Introduction
The franciscana dolphin Pontoporia blainvillei (Gervais & d'Orbigny, 1844) is a small cetacean endemic to the western South Atlantic Ocean, with a distribution ranging from south-eastern Brazil (Siciliano et al., Reference Siciliano, Di Beneditto and Ramos2002) to Golfo Nuevo, Argentina (Crespo et al., Reference Crespo, Harris and Gonzalez1998). Franciscana dolphins are primarily coastal, inhabiting waters beyond the surf zone and out to the 30 or 50 m isobath, depending on the region (Crespo, Reference Crespo, Perrin, Würsig and Thewissen2009; Secchi, Reference Secchi, Wilson and Mittermeier2014). Furthermore, diet seems to play an important factor for franciscana dolphin distribution. For example, Gomez & Cassini (Reference Gomez and Cassini2015) when analysing different variables (e.g. sea surface temperature, salinity, prey availability) to estimate their effect on the species distribution, found that the prey Cynoscion guatucupa represented the most important variable. There is strong evidence that franciscana dolphin is not continuously present throughout its distribution in Brazil (Siciliano et al., Reference Siciliano, Di Beneditto and Ramos2002; Danilewicz et al., Reference Danilewicz, Zerbini, Andriolo, Secchi, Sucunza, Ferreira, Denuncio and Flores2012), and there are two hiatuses proposed on the south-eastern Brazilian coast (Amaral et al., Reference Amaral, Danilewicz, Zerbini, Di Beneditto, Andriolo, Alvares, Secchi, Ferreira, Sucunza, Borges-Martins and de Oliveira Santos2018). Due to considerable population level differences (both genetic and morphological), Secchi et al. (Reference Secchi, Danilewicz and Ott2003) divided the franciscana dolphin area of distribution into four Franciscana Management Areas (FMAs). The species is classified as ‘Vulnerable’ in the IUCN Red List of Threatened Species throughout its range (Zerbini et al., Reference Zerbini, Secchi, Crespo, Danilewicz and Reeves2017), principally as a consequence of incidental mortality in fisheries. Within the study area (Franciscana Management Area III), along the Rio Grande do Sul (RS) state coast – southern Brazil, this species has been experiencing unsustainable rates of bycatch in gillnets for at least four decades (Moreno et al., Reference Moreno, Ott and Danilewicz1997; Ott et al., Reference Danilewicz, Rosas, Bastida, Marigo, Muelbert, Rodríguez, Lailson-Brito, Ruoppolo, Ramos, Bassoi, Ott, Caon, Da Rocha, Catão-Dias and Secchi2002; Secchi et al., Reference Secchi, Danilewicz and Ott2003; Secchi & Fletcher, Reference Secchi and Fletcher2004; Prado et al., Reference Prado, Kinas and Secchi2013, Reference Prado, Mattos, Silva and Secchi2016, Reference Prado, Kinas, Pennino, Seyboth, Silveira, Ferreira and Secchi2021). At a regional level, franciscana dolphins are listed as ‘Critically Endangered’ in the study region (Rio Grande do Sul State, Decree No. 51.797/2014).
Franciscana dolphin females are larger than males and lactation lasts for around nine months (Danilewicz et al., Reference Danilewicz, Rosas, Bastida, Marigo, Muelbert, Rodríguez, Lailson-Brito, Ruoppolo, Ramos, Bassoi, Ott, Caon, Da Rocha, Catão-Dias and Secchi2002), with calves taking solid food from around their third month of age (Pinedo et al., Reference Pinedo, Praderi and Brownell1989; Danilewicz et al., Reference Danilewicz, Rosas, Bastida, Marigo, Muelbert, Rodríguez, Lailson-Brito, Ruoppolo, Ramos, Bassoi, Ott, Caon, Da Rocha, Catão-Dias and Secchi2002; Secchi, Reference Secchi, Wilson and Mittermeier2014). The majority of franciscana dolphin prey belong to three main taxonomic groups: fish (~80%), crustaceans (~10%) and molluscs (~10%) (Brownell, Reference Brownell1975; Pinedo, Reference Pinedo1982; Rivero et al., Reference Rivero, Bastida and Rodriguez2000; Rupil et al., Reference Rupil, Barbosa, Marcondes, de Carvalho and Farro2019). Previous dietary studies indicate that this dolphin preys predominantly upon bottom-dwelling juvenile teleosts, squid and shrimp (Ott, Reference Ott1994; Bassoi, Reference Bassoi1997; Oliveira et al., Reference Oliveira, Pinheiro and Rosas1998; Di Beneditto, Reference Di Beneditto2000; Campos et al., Reference Campos, Lopes, da Silva and Santos2020), and several studies suggest that the species is a generalist and opportunistic predator (e.g. Danilewicz et al., Reference Danilewicz, Rosas, Bastida, Marigo, Muelbert, Rodríguez, Lailson-Brito, Ruoppolo, Ramos, Bassoi, Ott, Caon, Da Rocha, Catão-Dias and Secchi2002; Cremer et al., Reference Cremer, Pinheiro and Simões-Lopes2012; Paso-Viola et al., Reference Paso-Viola, Denuncio, Negri, Rodríguez, Bastida and Cappozzo2014). Nevertheless, there are few inferences about intrapopulation prey preferences or diet specialization for franciscana dolphins (e.g. Bassoi, Reference Bassoi1997; Troina et al., Reference Troina, Botta, Secchi and Dehairs2016; Henning et al., Reference Henning, de Sa Carvalho, Pires, Bassoi, Marigo, Bertozzi and Araújo2017).
