INTRODUCTION
Gastropoda are one of the most species-rich macrofaunal taxa from hydrothermal vents (Warén et al., Reference Warén, Bouchet, von Cosel, Desbruyères, Segonzac and Bright2006), and form a dominant component of assemblages at vent fields in western Pacific back-arc basins and on the Central and South-west Indian Ridges (e.g. Desbruyères et al., Reference Desbruyères, Alayse-Danet and Ohta1994; Galkin, Reference Galkin1997; Kojima et al., Reference Kojima, Segawa, Fijiwara, Fujikura, Ohta and Hashimoto2001; Van Dover et al., Reference Van Dover, Humphris, Fornari, Cavanaugh, Collier, Goffredi, Hashimoto, Lilley, Reysenbach, Shank, Von Damm, Banta, Gallant, Gotz, Green, Hall, Harmer, Hurtado, Johnson, McKiness, Meredith, Olson, Pan, Turnipseed, Won, Young and Vrijenhoek2001; Tao et al., Reference Tao, Lin, Guo, Chen, Wu, Han, German, Yoerger, Zhou, Li and Su2012). A substantial research effort has sought to elucidate the systematics and higher phylogeny of vent/seep gastropods and to characterize their diversity, biogeography and life-history biology (see Sasaki et al., Reference Sasaki, Warén, Kano, Okutani and Fujikura2010 for recent review).
More than 200 species of gastropods from at least 100 genera and 35 families have been recorded from deep-sea chemosynthetic environments of the world's oceans (Sasaki et al., Reference Sasaki, Warén, Kano, Okutani and Fujikura2010). Some of these species also inhabit non-chemosynthetic environments but have been recorded in much greater densities from chemosynthetic assemblages (see Sasaki et al., Reference Sasaki, Warén, Kano, Okutani and Fujikura2010), but others are endemic to vents and seeps at the species and higher taxonomic levels (e.g. Warén & Bouchet, Reference Warén and Bouchet2001).
Skeneinae Clark, Reference Clark1851 was originally treated as a separate family (‘Skeneidae’) in Trochoidea Rafinesque, Reference Rafinesque1815 (e.g. Hickman & McLean, Reference Hickman and McLean1990). More recent anatomical and molecular studies showed, however, that ‘Skeneidae’ was polyphyletic and that many genera should be reassigned (e.g. Bouchet et al., Reference Bouchet, Racroi, Fryda, Hausdorf, Ponder, Valdes and Warén2005; Kano, Reference Kano2008). Bouchet et al. (Reference Bouchet, Racroi, Fryda, Hausdorf, Ponder, Valdes and Warén2005) ranked Skeneinae as a subfamily of the Turbinidae Rafinesque, Reference Rafinesque1815, an arrangement maintained by Williams et al. (Reference Williams, Karube and Ozawa2008) in the newly defined Turbinidae. The rank of the Skeneinae remains uncertain and is under discussion (Williams, in press).
To date, all turbinids endemic to deep-sea chemosynthetic environments belong to either the Skeneinae or the Margaritinae Thiele, Reference Thiel1924 (Sasaki et al., Reference Sasaki, Warén, Kano, Okutani and Fujikura2010; see Table 1). Some colloniids, such as Cantrainea Jeffreys, Reference Jeffreys1883, also live in seeps and vents (e.g. Warén & Bouchet, Reference Warén and Bouchet1993, Reference Warén and Bouchet2001; Sasaki et al., Reference Sasaki, Warén, Kano, Okutani and Fujikura2010). The family Colloniidae Cossmann, Reference Cossmann1917 was classified as a subfamily within the Turbinidae (e.g. Hickman & McLean, Reference Hickman and McLean1990; Bouchet et al., Reference Bouchet, Racroi, Fryda, Hausdorf, Ponder, Valdes and Warén2005), but was moved recently to familial rank in the superfamily Phasianelloidea Swainson, Reference Swainson1840 (Williams et al., Reference Williams, Karube and Ozawa2008).
