muticum may alleviate native host organisms from parasites more than the native F. vesiculosus which is found to grow in the same locality. Dilution effects on cercariae of H. elongata were also caused by other invasive species, e.g. the Pacific oyster Crassostrea gigas and the American slipper limpet Crepidula fornicata (this study; Thieltges et al., Reference Thieltges, Reise, Prinz and Jensen2009). Similar effects have also been observed in other trematode species. For example, the invasive barnacle Austrominius modestus interferes with the transmission of cercariae of Echinostephilla patellae and Parorchis acanthus (Prinz et al., Reference Prinz, Kelly, O'Riordan and Culloty2009). Likewise, the invasive algae Caulerpa taxifolia interfere with the transmission of various trematode species, presumably due to toxic exudates (Bartoli & Boudouresque, Reference Bartoli and Boudouresque1997). These examples point to the potential importance of invasive species in mediating parasite–host interactions with potential ‘positive’ effects on native hosts in contrast to the usually perceived ‘negative’ effects (Harvell et al., Reference Harvell, Aronson, Baron, Connell, Dobson, Ellner, Gerber, Kim, Kuris, McCullum, Lafferty, McKay, Porter, Pascual, Smith, Sutherland and Ward2004; Kopp & Jokela, Reference Kopp and Jokela2007; Kelly et al., Reference Kelly, Paterson, Townsend, Poulin and Tompkins2009; Keesing et al., Reference Keesing, Belden, Daszak, Dobson, Harvell, Holt, Hudso, Jolles, Jones, Mitchell, Myers, Bogich and Ostfeld2010).
In conclusion, the experiments in this study have produced an extended inventory of dilutors, and indicate that there are many non-host species that interfere with the transmission of cercarial stages of H. elongata. Together with published data from other marine parasites and systems this suggests that trematode transmission can be interfered with by a multitude of organisms. However, despite increasing evidence of biotic factors interfering with parasite transmission pathways, there is still much that is unknown about how and under what conditions organisms remove the most parasites. Therefore, the future challenge is to determine the mechanisms which result in a reduction in disease risk caused by diluting organisms, and to understand the effects of whole communities on pathogen transmission pathways.
ACKNOWLEDGEMENTS
We would like to thank Hans Witte, Siem Gieles, Rob Dekker, Anouk Goedknegt and Andreas Wasser for their assistance with collecting the organisms. We also thank the two referees for their comments.