INTRODUCTION
Modelling species distribution is a valuable tool of biological conservation efforts, especially predictive models of marine predators, due to the logistical difficulties of monitoring their distributions at sea. For instance, managers of whale and dolphin populations can benefit from accurate model-derived predictions of cetacean habitat to mitigate anthropogenic effects, such as fisheries by-catch (Torres et al., Reference Torres, Rosel, D'Agrosa and Read2003) and the impacts of habitat alterations on ecosystem function (Baumgartner & Mullin, Reference Baumgartner and Mullin2001; D'Amico et al., Reference D'Amico, Bergamasco, Zanasca, Carniel, Nacini, Portunato, Teloni, Mori and Barbanti2003), in order to protect critical habitat (Hooker et al., Reference Hooker, Whitehead and Gowans1999; Cañadas et al., Reference Cañadas, Sagarminaga, de Stephanis, Urquiola and Hammond2005; Gregr & Trites, 2011) and understand the ecology of these animals (Hamazaki, Reference Hamazaki2002). By assuming that the distribution of cetaceans is non-random relative to environmental variability, predictive models of cetacean distribution typically identify the ecological relationships between the environment and species habitat selection.
Killer whales, Orcinus orca (Linnaeus, 1758) have a widespread distribution throughout the world's oceans and seas, from polar waters to the equator (Leatherwood & Dalheim, Reference Leatherwood and Dalheim1978; Heyning & Dahlheim, Reference Heyning and Dahlheim1988; Forney & Wade, Reference Forney, Wade and Estes2007). They are known to be common in many coastal areas, particularly at high latitudes, probably due to higher ocean productivity (Forney & Wade, Reference Forney, Wade and Estes2007), but they also occur in offshore and tropical waters (Leatherwood & Dalheim, Reference Leatherwood and Dalheim1978; Forney & Wade, Reference Forney, Wade and Estes2007). Killer whales are known to present seasonal movement patterns, often associated with increased prey availability in the north-east Pacific (Braham & Dahlheim, Reference Braham and Dahlheim1982; Baird & Dill, Reference Baird and Dill1995), North Atlantic (Sigurjónsson & Leatherwood, Reference Sigurjónsson and Leatherwood1988) and South Atlantic (Iñiguez, Reference Iñiguez2001). In the Atlantic, they are common around the northern part of the British Isles, along the Norwegian coast and throughout the eastern North Atlantic. They occasionally enter the North Sea and Skagerrak Strait (Reid et al., Reference Reid, Evans and Northridge2003). Killer whales are regularly seen in the Strait of Gibraltar and adjacent Atlantic waters (Horozco, Reference Horozco1598; Aloncle, Reference Aloncle1964a; Casinos & Vericad, Reference Casinos and Vericad1976; Bayed & Beaubrun, Reference Bayed and Beaubrun1987; Guinet et al., Reference Guinet, Domenici, de Stephanis, Barrett-Lennard, Ford and Verborgh2007; de Stephanis et al., 2008; Esteban, Reference Esteban2008; Foote et al., Reference Foote, Vilstrup, de Stephanis, Verborgh, Nielsen, Deaville, Kleivane, Martín, Miller, Øien, Pérez-Gil, Rasmussen, Reid, Robertson, Rogan, Similä, Tejedor, Vester, Víkingsson, Willerslev, Gilbert and Piertney2011). Conversely, killer whales are considered as a rare species in the Mediterranean Sea (Casinos & Vericad, Reference Casinos and Vericad1976; Raga et al., Reference Raga, Raduán and Blanco1985; Bayed & Beaubrun, Reference Bayed and Beaubrun1987; Notarbartolo di Sciara, Reference Notarbartolo di Sciara1987; Notarbartolo di Sciara & Birkun, Reference Notarbartolo di Sciara and Birkun2010).