Table 1. Turbinid gastropods described from extant hydrothermal vents/cold seeps up to the end of 2011 (confirmed locations and fully described species only).

CIR, Central-Indian Ridge; GoM, Gulf of Mexico; JdFR, Juan de Fuca Ridge; LB, Lau Basin; MAR, Mid-Atlantic Ridge; MCSC, Mid-Cayman Spreading Centre; NFB, North Fiji Basin; OT, Okinawa Trough; SB, Sagami Bay.
The Von Damm Vent Field is an active, high-temperature hydrothermal system, situated in a unique off-axis setting on the upper slopes of an oceanic core complex at 2300 m depth (Connelly et al., Reference Connelly, Copley, Murton, Stansfield, Tyler, German, Van Dover, Amon, Furlong, Grindlay, Hayman, Hühnerbach, Judge, Le Bas, McPhail, Meier, Nakamura, Nye, Pebody, Pedersen, Plouviez, Sands, Searle, Taws and Wilcox2012). The Von Damm Vent Field supports an abundant faunal assemblage that is dominated by dense aggregations of the shrimp Rimicaris hybisae Nye, Copley & Plouviez, Reference Nye, Copley and Plouviez2012 and includes small skeneimorph gastropods. During a recent research cruise to the Mid-Cayman Spreading Centre a piece of sulphide chimney was sampled from the Von Damm Vent Field. On the surface of the sampled sulphide chimney were several small gastropods of one species, Iheyaspira bathycodon sp. nov., which is described herein. In addition to enhancing existing knowledge about biodiversity, characterizing the composition of faunal assemblage at Mid-Cayman Spreading Centre vents has the potential to elucidate the factors determining vent biogeography of this region.
MATERIALS AND METHODS
Specimens were collected from the Von Damm Vent Field (2300 m) at the Mid-Cayman Spreading Centre, Caribbean, during the 44th voyage of RRS ‘James Cook’ (April 2010). All specimens were picked from the surface of a sample of sulphide chimney, collected by the hydraulic grab of HyBIS (Hydraulic Benthic Interactive Sampler), a manoeuvrable TV grab sampler. Specimens for molecular analysis were immediately placed in 100% ethanol and the shell and operculum were subsequently removed. Specimens for morphological study were fixed in 10% neutralized formalin, subsequently transferred to 90% Industrial Methylated Spirits and measured to the nearest 0.1 mm using Vernier callipers (see Table 2).
Table 2. Morphological variation in Iheyaspira bathycodon sp. nov.
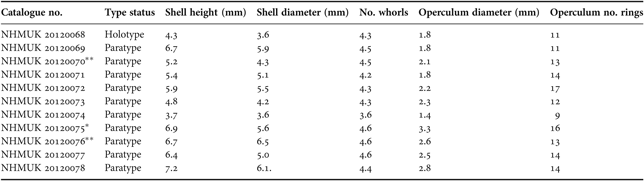
*, soft parts dissected out of shell; **, soft parts dissected out of shell and used for scanning electron microscopy.
Specimen [NHMUK 20120076] was dissected for scanning electron microscopy (SEM) of shell, operculum and radula. The shell and operculum were placed in an ultrasonic cleaning bath for three minutes. The mantle tissue was dissolved in potassium hydroxide diluted in water to expose the radula. The shell, operculum, radula and ctenidium were mounted uncoated onto an aluminium stub and micrographs were taken with a Hitachi TM3000 tabletop microscope. For SEM of soft parts, specimen [NHMUK 20120070] was dehydrated through a graded ethanol series, critical point dried and sputter coated with gold palladium prior to examination with a FEI Quanta 200 scanning electron microscope at accelerating voltage of 10 kV.
