INTRODUCTION
The gladius is the internal modified shell in squids of the orders Myopsida and Oegopsida. It originates during embryonic development located in the dorsal midline of the mantle. Throughout life it grows continuously through the accretive deposition of β-chitin in association with proteins (Hunt & Nixon, Reference Hunt and Nixon1981) and is composed of outer, intermediate and inner layers (Arkhipkin et al., Reference Arkhipkin, Bizikov and Fuchs2012). Regular growth increments have been observed in all of these layers and were considered of potential value for age and growth studies (LaRoe, Reference LaRoe1971; Arkhipkin & Bizikov, Reference Arkhipkin, Bizikov, Jereb, Ragonese and Boletzky1991; Bizikov, Reference Bizikov, Jereb, Ragonese and Boletzky1991; Jackson et al., Reference Jackson, Arkhipkin, Bizikov, Hanlon, Okutami, O'Dor and Kubodera1993; Perez et al., Reference Perez, O'Dor, Beck and Dawe1996, Reference Perez, Aguiar and Santos2006; Zaleski, Reference Zaleski2010).
In squids of the families Ommastrephidae and Loliginidae, the gladius structure is mainly formed by the intermediate layer that grows longitudinally and correlates highly with the mantle length growth (Bizikov, Reference Bizikov, Jereb, Ragonese and Boletzky1991; Jackson et al., Reference Jackson, Arkhipkin, Bizikov, Hanlon, Okutami, O'Dor and Kubodera1993). In some species (e.g. Sthenoteuthis ovalaniensis, Illex argentinus, Illex illecebrosus, Doryteuthis plei and Lolliguncula brevis) growth increments of the intermediate layer have been demonstrated to be daily deposited and their width regarded as proxies for somatic growth (Arkhipkin & Bizikov, Reference Arkhipkin, Bizikov, Jereb, Ragonese and Boletzky1991; Bizikov, Reference Bizikov, Jereb, Ragonese and Boletzky1991; Perez et al., Reference Perez, O'Dor, Beck and Dawe1996, Reference Perez, Aguiar and Santos2006; Zaleski, Reference Zaleski2010). Because cephalopod growth rates are influenced by body size, feeding rates and temperature (Forsythe & van Heukelem, 1987), growth rate variability, as assessed by the reconstruction of gladius growth, integrates the effects of size (absolute growth) and environmental conditions (e.g. temperature and food availability) experienced by individual squid during a certain period of its lifetime (Perez & O'Dor, Reference Perez and O'Dor1998, Reference Perez and O'Dor2000). In that sense the gladius analysis can and has been used to describe life history events and to address ecological and population processes (see review in Arkhipkin & Perez, Reference Arkhipkin, Perez, Rodhouse, Dawe and O'Dor1998).
Particularly interesting has been the application of this technique to understand early life ecology of a highly migratory ommastrephid squid, Illex illecebrosus in the north-west Atlantic. Paralarvae occur in the tropical waters off the coast of Florida and are transported northwards by the Gulf Stream to recruit in the continental shelf off Nova Scotia and Newfoundland. During that process individuals undergo important life history transitions and experience sharp temperature and food gradients as determined by the Gulf Stream and shelf/slope fronts (O'Dor, Reference O'Dor and Boyle1983). Gladius growth was shown to respond to food and temperature variability in the laboratory (Perez et al., Reference Perez, O'Dor, Beck and Dawe1996). More importantly, in wild population samples, the acceleration in gladius growth changed during early life transitions and growth conditions are favoured as juveniles attained more productive areas in the shelf/slope front (Perez & O'Dor, Reference Perez and O'Dor2000).
In the south-west Atlantic another ommastrephid, Illex argentinus displays a similar life history pattern combining spawning migrations to the transport of the Malvinas/Falkland Current and the dynamics of the Subtropical Convergence (Hatanaka et al., Reference Hatanaka, Kawahara, Uozumi and Kasahara1985). The species is distributed from Rio de Janeiro to Southern Argentina, and important concentrations occur on the continental shelf and slope waters south of 35°S in the Patagonian Shelf and around the Falkland/Malvinas Islands (Brunetti et al., Reference Brunetti, Ivanovic, Elena and Boschi1998). Within that distribution range, life-history attributes have been found to vary in space and time and several geographically- and seasonally-established population groups have been differentiated (see review in Haimovici et al., Reference Haimovici, Brunetti, Rodhouse, Csirke, Leta, Rodhouse, Dawe and O'Dor1998).
