Introduction
How and to what extent climatic variations can cause stress to eurythermal bivalve species is still not fully understood. This is the case because these variations relate to the world's polar and subpolar regions, and the Arctic is increasingly a focal point due to escalating human activities in these regions, as well as global warming, which is affecting the marine ecosystem. In contrast to temperate zones, northern areas are typically exposed to drastic changes in temperature (from +18.0 down to −2.0°C for water) and short days (absence of sun from 14 to 44 days annually). As a consequence, arctic and subarctic animals feel the effects of pronounced seasonal changes more than do the animals who inhabit temperate zones.
In several temperate bivalves, seasonal changes resulting in a lower metabolic rate (MR) in winter than in summer have been noted (DeVooys, Reference DeVooys1976; Lesser & Kruse, Reference Lesser and Kruse2004; Jansen et al., Reference Jansen, Pronker, Kube, Sokolowski, Sola, Marquiegui, Schiedek, Bonga, Wolowicz and Hummel2007). In species that inhabit temperate zones, these variations have been attributed to a decrease in daytime duration during winter and a commensurate increase in the inactive (low energy expenditure) phase of the daily cycle (Bayne, Reference Bayne1973), as well as to reduced food intake (Smaal et al., Reference Smaal, Vonck and Bakker1997; MacDonald & Ward, Reference MacDonald and Ward2009). Further variations are caused by seasonal variations in body mass (Sukhotin, Reference Sukhotin1989) and food quality and accessibility (Bayne & Widdows, Reference Bayne and Widdows1978; Thompson, Reference Thompson1984), in addition, possibly, to reproductive status (Hatcher et al., Reference Hatcher, Grant and Schofield1997). In combination, these factors are all part of a generalized adaptive strategy aimed at minimizing energy expenditure and food requirements during the winter months. But, for the most part, the above investigations dealt with temperate species and were carried out under laboratory conditions. Laboratory experiments generally do not reflect the complex conditions to which organisms are naturally subjected, such as interactions among and within species and the physical dynamics of the system (Underwood & Peterson, Reference Underwood and Peterson1988). However, there may be problems associated with interpreting the results of short studies in situ due to additional stresses. The potential confounding influences of short-term stress effects can be avoided by focusing research questions on larger-scale issues.
Blue mussels and horse mussels are considered ideal sentinel species in coastal and shelf ecosystems, respectively. Existing knowledge on the physiology and biochemistry of these species, especially those which inhabit the Arctic region, and the influence of environmental variables on associated processes is often weak or lacking. Mytilus edulis inhabits depths of 0.0 m (intertidal) or below, whereas Modiolus modiolus occurs at depths of no less than 5–6 m (Schweinitz & Lutz, Reference Schweinitz and Lutz1976; Naumov, Reference Naumov2006). Presumably, the differences in the ecotopes of those molluscs may be reflected in the behaviour strategy, physiology and energy costs associated with responding to environmental stress, growth and reproduction, which, in turn, are often related to metabolic efficiencies (Coleman & Trueman, Reference Coleman and Trueman1971; Jokumsen & Fyhn, Reference Jokumsen and Fyhn1982).
Oxygen consumption is an often used and direct index of MR (Seifert & Chapman, Reference Seifert and Chapman2006; Melzner et al., Reference Melzner, Gobel, Langenbuch, Gutowska, Portner and Lucassen2009), but heart rate (HR) can also be used as a measure of metabolic and physiological functions (Lowe & Trueman, Reference Lowe and Trueman1972; Campbell et al., Reference Campbell, Fraser, Peck, Bishop and Egginton2007). Cardiac activity has proven to be a reliable estimator of MR in limpets (Marshall & McQuaid, Reference Marshall and McQuaid1992; Santini et al., Reference Santini, De Pirro and Chelazzi1999) and blue mussels (Bakhmet, Reference Bakhmet2017). Moreover, although the heart of bivalves is myogenic (Jones, Reference Jones, Saleuddin and Wilbur1983), their cardiac activity may be modulated by various endogenous and exogenous factors, which represents a highly adaptive phenomenon, as HR plays a crucial role in a number of physiological processes (Joosse & Geraerts, Reference Joosse, Geraerts and Saleuddin1983). As a consequence, HR monitoring has been used extensively to study the physiological responses of molluscs to internal and environmental fluctuations (Romero & Hoffmann, Reference Romero and Hoffmann1991, Reference Romero and Hoffmann1996; Rovero et al., Reference Rovero, Hughes and Chelazzi1999; Rizzatti & Romero, Reference Rizzatti and Romero2001). However, there is scope for further research investigating the magnitude and occurrence of stress-induced changes in HR of Bivalvia. The prevalence, repeatability and longevity of these phenomena, the variability between individuals and the relationship of these factors to changes in MR are all areas in need of further work, as is the overall question of how these effects might influence energy budgets (Green, Reference Green2011). It should be underlined that most of those studies have dealt with laboratory conditions. The results of such studies may not be representative of the natural situation, and more authors are now placing emphasis on the measurement of physiological rates under natural conditions, preferably in the field (Bayne et al., Reference Вауnе, Widdows and Newell1978; Bakhmet, Reference Bakhmet2017).