Genomic DNA was extracted from eight specimens using the cetyltrimethyl ammonium bromide (CTAB) extraction procedure (Doyle & Dickson, Reference Doyle and Dickson1987). A region of mitochondrial cytochrome oxidase subunit I gene (COI) was amplified by polymerase chain reaction (PCR) performed in 20 µl final volume using universal primers (Folmer et al., Reference Folmer, Black, Hoeh, Lutz and Vrijenhoek1994) and the following conditions: 1X buffer reagent (200 mM Tris pH 8.8, 500 mM KCl, 0.1% Trixton X-100, 2 mg/ml bovine serum albumen), 2 mM MgCl2, 0.2 mM of each dNTP, 0.5 mM of each primer, 1 U Taq DNA polymerase (Bioline), 5 µl of template DNA and sterile H2O to final volume. Thermal cycling conditions were: 94°C/2 minutes; followed by 5 cycles at (94°C/35 seconds; 45°C/35 seconds; 72°C/1:20 minutes) and 35 cycles at (94°C/35 seconds; 50°C/35 seconds; 72°C/1:20 minutes) with a final extension of 72°C/10 minutes.
For the 16S ribosomal DNA gene (16S), PCR amplifications were performed in 20 µl final volume using 16Sar and 16Sbr primers (Palumbi, Reference Palumbi, Hillis, Moritz and Mable1996) and the following conditions: 1X buffer reagent (same as for COI), 2.5 mM MgCl2, 0.13 mM of each dNTP, 0.38 mM of each primer, 1 U Taq DNA polymerase (Bioline), 2.5 µl of template DNA and sterile H2O to final volume. Thermal cycling conditions were: 94°C/4 minutes; 30 cycles at (94°C/30 seconds; 52°C/1 minute; 72°C/2 minutes) and 72°C/5 minutes.
Polymerase chain reaction amplifications of the 18S ribosomal DNA gene (18S) were performed using the primer pair 5′-CACAGTGAAACTGCGAATGG-3′ and 5′-CAAATGCTTTCGCTGTAGGG3′ (this study) in a 20 µl final volume amplification mixture as described for COI. Thermal cycling conditions were: 95°C/5 minutes followed by 30 cycles at (94°C/1 minute; 60°C/1 minute; 72°C/2 minutes; 72°C/2 minutes).
Purifications and sequencing were performed as described by Nye et al. (Reference Nye, Copley and Plouviez2012). Sequence strands were proofread and assembled with CodonCode Aligner, version 3.7.1 (CodonCode Corporation, Dedham, MA, USA), to produce a continuous fragment. The 16S and 18S partial rDNA sequences were compared with those of other gastropods available in GenBank using the BLAST program (NCBI Basic Alignment Search Tool). The COI partial sequence of the new species was also compared with those of other trochoids of Suzanne William's published and unpublished dataset of deep-sea gastropods (Williams et al., Reference Williams, Karube and Ozawa2008; S. T. Williams, personal communication). Phylogenetic trees were constructed with MEGA5 (Tamura et al., Reference Tamura, Peterson, Peterson, Stecher, Nei and Kumar2011) using both maximum-likelihood (ML) (Kimura, Reference Kimura1980) and neighbour-joining (NJ) (Saitou & Nei, Reference Saitou and Nei1987) methods on 425- and 803-base pair (bp) alignments for 16S and 18S respectively. Bootstrap values were calculated on 1000 re-sampling replicates.
The GenBank accession numbers for the partial sequences of COI, 16S and 18S regions from the new species are JQ306326, JQ306327 and JQ306328 respectively.
SYSTEMATICS
Order VETIGASTROPODA Salvini-Plawen, Reference Salvini-Plawen1980
Superfamily TROCHOIDEA Rafinesque, Reference Rafinesque1815
Family TURBINIDAE Rafinesque, Reference Rafinesque1815
Subfamily SKENEINAE Clark, Reference Clark1851
Genus Iheyaspira Okutani, Sasaki & Tsuchida, Reference Okutani, Sasaki and Tsuchida2000
Iheyaspira bathycodon sp. nov. Nye, 2012
(Figures 1–5)

Fig. 1. Iheyaspira bathycodon sp. nov., shell, from the Von Damm Vent Field, Mid-Cayman Spreading Centre, Caribbean, 2300 m. (A) Holotype [NHMUK 20120068]; (B) holotype, lateral view [NHMUK 20120068]; (C) holotype, basal view [NHMUK 20120068]; (D) paratype [NHMUK 20120072]; (E) holotype, apical view [NHMUK 20120068]; (F) holotype, sub-apical view [NHMUK 20120068]. Scale bars: A–F = 1 mm.