Intrapopulation foraging preferences can be attributed to differences between sexes (e.g. ecological sexual dimorphism; Shine, Reference Shine1989) and/or age groups (e.g. ontogenetic niche shifts; Polis, Reference Polis1984). Sexual differences in body size, as in the case of franciscana dolphins, distinct physiological demands e.g. pregnancy and lactation, and parental guidance of juveniles could lead to differences in diet among males and females. A common inference about age group (mature and immature individuals) or size class specificity in diet is that this separation may reduce exploitation competition for food (Polis, Reference Polis1984), and increase individual fitness by avoidance of competitive selection (Wrona et al., Reference Wrona, Davies and Linton1979). Thus, differences in body size and resource use may allow competitive coexistence both between gender and age groups (Keast, Reference Keast1977; Maiorana, Reference Maiorana1978), or increasing niche width of a species (Polis, Reference Polis1984). Investigating franciscana dolphin diet composition, preference and variation is crucial to fully understand its dietary requirements and foraging, dietary niche characterization and its role in community and ecosystem functioning (Bearhop et al., Reference Bearhop, Adams, Waldron, Fuller and MacLeod2004; Secchi, Reference Secchi, Ruiz Garcia and Shostell2010).
The aim of this research was to investigate spatio-temporal diet variation with emphasis on assessing if intraspecific differences in feeding regimes exist between sexes and sexual maturity stages of franciscana dolphins for two distinct geographic areas off the southern Brazilian coast.
Materials and methods
Franciscana dolphin data
Samples were collected from dolphins incidentally killed in coastal gillnet fishing operations from Rio Grande (32°08′S 52°05′W; N = 172) and from Tramandaí (29°58′S 50°07′W; N = 98) southern Brazil, between 1994 and 2000 (Figure 1). The division of the study region into southern and northern coastal sites was based on the fishing grounds used by the vessels from Rio Grande and Tramandaí, respectively (see Moreno et al., Reference Moreno, Tavares, Danilewicz, Ott and Machado2018 and Boffo & Reis, Reference Boffo and Reis2003). Data on location, date of capture event, sex, total body length and weight were recorded. Individuals were categorized as sexually mature or immature based on gross and histological examination of gonads (N = 102; Botta et al., Reference Botta, Secchi, Muelbert, Danilewicz, Negri, Cappozzo and Hohn2010), or inferred from total body length (sexually mature female >138.9 cm and sexually mature male >128.2 cm; N = 168; Danilewicz et al., Reference Danilewicz, Secchi, Ott and Moreno2000).