Thus, the main objective of this study was to monitor closely in situ, over a full year cycle, the temporal changes in the basic biology (mainly relative MR) of the selected bivalve species (M. edulis L. and M. modiolus L.) and how they are affected by specific physical and physicochemical (for example, light, temperature, ice cover, salinity and oxygen) and biological (for example, food availability) parameters of ambient seawater. In this study, we pose the following question: does variability in food supply and reproductive status affect the organismal performance of molluscs when they are exposed to different temperatures? Recent studies have also incorporated organismal performance, especially as it is affected by food supply, as a significant factor that contributes to intertidal community structure (Dahlhoff & Menge, Reference Dahlhoff and Menge1996). Organismal performance can be framed as a bottom-up effect that can be quantified for key organisms in any marine habitat, but especially for intertidal species, by examining their thermal optima and limits (Somero, Reference Somero2002), metabolic performance along environmental gradients or changes in life-history characteristics associated with organismal fitness (Petes et al., Reference Petes, Menge and Harris2008). Also, does phenotypic plasticity which we estimated on the base of HR play a major role in the response of mussels to differences in food availability or seawater temperatures in the field? To achieve the goal of answering the above questions, the White Sea was chosen. The White Sea is characterized by a surface water layer temperature ranging from −1.5°C to +15–17°C during the year and thick ice-cover (up to 60 cm) from January to May, making it a typical sub-polar water body.
Materials and methods
Sample collection and HR recording
This study was conducted at the White Sea Biological Station of the Zoological Institute, Russian Academy of Science (RAS), between March 2013 and March 2014. The molluscs were collected from the Kandalaksha Gulf area of the White Sea (Russia, 66°20′N 33°40′E). Blue mussels of one size class were collected from Chupa Bay in the White Sea. Subtidal animals (shell length: 54–64 mm) were randomly collected from artificial substrates (suspended culture) at a depth of 2–3 m, and intertidal mussels (shell length: 38–48 mm) were collected from the centre of the tidal zone. Horse mussels (shell length: 65–95 mm) were sampled from the rocky subtidal zone at a depth of ~5–8 m using scuba. Immediately after collection, the molluscs were placed into plastic tanks with aerated seawater of standard salinity for the White Sea (25 PSU). On the same day, CNY70 sensors (Vishay siliconix) consisting of a phototransistor axially coupled with an infrared light-emitting diode were glued (using acrylic glue) to the shell of each animal, against each mollusc's heart (at the posterior end of the hinge region). Subsequently, some of the molluscs (subtidal (Group 1) and intertidal (Group 2) blue mussels and horse mussels) were placed into the cages (rigid-jointed framework covered up by net with mesh of 2 × 2 cm), and the cages were lowered into the sea at a depth of ~3 m (for the blue mussels) and ~6 m (for the horse mussels). Some intertidal blue mussels (now known as ‘real intertidal blue mussels’ (Group 3)) were placed at the intertidal zone (middle part of intertidal zone). The distance between location of collection and place of HR monitoring was not more than 500 m.