Fig. 2. Iheyaspira bathycodon sp. nov., paratype 08 [NHMUK 20120076] from the Von Damm Vent Field, Mid-Cayman Spreading Centre, Caribbean, 2300 m. (A) Operculum; (B) radula; (C) radula: lateral teeth; (D) radula: marginal teeth. Scale bars: A = 1 mm; B = 100 µm; C, D = 50 µm.
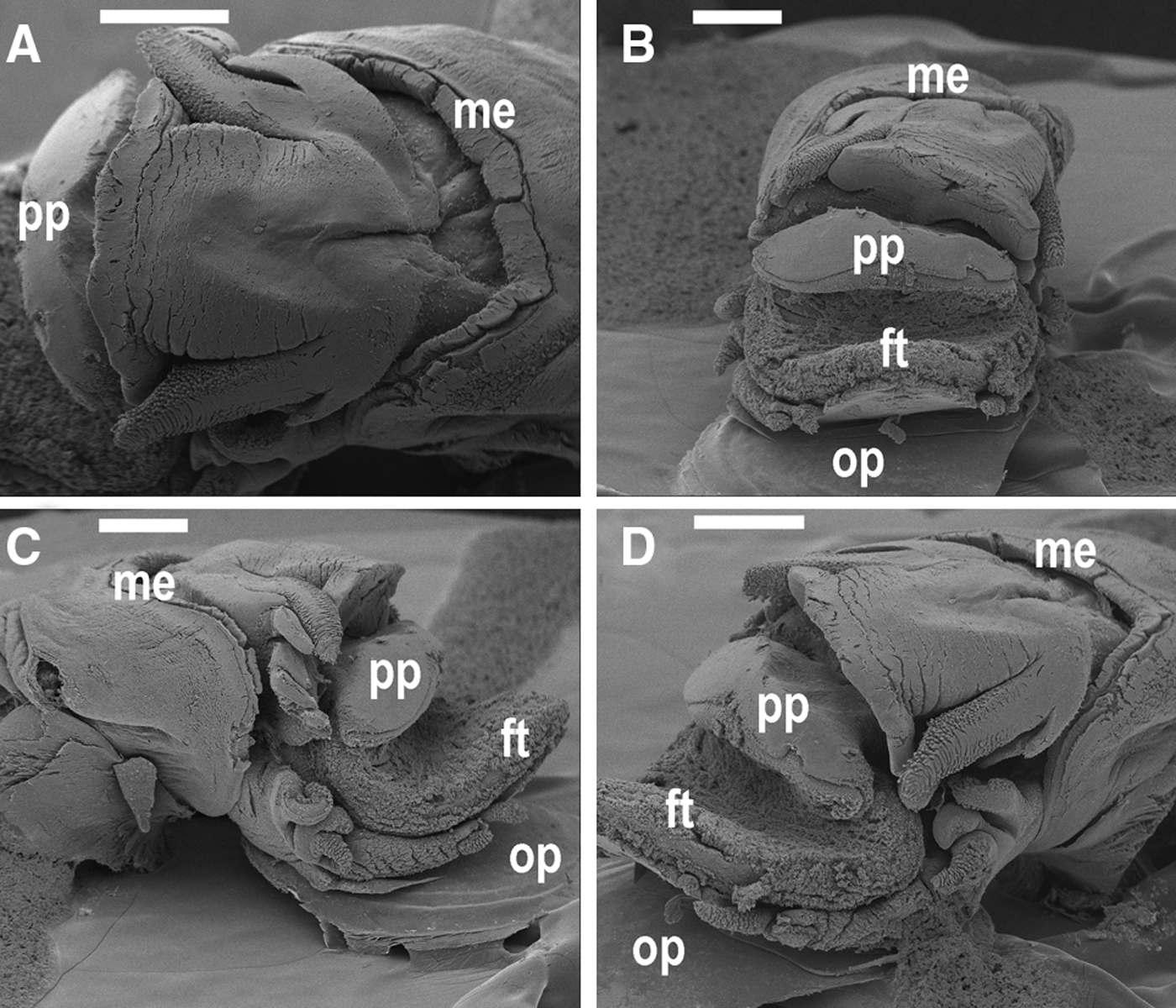
Fig. 3. Iheyaspira bathycodon sp. nov., paratype 02 [NHMUK 20120070] from the Von Damm Vent Field, Mid-Cayman Spreading Centre, Caribbean, 2300 m. Scanning electron microscopy micrographs of head-foot. (A) Dorsal view; (B) anterior view; (C) right side, lateral view; (D) left side, anterolateral view. Abbreviations used: ft, foot; me, mantle edge; op, operculum; pp, parapodium. Scale bars: A = 1 mm; B–D = 500 µm.