Off Brazil, a ‘tropical/subtropical’ subset of these population groups has long been described, and recently targeted by the fishing industry (Santos & Haimovici, Reference Santos and Haimovici1997; Perez et al., Reference Perez, Silva, Schroeder, Schwarz and Martins2009). In contrast with I. illecebrosus and the ‘temperate’ Patagonian population groups of I. argentinus, these squids have generally been characterized by a shorter life span (~6 months) with paralarvae, juvenile and adults concentrating in the continental shelf, shelf break and slope respectively (Haimovici et al., Reference Haimovici, Brunetti, Rodhouse, Csirke, Leta, Rodhouse, Dawe and O'Dor1998; Schwarz & Perez, Reference Schwarz and Perez2012). Seasonal growth variation during early life have been evidenced by the analysis of gladius growth patterns and were associated with a year-round spawning and the environmental fluctuations of the shelf break/slope waters off southern Brazil (Schroeder & Perez, Reference Schroeder and Perez2010; Schwarz & Perez, Reference Schwarz and Perez2012). In the present study these patterns are further explored to assess size- and age-dependent life history events in the shelf break-slope waters off southern Brazil. A preliminary analysis was presented by Bizikov (Reference Bizikov, Jereb, Ragonese and Boletzky1991) and involved specimens collected in Argentine Shelf and slope waters. This study addresses the patterns of growth in a tropical–subtropical environment at the northern extreme of the species distribution range in the south-west Atlantic.
MATERIALS AND METHODS
Biological samples and fishery data
Biological samples of Illex argentinus were obtained from the catches of 34 trawlers that operated off the Brazilian coast between 22–33°S and 45–722 m depth from 2001 to 2008. Part of the examined samples was collected from landings of national trawlers at the harbours of Santa Catarina State (south Brazil) (Perez et al., Reference Perez, Lucato, Andrade, Pezzuto and Rodrigues-Ribeiro1998). Information on the fishing area, effort (mean trawl duration, number of trawls per day and trip duration) and total catch were obtained during interviews with skippers at the time of the landings. Additional samples were obtained during commercial fishing operations and trawl surveys conducted by the international trawl fleet and research vessels, respectively (Table 1). These had information on trawl position (latitude–longitude–depth), date, time and fishing effort (trawling hours) and were deep-frozen for later analysis in the laboratory.
Table 1. Gladii of Illex argentinus captured in south-southern (22–33°S) Brazil used in the evaluation of age and growth studies. N IND, individuals of indeterminated sex; N ♂, males; N ♀, females; GL, gladius length range.
In the laboratory, mantle length (ML) and body weight (BW) were recorded to the nearest millimetre and gram, respectively. After dissection of the mantle, gladius and statoliths were both extracted. Gladius length was measured in millimetres (GL) and stored in plastic bags with formalin (4%). Statoliths were stored in ethanol 70% (see Schroeder & Perez, Reference Schroeder and Perez2010 and Schwarz & Perez, Reference Schwarz and Perez2010 for details). Males and females were differentiated and maturity stages were assigned according to the macroscopic scale proposed by Brunetti (Reference Brunetti1990). This scale defined seven and eight maturity stages for males and females respectively including: immature (Stages I and II); in maturation (Stage III); early maturity (Stage IV); advanced maturity (Stage V); spawning (Stage VI for males and Stages VI and VII for females); and spent (Stage VII for males and Stage VIII for females).
Gladius growth interpretation
The preparation and interpretation of gladius growth increments followed the procedures previously defined by Perez et al. (Reference Perez, O'Dor, Beck and Dawe1996) for I. illecebrosus. Gladii were washed in clean water and dried in paper tissues. Growth increments were observed directly on the gladius plate (intermediate layer) over the central rachis using a dissecting microscope (40×) connected to an image analysing system (Image-Pro Plus Media Cybernetics®). Increments were identified in the anterior, most recently deposited end (head) and counted backwards, towards the posterior end (fins) (Figure 1) until the increments become faint due to overgrowth (Perez et al., Reference Perez, O'Dor, Beck and Dawe1996). Readability of both the entire structure and along the gladius length was assessed. The relation between gladius and somatic growth was tested by fitting linear and power models to MLxGL and BWxGL relationships, respectively, using the least squares method.

Fig. 1. Schematic representation of the series of growth increments (GINC) measured over the dorsal surface of the gladius and the back-calculation of gladius growth.