The molluscs’ HR was monitored using the non-invasive technique of Depledge & Andersen (Reference Depledge and Andersen1990), and the electric contacts of the sensors were waterproofed using dental paste. After filtration and amplification, the signal was transmitted to a ScopeMeter® 125 portable digital oscilloscope (Comarc Fluke Co, GB) and then automatically recorded onto a computer hard disk at set time intervals (De Pirro et al., Reference De Pirro, Santini and Chelazzi1999; Santini et al., Reference Santini, Williams and Chelazzi2000). The initial mean HR was obtained from three recordings for each mollusc (each recording 60 s long) every 4 days throughout the experiment. The HR, expressed in beats per minute ± SEM, was determined manually from the heart contraction visualized on the computer display. For the real intertidal mussels in summer time, long-time monitoring was carried out. Estimations of HR were realized every 5 min over 2 days. The same individual mussels were monitored throughout the year.
Monitoring of abiotic factors
The temperature and salinity of the ambient water was monitored continuously by means of U22-001 and U24-002-C data loggers (Onset HOBO Data Loggers, USA), which were located near the experimental mussels at a depth of 3 m.
Monitoring of biotic factors (reproduction status, phytoplankton concentration)
Studies of reproduction
For the analysis of the sexual cycle, subtidal and intertidal blue mussels were collected monthly (during spawning time every 10 days). The condition of the reproductive glands was evaluated visually and examined on microscope slides of samples taken for 10 adult individuals. The gonads were analysed histologically to determine the gender of the collected mussels and to identify their maturation stages. The histological procedure was conducted using classical techniques (Humason, Reference Humason1967). Specifically, the gonad tissues were dehydrated through immersion in a series of ethanol concentrations followed by chloroform-paraffin wax, and they were then embedded in paraffin wax. Sections of 5 µm were prepared using a microtome and stained with haematoxylin.
Gametogenesis is a continual process, but for convenience of description, it can be divided into several stages. The stage of greatest interest is the spawning period of the mussel settlement as a whole. The subdivisions of the sexual cycle proposed by Chipperfield (Reference Chipperfield1953) were applied in this work to the analysis of gonad development. In this scheme, the summer periodicity of M. edulis gametogenesis was briefly described in Antsulevich et al. (Reference Antsulevich, Maximovich and Vuorinen1999): Stage 0 was determined as the period of gonadal restoration after spawning, when the gonad was fully filled up with connective tissues and a few unreleased gametes (spermatozoa/ova) that were about to resorb; Stage I was determined as early gametogenesis, when the follicle walls started to develop and immature gametes (spermatogonia/oogonia) were formed; Stage II was determined as active gametogenesis when the follicles increased in size and started to be filled with the developing gametes (the follicles occupied about 50% of the gonadal section); Stage III was determined as maturity, when the follicles reached maximum size and were filled with completely mature gametes and when the interfollicular connective tissue had disappeared almost completely. Pre- and post-spawning of the gonads can be described in the following stages: IIIA1, when the follicle occupies most of the gonadal section; IIIA2, which occurs immediately before the spawning; IIIB, which involves partial or complete discharge of the gametes; IIIC, where the gonad tissue is restored after non-completed discharge of the gametes; and IIID, which involves repeated or completed discharge of the gonads (though in our collections, such individuals were not found).
Phytoplankton concentration
During each HR registration, water samples were taken. To determine the concentration of phytoplankton, the water samples was collected manually using a Niskin bottle at a depth of ~3 m during maximum high tide from March 2013 to March 2014 (the sampling interval was 3–5 days). A sample volume of 200–250 ml and a depth of ~3 m were applied. The samples were fixed using Lugol's solution with added glutaraldehyde. The number of algae was determined by means of direct Nageotte (0.1 ml volume) and Naumann (1 ml volume) cell counts using a light microscope. Trophic status was assigned to algae on the basis of their own long-term fluorescent microscopy data that had been obtained from the study area and according to the literature. It should be noted that unlike the vast majority of autotrophic diatoms, almost all dinoflagellates are mixotrophs or heterotrophs (Stoecker, Reference Stoecker1999; Jeong et al., Reference Jeong, Yoo, Kim, Seong, Nam Kang and Kim2010). The amount of biomass in the carbon units was calculated based on the volume of cells (Menden-Deuer & Lessard, Reference Menden-Deuer and Lessard2000).