Fig. 4. Iheyaspira bathycodon sp. nov., paratype 02 [NHMUK 20120070] from the Von Damm Vent Field, Mid-Cayman Spreading Centre, Caribbean, 2300 m. Scanning electron microscopy micrographs of head-foot, right side. (A) Head, dorsolateral view; (B) head appendages, anterolateral view; (C) close-up of cephalic tentacle, lateral view; (D) head appendages and neck lobes, anterolateral view; (E) epipodial tentacles 1–3, dorsolateral view; (F) epipodial tentacles 4–5, lateral view; (G) epipodial tentacle 3, anterolateral view; (H) papillae on epipodial tentacle 3. Abbreviations used: ct, cephalic tentacle; es, eyestalk; et, epipodial tentacle; ft, foot; me, mantle edge; nl, neck lobe; op, operculum; pp, parapodium. Scale bars: A, D, F = 200 µm; B, E = 100 µm; C, G = 50 µm; H = 20 µm.

Fig. 5. Iheyaspira bathycodon sp. nov., paratype 02 [NHMUK 20120070] from the Von Damm Vent Field, Mid-Cayman Spreading Centre, Caribbean, 2300 m. Scanning electron microscopy micrographs of head-foot, left side. (A) Head, dorsolateral view; (B) head appendages, lateral view; (C) tip of cephalic tentacle, lateral view; (D) papillae on cephalic tentacle; (E) epipodial tentacles 1–3, dorsolateral view; (F) epipodial tentacles 4–5, dorsolateral view. Abbreviations used: ct, cephalic tentacle; es, eyestalk; et, epipodial tentacle; ft, foot; me, mantle edge; nl, neck lobe; op, operculum. Scale bars: A = 200 µm; B, E, F = 100 µm; C = 50 µm; D =20 µm.
TYPE MATERIAL
Holotype and paratypes deposited in the Natural History Museum, UK (NHMUK) [NHMUK 20120068–20120078]. All type material collected from the surface of a piece of sulphide chimney sampled from the Von Damm Vent Field, Mid-Cayman Spreading Centre, Caribbean (18° 22.605′N 81° 47.875′W), water depth 2300 m.
DESCRIPTION
Shell (Figure 1). Rounded, skeneiform, sturdy, height greater than width (see Table 2); maximum dimensions 7.2 mm height, 6.1 mm width [NHMUK 20120078]. Surface smooth, lacking pigmentation, with thin, beige-white periostracum. Surface and apical region, including the protoconch and early teleoconch, are corroded in most specimens. Protoconch too corroded for any details to be seen (Figure 1 E, F). Teleoconch whorls more than 2.5 in number, body whorl large. No nacre or lustre visible on exterior or interior of the shell. Umbilicus open and deep, clearly visible in basal view. Peristome smooth. Aperture large and circular with smooth outer lip.
Operculum (Figure 2A) moderately thin, corneous, and yellowish-brown; multispiral with a central nucleus and short growing edge, with a good fit to the aperture. Opercula retraction is deep.