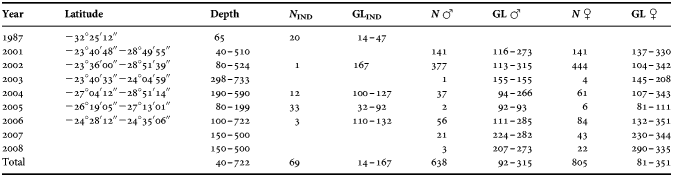
The accuracy of growth increments counts were obtained from a comparison among three independent counts in a sub-sample of 30 gladii conducted by three different readers. The consistency of the counts was estimated by calculating the coefficient of variability (CV; Chang, Reference Chang1982), the average percent error (APE.; Beamish & Fournier, Reference Beamish and Fournier1981) and by a one-way analysis of covariance (ANCOVA) between counts. Each ANCOVA was preceded by a test of homogeneity of slopes (Zar, Reference Zar1984).
The periodicity of gladius growth increment was investigated comparing the number of increments deposited in statolith and gladius of 84 individuals varying from 174 to 330 mm GL. This procedure assumed that statolith rings are daily deposited as demonstrated by Uozumi & Shiba (Reference Uozumi, Shiba, Okutani, O'Dor and Kubodera1993) and Sakai et al. (Reference Sakai, Brunetti, Ivanovic, Elena and Nakamura2004). The statoliths were prepared following procedures described in Schwarz & Perez (Reference Schwarz and Perez2010) and growth rings were examined under a microscope at 1000× magnification. In each squid, the number of statolith growth rings was compared to the number of gladius increments deposited anterior to 170 mm of the gladius length. A linear regression fitted to this data and the Ho: slope = 1 was tested using a t-test with 5% of significance (Zar, Reference Zar1984). If this hypothesis was statistically acceptable, it would imply that both structures deposited growth increments with the same periodicity.
Absolute growth reconstruction
Individual absolute growth was reconstructed measuring the distance between consecutive growth increments visualized (Figure 1) where the distance between one mark and the next correspond to one gladius growth increment (GINC). A series of growth increments, obtained from the most recently deposited at the anterior border backwards to the posterior sectors of the gladius plate, were submitted to a ‘low-pass’ filter in order to eliminate sharp discrepancies between following increments, normally produced by reading errors. In formula 1, each filtered growth increment (GINC′) resulted from a weighted average among three consecutive growth increments (GINC) measured on the gladius:
where, GINCi, is the inner filtered increment and GINCi-1 and GINCi+1 is the immediately anterior and posterior increments respectively where each filtered increment (GINC′) represented the absolute growth during one day (Perez et al., Reference Perez, O'Dor, Beck and Dawe1996). The length of each squid gladius at previous ages (GL′) was back calculated by subtracting its GL by all its visible increments. A mean absolute growth trajectory was reconstructed using the average GINC′ per 1 mm interval of GL′ reconstructed for all individuals and for males and females separately. The slopes of the absolute trajectories were compared between sexes by an ANCOVA that was preceded by a test of homogeneity of slopes.
Age-dependent growth
A growth curve was reconstructed from the cumulative number of growth increments counted in each centimetre of gladius length interval following Perez et al. (Reference Perez, O'Dor, Beck and Dawe1996). This procedure required: (1) the assumption of a daily deposition of gladius growth increments; and (2) that age of squid of 10 mm GL (the size at which gladius growth increments began to be seen) might be either 30 or 40 or 60 days, according to different results of statolith readings in juveniles of a sibling species, Illex illecebrosus (Hurley & Beck, Reference Hurley and Beck1979; Morris & Aldrich, Reference Morris and Aldrich1985; Balch et al., Reference Balch, Sirois and Hurley1988) and Illex argentinus (Arkhipkin & Scherbich, Reference Arkhipkin and Scherbich1991).
RESULTS
Gladius growth interpretation
A total of 1512 gladii were examined including juveniles (N = 69), with a mode in 70 mm GL, maturing and mature males (N = 638), with a more pronounced mode in 160 and a less evident one in 220 mm GL and females (N = 805), that presented a strong mode in 210 and a secondary between 290 to 310 mm GL) (Table 1). The 100–240 mm GL size-range included males and females in all gonad stages and above 200–240 mm GL mature squid predominated in both sexes (Figure 2). These were distributed in two modal groups, i.e. small female spawners (140–240 mm GL) and large female spawners (240–360 mm GL).

Fig. 2. Size-frequency and maturity stage distribution of males and females used in this work.