Statistical analysis
A single factor analysis of variance (ANOVA), with species (subtidal blue mussels, intertidal blue mussels under water, intertidal blue mussels on the littoral, horse mussels) as factor for each temperature index which animals met during our monitoring, and an ANOVAr with temperature as factor for every species, were applied to all measured parameters at a significance level of 5%. To reveal the periodic components of HR the obtained data series were treated using the fast Fourier transformation (FFT). Pearson correlation analysis was used to examine the relationships between the HR and temperature of ambient seawater (Sokal & Rohlf, Reference Sokal and Rohlf1995). Unless specified, values are given as means ± standard error (SE).
Results
All the molluscs showed a stable and clear heart rhythm over the full year. However, due to severe conditions (ice cover and storms), cardiac activity in the real intertidal blue mussels (Group 3) could only be monitored for half of the year (from July to November).
The HR was recorded over the temperature ranges of −0.8 to +17.2°C between the months of March and July and +17.2 to −1.2°C between the months of July and March, respectively. The cardiac activity or HR – in other words, the power of contractions – in the molluscs responded in the expected manner to changes in temperature (Figure 1). In the case of all animals, the strongest linear correlation was found between temperature and HR (Table 1). The equations of all the blue mussels were similar, while the regression line for the horse mussels differed significantly (t = 8.76; P < 0.01). In spite of the highly significant correlation between temperature and the molluscs’ cardiac activity, we identified some time periods during which the HR and temperature changes did not correlate. The first period began on 23 April, when the cardiac activity of the subtidal animals (Group 1) increased significantly (df = 17; F = 4.72; P < 0.05) on the basis of one temperature level. The second period was observed from 8 June to 19 June: Based on a temperature increase from 8 to 12°C, the molluscs’ cardiac activity did not change, and the HR of subtidal blue mussels (Group 1) even showed a significant decrease (df = 24; F = 1.10; P < 0.05). The third period was between 18 July and 23 August; in this case, the temperature did not change, while the HR of the molluscs decreased significantly in the subtidal blue mussels (Groups 1 and 2) (df = 31; F = 3.56; P < 0.01), intertidal blue mussels (Group 3) (df = 17; F = 2.34; P < 0.01) and horse mussels (df = 21; F = 2.76; P < 0.01) (Figure 1).

Fig. 1. Changes in the molluscs’ HR over a full year. Black thick line – subtidal blue mussels (Group 1); dotted line – intertidal blue mussels under water (Group 2); dark grey line – horse mussels; light grey line – real intertidal mussels (Group 3); black thin line – temperature.
Table 1. Parameters of the following equation: HR = b × t + a

HR, in beats per minute; t, temperature of ambient seawater; N, number of experimental mussels; b and a, coefficients of equations; r, correlation coefficient; R 2, determination coefficient; P, confidence level.
a Intertidal M. edulis at a depth of 3–4 m (Group 2).;
b Real intertidal M. edulis (Group 3).
c Subtidal M. edulis (Group 1).
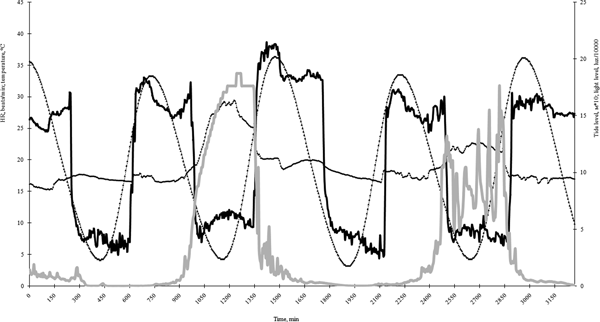
There was no difference in HR between the subtidal (Group 1) and intertidal (Group 2) (placed under the water) blue mussels over the entire duration of monitoring, with one exception: on 23 April, the cardiac activity of the subtidal (Group 1) animals was significantly higher than that of the intertidal (Group 2) animals (N = 17; F = 4.72; P < 0.05) (Figure 1). Conversely, cardiac activity in M. modiolus was significantly lower than in M. edulis from 9 May (df = 24; F = 7.32; P < 0.01) to 25 November (df = 13; F = 2.07; P < 0.05) (Figure 1). At the same time, the HR of the real intertidal M. edulis (Group 3) was significantly higher than that of the subtidal (Group 1) and intertidal (Group 2) blue mussels placed under the water (df = 28; F = 5.78; P < 0.01 and subsequently df = 19; F = 3.97; P < 0.05, respectively). During the winter and spring months (from December to April), the HRs of all the molluscs were similar (Figure 1). Extensive monitoring of the HRs of the real intertidal blue mussels (Group 3) revealed periodic fluctuations of cardiac activity, and this correlated strongly with tidal fluctuations. A sharp rise in HR was noted under immersed conditions, while there was a drop in cardiac activity in the case of air exposure (to the point of cardiac arrest in the case of some animals) (Figure 2). It should be noted that peak HR can be seen immediately after re-immersion and just before immersion (Figure 2). Using FFT one pattern of HR oscillations was revealed with periods of 12.25 h.