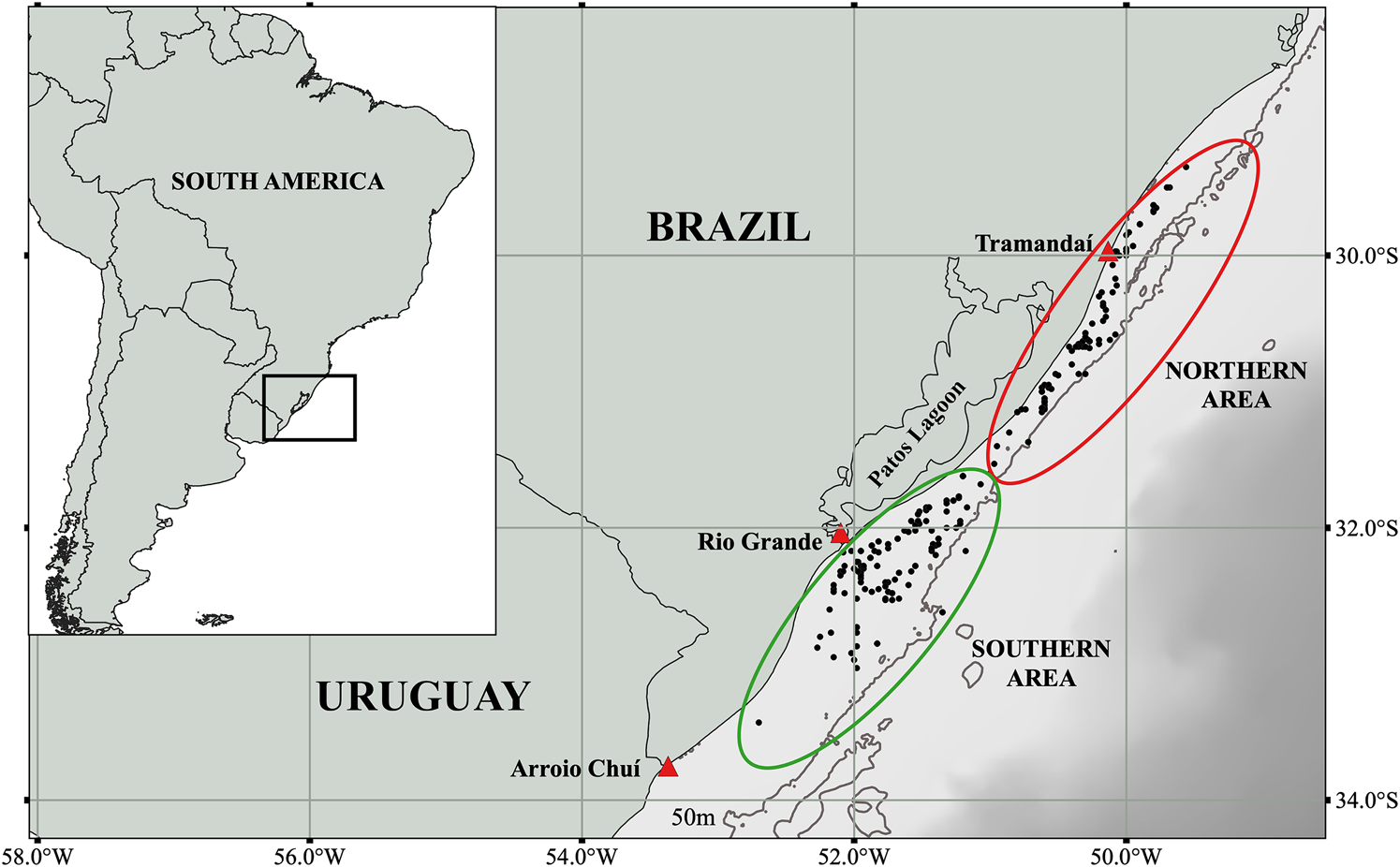
Fig. 1. Locations of franciscana dolphin (Pontoporia blainvillei) incidental by-catch events along the southern Brazilian coast (N = 270). The division of the study region into the northern (red ellipsoid) and southern (green ellipsoid) areas was based on the areas fished by the fishing vessels operating from Tramandaí and Rio Grande cities, respectively.
Sample data
The stomachs (including the main and pyloric chambers) were excised and opened for the diet analysis. The main contents analysed were otoliths, cephalopod beaks and crustacean remains (mainly cephalothorax). Otoliths and cephalopod beaks were measured with a microscope equipped with an ocular micrometer (0.1 mm scale). The total otolith length (LO) was considered as an estimate of the original size of fish prey species (total fish length). To estimate the original size of cephalopod species (mantle length), we measured the upper rostral beak length (URL) and lower rostral beak length (LRL) for squids and sepiolids; and upper hood beak length (UHL) and lower hood beak length (LHL) for octopuses. Reference collections of fish otoliths (Lucato, unpubl. data) and cephalopod beaks (Santos, Reference Santos1999) for the study area were available in the Laboratório de Recursos Pesqueiros Demersais e Cefalópodes, Universidade Federal do Rio Grande (FURG). The collections allowed correct prey identification and reliable regression equations to estimate the original length (mm) and weight (g) of the prey species at time of ingestion. The reconstructed size (mm) and weight (g) were calculated for 31 prey species. Specialists from FURG and Universidade Católica do Rio Grande do Sul identified crustacean specimens, but we were not able to reconstruct their size and weight data.