Linear and power models adequately described the relationship established between GLxML and GLxBW, respectively (Figure 3). The analysis of the residuals generated by the fitted models revealed that the MLxGL (Figure 3B) relationship achieved homoscedasticity, therefore, the variance of GL remained constant while ML increased. However, in the relationship GLxBW residuals showed heteroscedasticity, where variance of BW increased with an increase on GL. This increase was pronounced in individuals larger than 250 mm GL (Figure 3D), and the fitted model poorly explained the variability of the large individuals.

Fig. 3. Relationship between mantle length (ML), gladius length (GL) and body weight (BW): (A) the linear relationship found (ML = 1.70 + 0.99 (GL) (r2 = 0.98)); (B) residuals; (C) the power relationship found (BW = 6E-05 (GL)2.7876 (r2 = 0.95); (D) residuals.
Growth increments were readable in 99% of the extracted gladii, and could be counted on the anterior half (57 ± 8%) of its length. Increments deposited in the early posterior region of the gladius plate were faint and could not be enumerated. Values of APE and CV found among three distinct readers were, respectively, 8.04 and 10.84%.
The ANCOVA applied did not find significant differences among readers (Table 2) reinforcing the accuracy of the counts. Because gladius counts for each squid included all increments deposited anterior to 170 mm GL, this assumption predicts that the fitted line should intercept the y axis at an age estimate for squids of 170 mm GL in statoliths counts. The slope obtained (b = 0.99) was not significantly different from the hypothesized slope = 1, and the intercept of the fitted line was 160.14 statolith rings. The number of statolith increments estimated for I. argentinus of 170 mm GL analysed in other studies in Brazilian waters (Schwarz & Perez, Reference Schwarz and Perez2010) was 164 increments (Schwarz, personal communication) which approximate the regression's intercept. Moreover, the accretion of 20 growth rings in statoliths is followed by 20 gladius growth increments, which suggests that both structures deposit growth increments with the same periodicity, probably daily (Figure 4).

Fig. 4. Linear relationship between growth rings counted in statolith and growth increments enumerated anterior to 170 mm gladius length (GL) in gladius of 84 individuals. The solid line represents the linear model fitted to the data set.
Table 2. Homogeneity of slopes and analysis of covariance (ANCOVA) tests for regressions between readers (factors). Reader 1 was used as covariate. Significant values of P are printed in bold. Degrees of freedom (DoF), mean squares (MS), and F ratios are also indicated.
Absolute growth
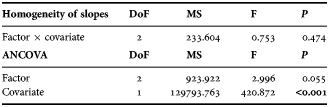
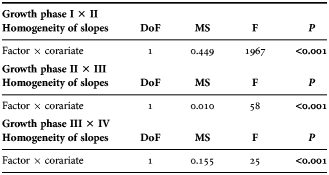
The examined material allowed the reconstruction of mean gladius growth rate variability along almost the entire size-range of the species (4–347 mm GL) (Figure 5A). Four growth phases were delimited by three growth discontinuities at 79 mm, 213 mm and 294 mm GL. The first three phases occupied together 85% of the GL range. These phases, however, could be differentiated by noticeable decreases in the acceleration of growth rates (principally from I to II) as confirmed by a test of homogeneity of slopes (ANCOVA) conducted for grouped sexes (Table 3). In the last phase at GL over 294 mm, absolute growth rate variability increased and stabilized at 1.43–1.99 mm day−1 (Figure 5A).

Fig. 5. (A) Mean absolute growth curves reconstructed for males and females and the growth phases identified. i, ii, iii are the growth intervals compared between males and females; (B–D) are the adjusted means for males and females in i, ii and iii, respectively.
Table 3. Homogeneity of slopes and analysis of covariance tests for regressions between growth phases for grouped sexes (factors). Gladius length was used as covariate. Significant values of P are printed in bold. Degrees of freedom (DoF), mean squares (MS), and F ratios are also indicated.
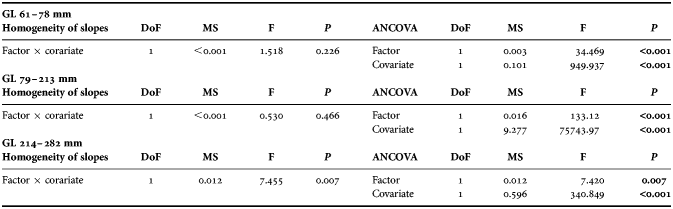
Males and females presented different gladius growth patterns during the reconstructed period, as confirmed by the test of homogeneity of slopes (ANCOVA) (Figure 5A; Table 4). Males and females absolute growth patterns started to differ at around 60 mm GL. Until 213 mm GL, males exhibited absolute growth rates significantly higher than females (Figure 5B and C). Between 214 and 282 mm GL this pattern was inverted, with females continuing to grow fast while males passed to grow less accelerated from this size (Figure 5D).