Fig. 2. Changes in the intertidal (Group 3) blue mussels’ HR during tidal fluctuations. Black thick line – HR; dotted line – tide level; grey thick line – light level; black thin line – temperature.
Investigations of gonad tissues revealed some regularity in reproduction. Stage I (absence of gametogenesis) was indicated for all molluscs from September to the end of April. Stage II (active gametogenesis) continued until the end of May. The gonads of all mussels reached maturity (Stage IIIA) by the start of June. Ten days later, the gonads of about half of all the blue mussels appeared to be in pre-spawning condition, and a week later, they were in after-spawning condition. At the same time, the gonads of the horse mussels were left half empty. Up to mid-July, observation showed either gonad restoration (mainly in males in Stage IIIC) or development of the gonads in after-spawning condition (Stages IIIB and IIID). One week later, some individuals were found to have gonads corresponding to Stage 0. In contrast, the gonads of the horse mussels remained at Stage IIIA up to mid-October. Then, in mid-November, partial resorption of gonads was observed.
On the surface of the White Sea (at a depth to 3 m), phytoplankton concentrations were generally found to be low over the whole year. However, an analysis of samples showed two peaks of extremely elevated phytoplankton concentration (as cells per ml, so μg of carbon per ml), namely, on 23 April and twice more on 21 May (Figure 3). Differences in species composition between those two rising phytoplankton concentrations were observed. The first peak mainly consisted of the diatoms Fragilariopsis oceanica and Pseudo-nitzschia seriata. The second was formed by Euglenoids, including Eutreptiella braarudii, E. gymnastica and the phototrophic dinoflagellate Heterocapsa rotundata.

Fig. 3. Changes in phytoplankton concentration over a full year. Black thick line – μg of carbon per ml; grey thick line – number of cells per ml/10 000).
Discussion
Here, we examined in detail the physiological ecology of intertidal and subtidal M. edulis and M. modiolus during a full year as a natural experiment (Diamond, Reference Diamond, Diamond and Case1986). We did this by measuring a physiological proxy (heart function) to ascertain the effects of food, reproduction status and temperature changes on blue mussels in different field sites and on horse mussels. Many laboratory studies have shown a positive correlation between MR, oxygen consumption and temperature, and as a result, much of the seasonal variation in biological activity has traditionally been related to an elevated environmental temperature. As noted above, a correlation has been experimentally established between the cardiac activity and metabolism levels of mussels. Similar positive correlations between heart function and temperature changes were shown in laboratory experiments for M. galloprovincialis, Anodonta cygnea, Aequipecten opercularis and other molluscs (Sommerville, Reference Sommerville1975; Kamenos et al., Reference Kamenos, Calosi and Moore2006; Kholodkevich et al., Reference Kholodkevich, Ivanov, Kuznetsova, Kurakin, Kornienko, Pan'kov and Khalatov2009).