In southern Spain, killer whales are observed in spring in the Gulf of Cadiz (Guinet et al., Reference Guinet, Domenici, de Stephanis, Barrett-Lennard, Ford and Verborgh2007; García-Tiscar, Reference García-Tiscar2009), when their main prey, Atlantic bluefin tuna (hereafter ABFT) (Thunnus thynnus), enters the Mediterranean Sea (Cetti, Reference Cetti1777; Sella, Reference Sella1929; Rodríguez-Roda, Reference Rodríguez-Roda1964) and in summer associated with the ABFT long-line fishery of the Strait of Gibraltar (Srour, Reference Srour1994; de la Serna et al., Reference de la Serna, Alot, Majuelos and Rioja2004), when ABFT return from their spawning areas on their way back to the Atlantic Ocean (Lozano, Reference Lozano1958; Aloncle, Reference Aloncle1964b; Rodríguez-Roda, Reference Rodríguez-Roda1964; de Stephanis et al., Reference Forney, Wade and Estes2008b). These killer whales belong to the same population as killer whales sampled in the Canary Islands, and are significantly different from other populations in the north-east Atlantic (North Sea, Iceland and Norway) (Foote et al., Reference Foote, Vilstrup, de Stephanis, Verborgh, Nielsen, Deaville, Kleivane, Martín, Miller, Øien, Pérez-Gil, Rasmussen, Reid, Robertson, Rogan, Similä, Tejedor, Vester, Víkingsson, Willerslev, Gilbert and Piertney2011). Additionally, differences in carbon and nitrogen stable isotopes ratios (García-Tiscar, Reference García-Tiscar2009) and parasite load (Mackenzie, Reference Mackenzie1999; Dwyer & Visser, Reference Dwyer and Visser2011) among pods from the Strait of Gibraltar–Canary Islands population suggest that it is a non-cohesive population that may not follow the same resource all year round.
Due to their small population size, the killer whales of the Strait of Gibraltar and contiguous waters have been recommended to be included in the ‘Critically Endangered’ category by ACCOBAMS–IUCN (Cañadas & de Stephanis, Reference Cañadas, de Stephanis, Reeves and Notarbartolo di Sciara2006). Likewise, the International Whaling Commission has recommended the implementation of a conservation plan for this subpopulation as soon as possible (IWC, 2007), and since 2011 the Spanish Ministry of Environment has considered the killer whales of the Strait of Gibraltar and the Gulf of Cadiz ‘Vulnerable’ in the Spanish Catalogue of Endangered Species. Both local (Andalusia) and Spanish authorities have recommended studying the spatial distribution of the species in southern Spain, to delineate it and the boundaries to be managed for a correct implementation of the conservation plan.
A great effort has been made to describe the distribution of marine mammals in the Alboran Sea and Strait of Gibraltar (Cañadas et al., Reference Cañadas, Sagarminaga, de Stephanis, Urquiola and Hammond2005; Cañadas & de Stephanis, Reference Cañadas, de Stephanis, Reeves and Notarbartolo di Sciara2006; Cañadas & Hammond, Reference Cañadas and Hammond2006; Guinet et al., Reference Guinet, Domenici, de Stephanis, Barrett-Lennard, Ford and Verborgh2007; de Stephanis, et al., Reference de Stephanis, Cornulier, Verborgh, Salazar Sierra, Pérez Gimeno and Guinet2008a, Reference de Stephanis, García-Tiscar, Verborgh, Esteban Pavo, Pérez, Minvielle-Sebastia and Guinetb; Verborgh et al., Reference Verborgh, de Stephanis, Pérez, Yaget, Barbraud and Guinet2009; Foote et al., Reference Foote, Vilstrup, de Stephanis, Verborgh, Nielsen, Deaville, Kleivane, Martín, Miller, Øien, Pérez-Gil, Rasmussen, Reid, Robertson, Rogan, Similä, Tejedor, Vester, Víkingsson, Willerslev, Gilbert and Piertney2011), but little is known about the distribution of killer whales in the whole study area, as only one publication describes the summer distribution in the central area of the Strait of Gibraltar (de Stephanis et al., Reference de Stephanis, Cornulier, Verborgh, Salazar Sierra, Pérez Gimeno and Guinet2008a), and no information is available for the rest of the Alboran Sea or the Gulf of Cadiz. This study aims to understand the spatial distribution of killer whales in the southern Iberian Peninsula in relation to different environmental variables, both in spring and summer, from data series compiled from research, whale watching vessels and opportunistic data.