Table 4. Homogeneity of slopes and analysis of covariance (ANCOVA) tests for regressions between sexes (factor). Gladius length was used as covariate. Significant values of P are printed in bold. Degrees of freedom (DoF), mean squares (MS), and F ratios are also indicated.
Age-dependent growth
Growth increments deposited in the initial 10 mm of gladius growth were only visible in four juveniles of 14, 15, 19 and 20 mm GL. Although the number of growth increments deposited at juvenile stage was not visible in the gladius of adult squid, in these four individuals 32, 33, 48 and 79 growth increments counted in these gladius sectors are in accordance with the initial values assumed. Ages estimated were 368, 369, 384 and 415 days respectively, for the initial ages of 32, 33, 48 and 79 days (Figure 6).

Fig. 6. Growth curve reconstructed from the cumulative number of growth increments reconstructed for the initial ages of 32, 33, 48 and 79 days.
DISCUSSION
The patterns of use of the gladius as a tool for age and growth studies corroborated previous investigations conducted on Illex argentinus (Bizikov, Reference Bizikov, Jereb, Ragonese and Boletzky1991), Sthenoteuthis ovalaniensis (Arkhipkin & Bizikov, Reference Arkhipkin, Bizikov, Jereb, Ragonese and Boletzky1991; Bizikov, Reference Bizikov1995), Sepioteuthis lessoniana (Jackson et al., Reference Jackson, Arkhipkin, Bizikov, Hanlon, Okutami, O'Dor and Kubodera1993), Illex illecebrosus (Perez et al., Reference Perez, O'Dor, Beck and Dawe1996), Doryteuthis plei (Perez et al., Reference Perez, Aguiar and Santos2006) and Lolliguncula brevis (Zaleski, Reference Zaleski2010). Gladius plate was unsuitable for age determination because the number of growth increments deposited throughout life were not visible during the juvenile growth phase. Yet the daily nature of gladius growth increments of the intermediate shell layer was supported and a high correlation with body growth was established. Combined, these elements allowed the interpretation of gladius growth rate series as proxies for individual growth ‘histories’.
The most remarkable feature of I. argentinus absolute growth off the Brazilian coast was the continuous increase of growth increments, consistent throughout most of the species size-range (Brunetti et al., Reference Brunetti, Ivanovic, Elena and Boschi1998). This trend, however, is not homogeneous, but exhibits discontinuities, the most noticeable of them at approximately 80 mm GL. This transition may be related to the lifestyle shift to demersal habits in the outer shelf and also by environmental components (i.e. bottom temperature) as demonstrated in the present study. It is also interesting to note that gladius growth rates of I. illecebrosus also shift their accelerating pattern at a similar size (Perez & O'Dor, Reference Perez and O'Dor2000). Yet in this species (and geographical area) absolute growth rates (mm day−1) completely stabilize in larger squid, a pattern that has been principally attributed to important environmental transitions experienced by juvenile squid (Perez & O'Dor, Reference Perez and O'Dor2000). During early life, paralarvae of I. illecebrosus are concentrated offshore, mostly under the influence of Gulf Stream warm and oligotrophic waters (temperature >16oC). As juveniles move shoreward into shelf/slope front they experience a sharp temperature decrease as they become exposed to a larger food supply (O'Dor, Reference O'Dor and Boyle1983). At approximately 90 mm GL squid reach the Scotian shelf experiencing temperatures as low as 5–6oC during the spring time (Perez & O'Dor, Reference Perez and O'Dor2000). Such inshore movement seems energetically favourable, since their growth performance, measured as the wasted daily growth potential (the quantity of food required to achieve maximum growth), greatly improves (Perez & O'Dor, Reference Perez and O'Dor1998). Yet squid in cold and productive shelf environments grow at a constant rate.