To assess the importance of prey items in the diet of franciscana dolphin we calculated the Index of Relative Importance using the formula: IRI = (% N + % W) * % O (Pinkas et al., Reference Pinkas, Oliphant and Iverson1971). Here, % N is the percentage of numerical abundance of a particular prey recovered from all stomachs; % W is the percentage of reconstructed weight by a particular prey; and % O the proportion of stomachs that contained this particular prey species, regardless of weight or abundance. IRI values considering all the prey ingested were calculated for the southern and the northern areas separately (see Table 1).
Table 1. Numbers of franciscana dolphin analysed in this study

Statistical analysis
To investigate prey species abundance according to franciscana dolphin diet groups (sex and sexual maturity stage), we used GLM as it is a flexible generalization of ordinary linear regression that allows for response variables that have error distribution models other than a normal distribution (Venables & Dichmont, Reference Venables and Dichmont2004). The response variables prey species number (non-normal counts) were analysed based on a Poisson error structure. The explanatory categorical data ‘sex’ with two-level factor (male and female), ‘sexual maturity stage’ with two-level factor (mature and immature), ‘area’ with two-level factor (northern and southern) and ‘season’ with a four-level factor, used the log-link function and a linear variance-mean relationship (Graphen & Hails, Reference Graphen and Hails2002). Final models' selection was based on a stepwise procedure using the Akaike Information Criterion (AIC) (Crawley, Reference Crawley2012). Analysis of deviance was performed to obtain the values of degrees of freedom, residuals, and P, to identify significant relationships from the models.
We assessed the relationships between reconstructed prey length and weight (response variables) and franciscana dolphin sex, maturity stage, season and area (northern and southern) using linear models (LMs). Log-transformations were performed when the residuals contravened the assumption of normality, and model selection was based on stepwise procedure using the AIC (Crawley, Reference Crawley2012). From the LMs, analysis of variance tables were used to obtain the values of degrees of freedom, residuals, F, and P, to identify significant relationships.
Rényi diversity profiles was chosen to rank prey species comparing franciscana dolphin groups according to diversity (Kindt & Coe, Reference Kindt and Coe2005), since these profiles by the Rényi series provide different diversity measures to compare equivalently the group's diet. This index was calculated using the vegan package (Oksanen et al., Reference Oksanen, Blanchet, Friendly, Kindt, Legendre, McGlinn, Minchin, O'Hara, Simpson, Solymos, Stevens, Szoecs and Wagner2019).
Results
Franciscana dolphin and stomach contents data
The number of franciscana dolphin sampled for each area, season and ontogenetic groups are shown in Table 1 (for 26 dolphins in the northern area, sexual maturity and/or gender could not be achieved). Only nine of the analysed stomachs were empty, including eight stomachs from lactating calves. A total of 13,354 otoliths, 12,248 cephalopod beaks and 182 remains of crustaceans (mainly cephalothorax) were identified. Fish and cephalopods were the most important prey groups in the diet of franciscana dolphin (see Table 2). Bottom-dwelling teleosts (26 species) were the most numerous among the fishes, whereas shelf-demersal squid (3 species) and shelf-benthic octopus (2 species) were the predominant cephalopods (Table 2).
Table 2. A summary of the prey species composition and relative importance of prey items (% N, % W, % O and IRI) of franciscana dolphin diet from southern and northern coastal regions of the study area

*(% N + % W)*(% O), Pinkas et al. (Reference Pinkas, Oliphant and Iverson1971). **Could be also considered as secondary ingestion or post-mortem invasion.
Analysis of reconstructed prey lengths, discriminated by species, show considerable variation (Table 3), with some ingested fish (e.g. Trichiurus lepturus) much larger than the mean fish size consumed by franciscana dolphin. Some species minimum sizes are only 4–6 mm in length, which could be part of the diet of the prey itself rather than direct intake by the dolphin. Nevertheless, this is still doubtful as in some events we could find many of these tiny otoliths and no bigger otoliths or any remains of bigger prey which would indicate the predator of these small fishes. The mean and median reconstructed fish lengths are estimated to be 96.37 and 59.98 mm, respectively (N = 4443), which represents juvenile fish (Haimovici, Reference Haimovici1997a). The range of lengths was similar for both the northern and southern study areas. In contrast, the reconstructed cephalopod mantle lengths represent adult individuals (Santos, Reference Santos1999) (mean = 105.48 mm, median = 105.77 mm, N = 6192), within 97% of the beaks from the common squid (Doryteuthis sanpaulensis).