Our field study also showed a significant linear link of molluscs’ HR and the temperature of ambient seawater. However, the maintenance of metabolic processes during exposure to changes in thermal regimes that occur simultaneously with changes in food supply is now believed to be a confounding factor in the correlation of HR and temperature. In our investigation, this took place at the end of April and in the second part of May (Figure 3). The increase in subtidal blue mussels’ (Group 1) cardiac activity at the same time as a sharp rise in phytoplankton concentration (at the end of April) is in accordance with the results of our previous study (Bakhmet, Reference Bakhmet2017). It is obvious that food availability exerts greater control over respiratory rate and cardiac activity than temperature, as was shown earlier (Loo, Reference Loo1992). However, in contrast, no significant changes were observed in the intertidal blue mussels (Group 2) or horse mussels. It is well known that horse mussels initially have a lower basal metabolism than blue mussels do (Navarro & Thompson, Reference Navarro and Thompson1996) and, hypothetically, that molluscs cannot react to food availability in such a low temperature (−1.0°C).
The estimation of the intertidal mussels’ (Group 2) HR placed under water yielded some unexpected results. However, those molluscs had only been placed under water ~1 month prior to the test period, and in all likelihood, such a short time frame was insufficient for adaptation to new conditions. The second and more prominent increase in phytoplankton concentration provoked tachycardia in all molluscs, but elevated temperature was also a factor. Hence, at first glance, it is impossible to divide the influences of food availability and temperature in such a case. However, if we consider the further HR changes, namely, the absence of significant cardiac activity changes in parallel with a temperature decrease between 21 and 23 May (Figure 1), it becomes clear that the molluscs’ metabolism level remained at the same level due to the high concentration of phytoplankton. It should be underlined that a high HR remains a factor in all species of mollusc, in contrast to the first peak of phytoplankton concentration, which does not affect all molluscs. In our opinion, the main reason for a high HR in all the animals is the higher temperature and consequent higher MR, although it may be due to the difference between those two peaks in terms of species composition. First, Euglenoids, which dominated in the second peak, have no silicic shell and are consequently more calorific. Second, diatoms have the pigments Chla, Chlb, Chlc2, Chlc3 and fucoxanthin, whereas Euglenoids have the pigments Chla, Chlb and diadinoxanthin (Jeffrey et al., Reference Jeffrey, Wright, Zapata, Roy, Llewellyn, Egeland and Johnsen2011).
Thus, taking account of the positive correlation between molluscs’ HR and the temperature indices shown in Table 1, this is additional evidence that food availability exerts greater control over respiration rate and cardiac activity than temperature does (Loo, Reference Loo1992; Bakhmet, Reference Bakhmet2017). Moreover, the costs associated with acclimatizing to thermal stress could be offset by the availability of significantly higher concentrations of food that could maintain energy budgets while also allowing a surplus and positive scope for growth (Lesser et al., Reference Lesser, Witman and Sebens1994).
Reproduction status could be considered an additional confounding factor that may conceal the influence of temperature on the molluscs’ cardiac activity. Our study showed this effect during the spawning time of the blue and horse mussels, which took place from 10 June to ~17–20 June. During that period, there was a significant decrease in blue and horse mussels’ HR (df = 34, F = 1.62 and P < 0.001 for subtidal blue mussels (Group 1); df = 25, F = 1.07 and P < 0.001 for intertidal blue mussels (Group 2); and df = 24, F = 1.04 and P < 0.001 for horse mussels), while the temperature was left at the same level (Figure 1). Although we could not determine the exact time of spawning for each individual mussel, many authors (Chipperfield, Reference Chipperfield1953; Toro et al., Reference Toro, Thompson and Innes2002) note a prolonged period of mussel reproduction (up to several months). But in most cases, researches are focusing on the period within which gametes are recorded in the gonads of mussels. As was shown earlier (Maximovich, Reference Maximovich and Lukanin1985), of the total duration of mussels’ reproduction period in the White Sea from July to August, the actual spawning activity of these specimens in local habitats is realized within a few days (Maximovich, Reference Maximovich and Lukanin1985). Thus, the general tendency of spawning time is apparent, and as we have shown, it involves a step-by-step decrease in metabolic levels. This may be connected with some facts. First, the presence of sperm in water stimulates females to cease filtering and, respectively, feeding (Newell & Thompson, Reference Newell and Thompson1984). The positive connection between mollusc feeding behaviour and HR was shown earlier (Bakhmet, Reference Bakhmet2017), and we have observed this situation in our study. Second, as was established in some studies, the reproductive cycle can explain seasonal variations in oxygen consumption to a greater extent than temperature can. The sharp decrease in oxygen consumption after spawning was also shown (Bayne & Widdows, Reference Bayne and Widdows1978; Smaal et al., Reference Smaal, Vonck and Bakker1997). Essentially, this is the same set of events we observed in our investigation.