Soft parts (Figures 3–5). Animal pale white in colour. Head quite large, snout cylindrical, terminating in a broad tip with mouth positioned at the midline. One pair of cephalic tentacles of similar size to each other and equal in length to the snout; cephalic tentacles densely papillated with what appear to be sensory papillae (Figure 5D). Eyestalks subequal in length and width to cephalic tentacles, without visible papillae and eyes. Right eyestalk approximately one-half length of right cephalic tentacle (Figure 4A, B, D), left eyestalk approximately one-third length of left cephalic tentacle (Figure 5A, B). Cephalic lappets absent. Neck lobes arise from both basal sides of the head. Right neck lobe (Figure 4D) divided into elongate anterior (nl1) and posterior (nl2, nl3) tentacles. Left neck lobe composed of at least one undivided tentacle beneath the left eyestalk (Figure 5A, B). Foot equipped with five epipodial tentacles on both sides. Right side: first, second and third epipodial tentacles clustered together, similar in size, with dense papillae except on second (Figure 4E); fourth and fifth isolated from first three in middle part of epipodium between lobes of epipodial skirt, densely papillate (Figure 4F). Left side: first, second and third epipodial tentacles clustered together; first and third epipodial tentacles of similar size with dense papillae; second slightly smaller, without visible papillae (Figure 5E); fourth and fifth isolated from first three in middle part of epipodium, densely papillate (Figure 5F). Ctenidium monopectinate, attached along its whole length, with bursicles.
Radula (Figure 2B, D). Rhipidoglossate, bilaterally symmetrical, with the formula ∞ –9 –1 –9 –∞ (>20). Length ~3.1 mm, width ~384 µm, with at least 60 transverse rows along total length in paratype 08 [NHMUK 20120076]. Central tooth differentiated in form from lateral teeth; smooth-sided, bell-shaped, wider proximally than distally, with a single incurved central cusp (Figure 2B). Lateral teeth (Figure 2B, C) of similar size to central tooth, increasing in size outwards; with a long, rounded single central cusp, and an outer apical margin with several flanking denticles (>7); dentition attenuates towards the cusp and is strongest on the outermost lateral. Marginal teeth (Figure 2B, D) exceed twenty in number on both sides; cutting plate concave, terminating in a single short cusp; apical margins oblique, each with about 10–14 denticles that are longer and finer than those on the lateral teeth. Outermost marginal teeth in a row are smaller, with weaker dentition and straighter shafts. Marginal rows overlap each other.
No jaws are present.
COMPARATIVE REMARKS
The shell of Iheyaspira bathycodon sp. nov. is superficially similar to those of several other skeneimorph taxa, but the radula pattern appears to be unique in both number of teeth and shape.
The new species is closest in morphology to Iheyaspira lequios Okutani, Sasaki & Tsuchida, Reference Okutani, Sasaki and Tsuchida2000 (Turbinidae: Skeneinae), the type species of a monotypic genus. Affinities with I. lequios include conchological similarity and shared radula characters, most notably in the shape of the central tooth (see Okutuni et al., Reference Okutani, Sasaki and Tsuchida2000: Figure 2, p. 269). The new species does however exhibit several important dissimilarities to I. lequios: (1) shell: small (maximum 7.2 × 6.1 mm) rather than minute (maximum 5.7 × 5.4 mm in I. lequios), umbilicate, teleoconch with more than two whorls; (2) radula: central tooth bell-shaped, not rhombic/arrow-shaped; only nine (not twelve) pairs of lateral teeth; (3) eyestalks: reduced, subequal in length and width to cephalic tentacles, as opposed to well-developed, thicker than cephalic tentacles; (4) neck lobes: right neck lobe composed of three (not two) tentacles; (5) epipodial tentacles: five (not four) on both sides; left ET1 and ET3 densely papillate (I. lequios ET1-3 lack papillae). In Iheyaspira bathycodon sp. nov., ET2 and ET3 are very close together, arising as a pair from the same base; this is similar to I. lequios, and may be an epipodial sense organ.