Table 3. Analysis of prey species reconstructed lengths (mm) and weights (g) ingested by franciscana dolphins off the southern Brazilian coast (SE = standard error of the mean, SD = standard variation)

GLMs and LMs
GLM revealed most of the highly significant differences of prey species number were related to spatial and temporal explanatory variables (Table 4). Hence, the comparisons of intrapopulation groups (i.e. sex, sexual maturity stage) were made only within each study site (northern or southern), mitigating potential biases resulting from spatial differences. Moreover, season has a very important influence on diet variation (see Table 4). There were few significant differences between sexual maturity stages, and no significant differences in prey species numbers ingested between males and females in both study areas. Contrariwise, the linear models for reconstructed prey length and weights between the study areas, sex and sexual maturity showed significant differences for 86% of the analyses (Table 5). A detailed description of the significant results according to each explanatory variable (geographic, temporal and ontogenetic) is presented below.
Table 4. List of generalized linear models (GLMs) and the analysis of deviance according to study area (northern, southern), sex (male, female), sexual maturity stage (mature, immature), and seasonality (summer, autumn, winter and spring) for the numerical abundance of prey species recovered from the stomach contents of franciscana dolphins by-caught on the southern Brazilian coast

Table 5. List of linear models (LM) and the analysis of variance according to study area (northern, southern), sex (male, female), sexual maturity (mature, immature), and seasonality (summer, autumn, winter and spring) for reconstructed fish and cephalopod prey lengths (mm) and weights (g)

The estimated effects are average responses due to the given treatment combinations, having adjusted for all model terms. For instance, the interaction effects are changes in response after adjusting for the grand mean and both main effects.
Dietary variation by area
The main fish species in the diet of franciscana dolphin differed between the areas, with Stellifer rastrifer being the most important fish for northern and Cynosion guatucupa for the southern diet (see Tables 2 and 4). Southern animals had eaten larger and heavier cephalopods than northern franciscana dolphins, while the opposite was the case for fish (Table 5).
Dietary variation by season
The southern area showed twice as many significantly different prey species frequencies through seasons as the northern area (Table 4). Not surprisingly, the importance of warm water related fish and cephalopod species (e.g. Trichiurus lepturus, Peprilus paru, Argonauta nodosa) and cold water species (e.g. Anchoa marinii, Family Engraulidae, Doryteuthis sanpaulensis) were different through the seasons. The biggest fish (lengths and weights) ingested were found in the summer season, and the smallest fish were from winter months in both areas. This finding is mainly influenced by the bigger fish prey T. lepturus (see Table 3), with higher occurrence in summer and autumn, decreasing considerably in the winter. Additionally, the biggest cephalopods were consumed during spring and summer months equally between the areas. Hence, seasonal results of prey species sizes for both areas are similar, with bigger prey being consumed by franciscana dolphins during warmer months.
Dietary variation by sex
GLM analyses revealed that there were no differences in prey species preferences between male and female individuals for each site (Table 4), and Rényi index profiles exhibited high diversity similarity (Figure 2). Nevertheless, there were differences in reconstructed lengths and weights of cephalopod and fish specimens consumed by males vs females (Table 5). Females had consumed longer and heavier cephalopods and fish in the northern area than males, while the males had ingested longer and heavier cephalopods in the south (Figure 3). Moreover, considering males and females for the whole study area, there were no significant differences in the estimated mass of prey ingested (all samples, P < 0.001).

Fig. 2. Prey species richness and diversity profiles of Rényi comparing the franciscana dolphin groups (sex and sexual maturity). The Rényi diversity profile at scale 0 reflects species richness, at scale 1 the Shannon index, at scale 2 the Simpson index and at scale Inf the Berger–Parker index (the dominance of the most abundant prey species).

Fig. 3. Ontogenetic variation in reconstructed lengths of prey items (cephalopod beaks = 6192 and fish otoliths = 4443) recovered from the stomachs of by-caught franciscana dolphins from the two study areas (north = 98, south = 172) off the southern Brazilian coast. The horizontal line in the interior of the box is located at the median of the data. The height of the box is equal to the interquartile range, and the outliers are drawn individually, indicated as circles. All the points more than 1.5*(Inter-Quartile Range) are considered outliers.
Dietary variation by sexual maturity stage
Variation in the diet between sexually immature and mature franciscana dolphins was observed. The main fish species (C. guatucupa and S. rastrifer) and shrimp specimens ingested differed significantly between maturity stages in both the northern and southern areas (Tables 4 and 6). Prey species richness is higher for immature dolphins, as the importance of the main prey species ingested (Figure 2). Although sexually mature dolphins consumed a lower diversity of species (Figure 2), the ingested prey specimens had greater reconstructed lengths and weights (all prey, df = 4394, F = 457.9, P < 0.001, see Figure 3). For cephalopods, it was only in the north that sexually mature animals consumed larger prey than immature animals (Figure 3).