Our study considered the effects of species-specific differences in temperature on molluscs’ physiology. During the winter-spring and autumn-winter seasons under low temperatures (−0.8 to +1.7°C and +5.0 to −1.2°C, respectively), no differences in HR were observed in any of the animals. It should be emphasized that if an organism maintains a steady cardiac rhythm throughout severe winter conditions, this suggests that its metabolism remains active and does not fall into a period of resting activity. Therefore, the present research provides significant insights into the active behaviour of these subpolar benthic species during a polar night, and in this way, it extends the findings of recent reports. The most significant HR difference between the blue mussels (Groups 1 and 2) and the horse mussels in this study appeared at the temperatures of +1.7°C in spring and +5.0°C in autumn. Between those temperature points HR of horse mussels remains significantly lower than cardiac activity in blue mussels. The significant lower cardiac activity in the horse mussels’ HR compared with the blue mussels’ HR during summer time is connected with an initially lower basal metabolism level in M. modiolus (Navarro & Thompson, Reference Navarro and Thompson1996).
It is obvious, in our opinion, that the reason for the difference in spring at such a low temperature is connected with the bloom of phytoplankton concentration. This is additional evidence of food availability exerting greater control over respiration rate and cardiac activity than temperature. During the subsequent summer period, HR was at the same level in the subtidal and intertidal blue mussels (Groups 1 and 2). Here, if it is taken into account that these two animal groups are the same species and that M. edulis has a great capacity for acclimation, this reaction of the mussels could be expected.
Real intertidal blue mussels (Group 3) are exposed to significant changes in environmental conditions (for example, air and seawater temperatures) in such a way that physiological adaptations and the ability to acclimatize seasonally play a critical role in the survival of M. edulis and its ability to be a competitive dominant in the intertidal zone (Bayne, Reference Bayne2004). In our study, this was apparent in the significantly higher HR of the blue mussels and, consequently, the highest level of metabolism during immersion. The significant positive correlations between HR and oxygen consumption in invertebrates have been shown previously (Marshall & McQuaid, Reference Marshall and McQuaid1992; Santini et al., Reference Santini, De Pirro and Chelazzi1999; Bakhmet, Reference Bakhmet2017). Thus, we believe that the sharp increase in HR we observed in this study is connected with the oxygen debt accumulated in the animals’ tissues during air exposure. Further evidence of this tachycardia was found in the significant peak of HR that was observed immediately after re-immersion. However, the second peak of HR before the last return to the ambient seawater is difficult to explain. It looks as though the animals may foresee air exposure long before such exposure occurs, and, consequently, the mussels oversaturate the haemolymph with oxygen. This question should be researched in future studies.
Conclusion
This study shows that not only abiotic but also biotic factors have a significant effect on the physiological ecology of M. edulis from different ecological zones and on M. modiolus with respect to their ability to tolerate abiotic stress when exposed to varying amounts of food. In addition, our study supports the findings of Green (Reference Green2011) that more significant results must be obtained from cardiac activity monitoring based on more prolonged registration of HR. However, further analysis and additional laboratory experiments are required to clarify this assumption.
Acknowledgements
We are deeply grateful to the staff of the White Sea Biological Station at the Zoological Institute of the RAS. Also we wish to extend our warm thanks to Dr Jasmine Nahrgang, University of Tromsø.
Financial support
This work was supported by a Russian Foundation for Basic Research (projects no. 12-04-93081A and no. 16-04-00820 А) and by federal funding for government-ordered project theme no. 0221-2014-0033.
Ethical standards
This study was approved by the Ethics Committee at the Institute of Biology, Karelian Science Centre, and the experimental protocol conformed to international ethical standards (Portaluppi et al., Reference Portaluppi, Smolensky and Touitou2010).