The new species is also comparable with the turbinid Fucaria mystax Warén & Bouchet, Reference Warén and Bouchet2001 (Skeneinae), based on similarities in shell and radula characters, especially the shape of the central tooth (see Warén & Bouchet, Reference Warén and Bouchet2001: Figure 11C, p. 135). Iheyaspira bathycodon sp. nov. is differentiated from F. mystax by: (1) shell: umbilicus clearly visible in basal view, teleoconch with greater number of whorls (>2.5); (2) radula: central tooth bell-shaped, lacking drawn out and narrow anterior support; only nine (not eleven) pairs of lateral teeth; (3) eye stalks: do not encircle cephalic tentacles. Moreover, members of the genus Fucaria Warén & Bouchet, Reference Warén and Bouchet1993 are equipped with a coat of sensory papillae on the snout, a feature not observed in the new species.
DISTRIBUTION AND HABITAT
Known only from the type locality, the Von Damm Vent Field, Mid-Cayman Spreading Centre, Caribbean in 2300 m depth. See Connelly et al. (Reference Connelly, Copley, Murton, Stansfield, Tyler, German, Van Dover, Amon, Furlong, Grindlay, Hayman, Hühnerbach, Judge, Le Bas, McPhail, Meier, Nakamura, Nye, Pebody, Pedersen, Plouviez, Sands, Searle, Taws and Wilcox2012) for a description of the geological, geochemical and biological setting of the Von Damm Vent Field. Accompanying fauna observed in close proximity to the new species included the alvinocaridid shrimps Rimicaris hybisae Nye, Copley & Plouviez, Reference Nye, Copley and Plouviez2012 and Alvinocaris sp., the hippolytid shrimp Lebbeus sp., zoarcid fish and siboglinid polychaetes.
ETYMOLOGY
The species name bathycodon is derived from the Greek words for deep and bell, in reverence to the species’ deep-sea habitat and bell-shaped rachidian tooth.
MOLECULAR PHYLOGENY
Partial sequences of the COI (549 bp), 16S (505 bp) and 18S (803 bp) region of Iheyaspira bathycodon sp. nov. were consistent amongst specimens. Fixed and unique mutations were observed in the partial sequences of the COI, 16S and 18S regions in comparison with other trochoid taxa. When compared with partial sequences of COI in the gastropod dataset of Suzanne Williams (NHMUK) the partial COI sequence of the new species was near other trochoidean skeneimorph taxa and unique amongst any of the species available (Suzanne Williams, personal communication). Based on a 425-bp alignment of partial 16S sequences, NJ and ML phylogenetic trees have the same topologies and place the new species in the same clade (Turbinidae: Skeneinae) as Dillwynella cf. vitrea [AY163406.1], Protolira valvatoides [AY163405.1] and Protolira sp. [GQ160698.1], with 96% and 87% bootstrap support for NJ and ML methods respectively (Figure 6). Of the 16S partial sequences available in GenBank the new species is closest in evolutionary distance to D. cf. vitrea (14% divergence). Phylogenetic analyses on an 803-bp alignment of partial 18S sequences of turbinid species place the new species closest in evolutionary distance to D. planorbis [AB365310.1], with 39% and 42% bootstrap support for NJ and ML methods respectively. The new species exhibits 1.4% divergence from D. planorbis across an 803 bp of the 18S region.

Fig. 6. Neighbour-joining tree of turbinid gastropods based on a 425-bp alignment of partial nucleotide sequences from the mitochondrial 16S region with Buccinum tenuissimum (Caenogastropoda: Neogastropoda: Buccinoidea: Buccinidae) as outgroup. Evolutionary distances computed using the Jukes–Cantor method (Jukes & Cantor, Reference Jukes, Cantor and Munro1969) are represented by branch length; scale bar is proportional to inferred nucleotide divergence. Bootstrap support calculated on 1000 re-sampling replicates is shown by the numbers along the branches (NJ, plain text; ML, italic text). GenBank accession numbers are given after species names.