Table 6. A summary of the prey species composition and relative importance of prey items (N %, W %, O % and IRI) for sexual mature and immature individuals from southern and northern coastal regions of the study area

Discussion
Dietary variation by area
The study region is influenced by the Brazil–Malvinas Confluence (BMC), an important confluence of two distinct cold (Malvinas/Falklands) and warm (Brazil) currents, as well as variability in freshwater discharges from Patos Lagoon and subsurface upwellings (Piola et al., Reference Piola, Campos, Möller, Charo and Martinez2000; Möller et al., Reference Möller, Piola, Freitas and Campos2008). An important feature of the BMC is that its position oscillates seasonally and geographically along the study area, affecting the north and south coastal regions differently, which in turn influences the distribution and abundance of many franciscana dolphin prey species (Haimovici, Reference Haimovici1997a, Reference Haimovici1997b). Consequently, the most important prey of franciscana dolphin differed between the northern and southern regions of the study area, which was also reported in other studies comparing neighbourhood sites and franciscana sub-populations distributed in marine and estuarine areas in Argentina (Rodrígues et al., Reference Rodríguez, Rivero and Bastida2002; Denuncio et al., Reference Denuncio, Viola, Machovsky-Capuska, Raubenheimer, Blasina, Machado, Polizzi, Gerpe, Cappozzo and Rodriguez2017). Despite the fact that these are adjacent areas and some diet differences also include cephalopod beak presence and absence (and these structures can remain months in the stomach), it seems that the animals are not swimming to distant areas. However, these dietary geographic differences have been discussed before suggesting that franciscana dolphin may occupy, and possibly for long periods, small spatial ranges (Bordino et al., Reference Bordino, Siciliano, Bastida and Cremer2002, Reference Bordino, Wells and Stamper2008; Bassoi et al., Reference Bassoi, Shepherd, Secchi, Moreno and Danilewicz2020), suggesting some degree of residency (Crespo et al., Reference Crespo, Pedraza, Grandi, Dans and Garaffo2010).
Seasonal diet variation
The demersal ichthyofauna of the southern Brazilian shelf is transitional between tropical and temperate zones and variation of species richness and relative abundance follows seasonal temperature variations in coastal waters (>10°C) (Haimovici et al., Reference Haimovici, Martins and Vieira1996). The results of this study confirm this seasonal variation, and it is possible that the southern region could be more influenced by colder water masses than the northern area (see Table 4). Warmer seasons seem favourable for franciscana dolphins to feed upon bigger prey such as T. lepturus and cephalopods that were clearly the items that had contributed most to their diet during this period. The greater ingestion of prey biomass in warmer seasons could be explained by the needs to increase fat reserves (blubber), as energy storage for colder seasons. These diet results corroborate studies on the concentration of total lipid in the blubber of franciscana dolphins, being higher for summer/spring, 357.4 mg g−1, than in autumn/winter, 318.6 mg g−1 (Caon & Kucharski, Reference Caon and Kucharski2000).
Sex-related diet variation
Prey species diversity identified from stomach contents of male and female franciscana dolphins were similar in both areas (see Figure 2), as observed using stable isotope analysis (Troina et al., Reference Troina, Botta, Secchi and Dehairs2016). We did not find significant differences of prey species abundance ingested by males and females (see Table 2), but there were disparities in some species occurrence (see Table 7). Males from the northern coast appear to consume a higher number of species related to warm water masses from the Brazil Current (Haimovici, Reference Haimovici1997a, Reference Haimovici1997b), such as S. rastrifer and the cephalopods Doryteuthis plei and Argonauta nodosa. This is possibly related to the larger sample size of males, both in the northern and southern areas, during the warmer periods. This could also explain the ingestion of significantly larger cephalopods (by mantle length and weight) by males from the southern area (Haimovici & Perez, Reference Haimovici and Perez1991; Santos, Reference Santos1999). Females in both study areas also ingested shrimp species more frequently, which is in agreement with previous franciscana dolphin studies (Pinedo, Reference Pinedo1982; Ott, Reference Ott1994; Bassoi, Reference Bassoi1997; Henning et al., Reference Henning, de Sa Carvalho, Pires, Bassoi, Marigo, Bertozzi and Araújo2017), and possibly related to parental guidance of juveniles/immature individuals, which show a high prevalence of shrimps in their diet (Bastida et al., Reference Bastida, Rodríguez, Moreno, Pérez, Marcovecchio and Gerpe1992; Smith & Read, Reference Smith and Read1992; Danilewicz et al., Reference Danilewicz, Rosas, Bastida, Marigo, Muelbert, Rodríguez, Lailson-Brito, Ruoppolo, Ramos, Bassoi, Ott, Caon, Da Rocha, Catão-Dias and Secchi2002; Rupil et al., Reference Rupil, Barbosa, Marcondes, de Carvalho and Farro2019; this study). Furthermore, crabs (e.g. Loxopagurus loxocheles, Dardanus insignis, Pleocyemata) occurred more frequently in females, all of which are benthic species abundant in shallow coastal waters (Capítoli, Reference Capítoli1997). In terms of general prey numerical abundance, females consumed more fish than males, however, smaller fish specimens (recruits) were similar to the prey size ingested by immature dolphins. Overall, the findings of this study suggest that sexually mature females may be distributed closer to the coast than sexually mature males, at least in some periods (e.g. austral summer), as a result of juvenile guidance.