Contrastingly, paralarvae of I. argentinus off the Brazilian coast seem to concentrate on the shelf in association with coastal upwelling areas (Vidal et al., Reference Vidal, Haimovici and Hackbart2010). As they develop into juveniles and sub-adults they move offshore to the shelf break and slope areas where spawning individuals are found (Haimovici et al., Reference Haimovici, Brunetti, Rodhouse, Csirke, Leta, Rodhouse, Dawe and O'Dor1998). No temperature measures were available at the capture sites of the examined samples. However, when mean bottom temperature profiles, measured throughout the year along the sampled area (Haimovici et al, Reference Haimovici, Rossi-Wongtschowski, Bernardes, Fisher, Vooren, Santos, Rodrigues and Santos2008) are compared with the depth of capture of individuals used for individual growth reconstruction (Figure 7) it is observed that: (a) small individuals (GL <89 mm GL) concentrated below 150 m where temperatures oscillate around 15–16oC; and (b) as individuals increase in size they move into deeper sectors of the shelf break and slope experiencing progressively colder waters (14–7°C). In contrast to Illex illecebrosus, which linear absolute growth rates do not change with size in this colder environment (Perez & O'Dor, Reference Perez and O'Dor2000), those in I. argentinus increase with GL though with lower rate than among juveniles in warmer shelf waters.

Fig. 7. (A) Distribution of gladius length (GL) samples under 165 mm according to depth strata obtained from Haimovici et al. (2008); (B) GL distribution of the same samples was plotted against the mean temperature obtained in each stratum.
Another relevant factor associated with the growth rate trends observed in I. argentinus off Brazil refers to the potential interference of different spawning groups in the examined samples. According to reproductive patterns and statolith-derived age and hatching date data, most squid concentrations off Brazil were composed of ‘local’ spawning groups that live approximately half-year and spawn at small sizes (150–250 mm ML). Yet during winter–spring months concentrations of large (ML > 250 mm), nearly 1-year-old, mature males and females occur in the lower slope, possibly originated from migratory components of northern Patagonian Shelf spawning groups (Perez et al., Reference Perez, Silva, Schroeder, Schwarz and Martins2009; Schwarz & Perez, Reference Schwarz and Perez2012). Both spawning groups have been mixed to compose the absolute growth rate analysis (Figure 5) but were differentiated by the maturation data (Figure 2) and also by disruptions of the GLxBW relationships (Figure 3). As a consequence, gladius absolute growth phases would characterize growth patterns of ‘local’ spawning groups (phases I and II), large winter spawners (phase IV), and the mixture of both groups (phase III). The implications were of such effect were that:
(a) the discontinuity growth between phase II and III is unrealistic, i.e. not determined by real life-history transitions;
(b) large winter spawners may have a longer stable growth rate pattern (phase IV) such as the one observed in I. illecebrosus;
(c) ‘local’ spawners accelerate growth continuously to reach spawning sizes earlier within a 0.5 year life span, according to Schwarz & Perez (Reference Schwarz and Perez2012).
The growth curve as reconstructed by the cumulative growth increments in gladius differ from the one estimated for statolith (Schwarz & Perez Reference Schwarz and Perez2010) which typically follow the Sachs cycle of growth with increase of growth in the first part and decrease in growth rates in the second part of ontogenesis, represented by an s-shaped curve (Arkhipkin & Roa-Ureta, Reference Arkhipkin and Roa-Ureta2005). One possible explanation is that as mantle shrinks the gladius tend to bow, the result being that it is longer than the projected mantle length (Perez, Reference Perez1995). Nevertheless, the initial age estimates of 30–40 days corroborate the studies of Arkhipkin & Scherbich (Reference Arkhipkin and Scherbich1991) and Schwarz & Perez (Reference Schwarz and Perez2012) for I. argentinus, and the ages achieved for older individuals were one year.
Considering that the environmental factors such as temperature and nutrition directly affect the onset of maturity and indirectly affect growth (Boyle & Rodhouse, Reference Boyle and Rodhouse2005) and that species are likely to adapt to the temperature and environment at which they live, the accelerated absolute growth and earlier development of the gonads of the ‘local’ individuals observed in Perez et al. (Reference Perez, Silva, Schroeder, Schwarz and Martins2009) may represent a life-history strategy in which organisms grow fast, mature earlier and produced offspring sooner (Wood & O'Dor, Reference Wood and O'Dor2000). Our data show that in Southern Brazil Illex argentinus fully exploit high flexibility of life cycles particular for cephalopods (Boyle & Boletzky, Reference Boyle and Boletzky1996) and display both possible ways of use of annual changes in environment. One population lives generally for six months and another for a whole year that ensures the maximum sustainable use of highly variable food resources of the tropical shelf and upper slope.
ACKNOWLEDGEMENTS
The authors are indebted to all observers, captains, and crews who allowed this large body of data to be collected during their commercial operations off southern Brazil. We also thank Vladimir Laptikhovsky and three anonymous referees for their valuable critiques on the early versions of the manuscript.