DISCUSSION
The molecular and morphological analyses of specimens of this trochoid gastropod reveal the presence of a new species. The new species is similar in morphology to Iheyaspira lequios and Fucaria mystax (see above), both of which are known only from hydrothermal vents in the Pacific at water depths less than 1500 m (see Table 1). It is, however, excluded from both species by differences in the radula and appendage structure of the head-foot (see above). It is closest in morphology to I. lequios and, therefore, the most conservative approach is to give the new species the generic name Iheyaspira to indicate the similarity between the two species. Consistency between specimens of I. bathycodon sp. nov. in partial sequences of the COI, 16S and 18S regions confirm that they belong to a single species, but the presence of unique and fixed mutations in the sequences indicate that they are genetically distinct from all other genera and species in the GenBank database.
The systematic position of both Iheyaspira and Fucaria is uncertain because there are no sequences available in GenBank for either genus; however both genera are classified currently within the family Turbinidae and subfamily Skeneinae (e.g. Bouchet, Reference Bouchet, Bouchet, Gofas and Rosenberg2010 a, Reference Bouchet, Bouchet, Gofas and Rosenbergb; Sasaki et al., Reference Sasaki, Warén, Kano, Okutani and Fujikura2010). After redefining the Turbinidae, Williams et al. (Reference Williams, Karube and Ozawa2008) remarked that it is hard to determine morphological characters that are typical of this family, and even suggested that the Skeneinae could be considered as a group distinct from (but most closely related to) Turbinidae (Williams et al., Reference Williams, Karube and Ozawa2008). The rank of the Skeneinae is still under discussion and further work will elucidate the systematic position of this taxon (Williams, in press).
Morphological features of the Skeneinae shared with the new species include the monopectinate ctenidium and absence of any visible nacre. In addition, the shell of the new species bears superficial resemblance to that of other members of the Skeneinae, such as Protolira. In the GenBank database sequences for the Skeneinae are available presently for a few species only. Despite this impediment, comparative 16S results presented herein suggest the proximity of the new species to members of the Skeneinae, with strong bootstrap support for inclusion of the new species within this clade. This is further supported by comparative 18S results, whereby NJ and ML methods both place the new species closest in evolutionary distance to Dillwynella planorbis [AB365310.1], although this is with weak bootstrap support (39% and 42% for NJ and ML methods respectively).
The first right neck lobe tentacle (RNL1) in the new species may be modified (see Figure 4 B, D). Warén & Bouchet (Reference Warén and Bouchet1989) described a modified neck lobe tentacle in Bathymargarites symplector Warén & Bouchet Reference Warén and Bouchet1989 and interpreted this modified appendage as a penis. Collection of further specimens will enable the reproductive anatomy of the new species to be characterized.
The recent discovery of hydrothermal vents and chemosynthetic assemblages on the Mid-Cayman Spreading Centre has provided an opportunity to enhance existing knowledge about biodiversity in the deep sea. Iheyaspira bathycodon sp. nov. is the second new species to be described from the Von Damm Vent Field, and the tenth turbinid gastropod to be described from a hydrothermal vent environment to date (Table 1). Description of species from Mid-Cayman Spreading Centre vents, and further characterization of their faunal assemblages by future collections has the potential to elucidate the factors determining vent biogeography of this region.
ACKNOWLEDGEMENTS
The authors extend their thanks to those on-board the 44th voyage of RRS ‘James Cook', to P.A. Tyler and C.L. Van Dover for providing laboratory facilities at NOCS and DUML, and to A. Glover (NHMUK) and A. Page (Biomedical Imaging Unit, University of Southampton) for use of their microscopy facilities. A. Warén and S. Williams are also thanked for commenting on aspects of this work, which is supported by a UK NERC award (NE/F017774/1) to J.Copley and NASA ASTEP Grant (NNX09AB75G) to C.L. Van Dover. This paper benefitted from an anonymous referee who gave valuable comments for its improvement.