Table 7. A summary of the prey species composition and relative importance of prey items (% N, % W, % O and IRI) for male and female individuals from southern and northern coastal regions of the study area

The reconstructed mass of all prey combined was similar for both males and females in the whole study area. Female dolphins have greater body lengths and extra demands for energy during pregnancy and lactation, but notably adult males had similar values of total prey biomass. This suggests that males, in spite of their smaller body mass, might be consuming more prey biomass than females. Swimming and travelling could be an extra demand for energy, as suggested previously for males. This finding is consistent with total lipid concentrations in franciscana dolphin blubber in southern Brazil, which are higher in males (369.3 mg g−1) than females (296.9 mg g−1) (Caon & Kucharski, Reference Caon and Kucharski2000). Therefore, it might be possible that the males are using a different habitat for feeding than females, where males' movements would not be so restricted to the coast as females, or they could be swimming to deeper areas or further along the coast. These differences, however, should be further investigated.
Dietary variation by sexual maturity stage and prey size selection
Overall, sexually immature franciscana dolphins ingested smaller prey than sexually mature individuals (see Figure 3). The observed differences in reconstructed fish prey size (length and weight) at time of ingestion between maturity groups is likely to be a result of young animals consuming more juvenile fishes, and sexually mature animals consuming more of the subadult and adult size fishes. The majority of small teleosts consumed by immature dolphins were estimated to be the size typical of densely schooling demersal recruits (<50 mm) distributed throughout the coastal continental shelf off southern Brazil (Haimovici et al., Reference Haimovici, Martins and Vieira1996; Martins & Haimovici, Reference Martins and Haimovici2016; Pio et al., Reference Pio, Pezzuto and Wahrlich2016). Thus, it seems that immature franciscana dolphins are not only ingesting the most abundant and available resource in the area as well as easier prey to catch than marine shrimps, (Dall et al., Reference Dall, Hill, Rothlisberg and Sharples1990) one of the first prey consumed by immature dolphins.
In contrast, sexually mature franciscana dolphins appear to be feeding more selectively, consuming larger fish prey with greater biomass. Other studies demonstrated franciscana dolphin predation on a variety of teleosts with mean total length greater than 150 mm, representing the fish subadult sizes for many species (Ott, Reference Ott1994; Rivero et al., Reference Rivero, Bastida and Rodriguez2000; Bittar & Di Beneditto, Reference Bittar and Di Benedito2009; Machado et al., Reference Machado, de Oliveira, Ott, Haimovici, Cardoso, Milmann, Romero, dos Santos and Borges-Martins2020). An example is T. lepturus, which had a mean length of over 400 mm (subadults), and various specimens greater than 700 mm, categorized as adult size (Martins & Haimovici, Reference Martins and Haimovici1997). Overall, it seems likely that franciscana dolphins eat more of the most available prey in the area (Bassoi & Secchi, Reference Bassoi and Secchi2000), in many cases recruit teleosts (Haimovici et al., Reference Haimovici, Martins and Vieira1996), but when subadult and adult fish are also abundant (>100 mm, e.g. T. lepturus, Urophycis brasiliensis), adult dolphins appear to select such prey.
Additionally, franciscana dolphins do appear to select larger squid, because the average size of squid preyed on by this dolphin (106.5 mm, see Table 3) was higher than those found during research surveys in this area, especially in summer, where the mantle length average was 58.4 mm (Andriguetto & Haimovici, Reference Andriguetto and Haimovici1991; Santos, Reference Santos1999). Paso-Viola et al. (Reference Paso-Viola, Denuncio, Negri, Rodríguez, Bastida and Cappozzo2014) found that franciscana dolphin, mainly adult animals, do appear to select larger squid because most cephalopods consumed in southern Buenos Aires were mature individuals (>110 mm), and franciscana dolphins in the Babitonga Bay estuary (Brazil) ingested bigger cephalopods than the larger co-occurring Guiana dolphins (Cremer et al., Reference Cremer, Pinheiro and Simões-Lopes2012). In conclusion, franciscana dolphins have fairly opportunistic behaviour in terms of prey abundance and occurrence, although for some prey species the dolphin has a preference for bigger specimens, mainly selected from adult individuals.
Ontogenetic shifts in diet
Ontogenetic dietary differences may be the result of changing foraging or physiological abilities (Frainer et al., Reference Frainer, Huggenberger and Moreno2015), the relative importance of energy intake (Hin et al., Reference Hin, Harwood and de Roos2019) or differences in experience (i.e. culture). The prey consumed by sexually mature individuals in this study suggest active foraging behaviour and high mobility (Clarke, Reference Clarke1997; Santos & Haimovici, Reference Santos and Haimovici2001), and feeding on the larger individuals in the spawning grounds allows the predator to take protein at its maximum production and concentration (Clarke, Reference Clarke1997). Further, studies on caloric values of franciscana dolphin prey have shown that squid have greater gross energy content than fish (Di Beneditto et al., Reference Di Beneditto, dos Santos and Vidal2009). Energy requirements are higher for larger individuals, but the variations of feeding regimes between sexual maturity categories could also indicate a degree of adaptation to minimize prey competition within the population (Nikolsky, Reference Nikolsky1963). Minimizing interspecific competition for prey is also suggested by Teixeira et al. (Reference Teixeira, Botta, Daura-Jorge, Pereira, Newsome and Simões-Lopes2020) showing lower niche overlap between franciscana dolphins and two other coastal dolphin species.
In terms of physiological ability, the maturation of the echolocation system of young individuals is likely to be related to their prey preferences (Frainer et al., Reference Frainer, Huggenberger and Moreno2015), as well their experience in detecting prey. Passive listening would increase efficiency of capturing soniferous fish species, therefore, franciscana dolphins may obtain useful information by listening, such as prey identification and location, body size and number of fish present (Tellechea et al., Reference Tellechea, Perez, Olsson, Lima and Norbis2017), as was described for common bottlenose dolphins (Tursiops truncatus) in Florida (USA) (Barros & Wells, Reference Barros and Wells1998; McCabe et al., Reference McCabe, Gannon, Barros and Wells2010). This foraging behaviour would likely increase in efficiency as the dolphin ages, with sexually mature individuals being more experienced in both tracking and capturing their prey.
In summary, dietary variations between sex and sexual maturity groups of franciscana dolphin could indicate that these groups select certain prey types over others, potentially as a result of (1) ability to capture particular prey, (2) a degree of adaptation to minimize prey competition within the population, (3) differential use of habitat and/or (4) other unknown pattern of niche segregation as described for other marine species (e.g. Phillips et al., Reference Phillips, Silk, Phalan, Catry and Croxall2004).
Acknowledgements
We are indebted to Brazilian fishermen from Rio Grande, Tramandaí/Imbé and Itajaí who provided samples for this work. We thank all the staff of the Ecologia e Conservação da Megafauna Marinha Laboratory and Museu Oceanográfico ‘Prof. Eliézer C. Rios’ at the Universidade Federal do Rio Grande-FURG, the Grupo de Estudos de Mamíferos Aquáticos (GEMARS) and the Centro de Estudos Costeiros Limnológicos e Marinhos (CECLIMAR/UFRGS Litoral) for their assistance and support. We particularly thank Jorge Acevedo and two anonymous reviewers, whose comments greatly improved the manuscript content.
Financial support
The Conselho Nacional de Desenvolvimento Científico e Tecnológico-CNPq (Brazil) and University of Southampton (UK) provided financial support for M.B. (200404/ 01-6). CNPq also provided a Research Fellowship to ERS (PQ 310597/2018-8) and funding (Edital Universal, Grant n. 426502/2018-0). The Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) provided access to the Portal de Periódicos. Funding for fieldwork and data analysis were provided by Yaqu Pacha (Germany). This study is within the scope of the Projeto Toninhas do Sul/Ecomega-FURG/GEMAR/NEMA, apoiado pelo Fundo Brasileiro para a Biodiversidade (FUNBIO) and is a contribution of the Research Group Ecologia e Conservação da Megafauna Marinha (EcoMega/CNPq).