MATERIALS AND METHODS
Study area
The present study was carried out in the Gulf of Cadiz, Strait of Gibraltar and the Alboran Sea (Figure 1). It is found between 0°30′E and 9°30′W and between 37°30′ and 35°N, and is bounded to the north by the Iberian Peninsula and to the south by Africa. These areas display different bathymetric features. The Gulf of Cadiz has a wide, smooth shelf and slope. In contrast, the northern shelf of the Alboran Sea is very narrow, but has a very steep shelf and slope gradient. The Gulf of Cadiz, located in the eastern sector of the central North Atlantic, is concave in shape, with a NW–SE orientation (Roberts, Reference Roberts1970; Malod, Reference Malod1982). The physiographic profile of the margin includes a wide shelf (30–40 km) with a sea-floor slope of 0.2–0.32°, a shelf-break located at a water depth of between 140 and 120 m, and a smooth continental slope, with sea-floor gradients of 1.5° in the upper part and 0.5–1° in the middle and lower parts (Baraza et al., Reference Baraza, Ercilla and Nelson1999). The Strait of Gibraltar has a length of 60 km (on an E–W axis), and a mean width of 20 km. The shallowest depth, less than 300 m, is found in the main sill of Camarinal and its minimum width of around 14 km coincides with the contraction of Tarifa narrow. The bathymetry of the Strait is characterized by a west to east canyon, with shallower waters (200–300 m) found on the Atlantic side and deeper waters (800–1000 m) on the Mediterranean side. On the eastern side of the Strait of Gibraltar lies the Alboran Sea basin, at the western edge of the Mediterranean Sea. It is arc-shaped, and characterized generally by a complex physiography related to its tectonic history. The Spanish margin has a very narrow, steep shelf (5–10 km) up to the shelf-break at 110–120 m water depth, which establishes the boundary, with a shelf gradient of 0.5–0.7° and a slope of 2.3° (Ercilla et al., Reference Ercilla, Alonso and Baraza1992; Hernández-Molina, Reference Hernández-Molina1993; Hernández-Molina et al., Reference Hernández-Molina, Somoza, Rey and Pomar1994).

Fig. 1. Cetacean sighting distribution in the study area. In red killer whales sightings and in grey other cetacean sightings: (A) sightings in spring in the southern Iberian Peninsula; (B) sightings in spring in the Strait of Gibraltar; (C) sightings in summer in the southern Iberian Peninsula; (D) sightings in summer in the Strait of Gibraltar.
Data collection
Sightings from cetaceans collected between 2002 and 2012 in waters of the southern Iberian Peninsula were compiled. The datasets were separated in two datasets as killer whales have been described in different areas during different seasons: shallow waters in the Gulf of Cadiz in spring (April–June) (Guinet et al., Reference Guinet, Domenici, de Stephanis, Barrett-Lennard, Ford and Verborgh2007), and in the central waters of the Strait of Gibraltar in summer (July–September) (de Stephanis et al., Reference de Stephanis, Cornulier, Verborgh, Salazar Sierra, Pérez Gimeno and Guinet2008a). We have also based this separation on the migration pattern of their main prey, bluefin tuna (Cetti, Reference Cetti1777; Sella, Reference Sella1929; Rodríguez-Roda, Reference Rodríguez-Roda1964). Insufficient data were available to make robust spatial models for the rest of the year. A total of 11,276 records of cetaceans were available for this analysis coming from: (1) random transect sightings from a research boat performed by the non-governmental organization (NGO) ANSE (Asociación de Naturalistas del Sureste) in the Murcia region; (2) sightings collected by Alnitak since 2002, a research NGO which performed a random transect throughout the Alboran Sea (Cañadas et al., Reference Cañadas, Sagarminaga, de Stephanis, Urquiola and Hammond2005); (3) sightings collected by Alnilam since 2010, a research company which performed a random transect throughout the Alboran Sea; (4) sightings of CIRCE (Conservation, Information and Research on Cetaceans) since 2002, a research NGO that made random and line transects throughout the Strait of Gibraltar and Gulf of Cadiz (de Stephanis et al., Reference de Stephanis, Cornulier, Verborgh, Salazar Sierra, Pérez Gimeno and Guinet2008a); (5) sightings of TURMARES (Turismo Marítimo del Estrecho) since 2003, a whale watching company based in the Strait of Gibraltar; (6) sightings from Mar Ilimitado (Tourism and Research) since 2005, a whale watching and research company based in southern Portugal; (7) sightings from Consejería de Medio Ambiente de la Junta de Andalucía, a governmental institution of the local council of Andalucía since 2005, that performs random and line transects by boat and aeroplane throughout the entire Andalusian region; (8) sightings collected by the EBD-CSIC (Estación Biológica de Doñana – Consejo Superior de Investigaciones Científicas) since 2011, from a small research boat doing line transects in the Gulf of Cádiz; and (9) opportunistic data.
Environmental explicative models
Habitat preferences of killer whales within the study area were investigated. The relationships between the spatial occurrence of the whales, and environmental variables were assessed using generalized additive modelling (GAM) techniques (Hastie & Tibshirani, Reference Hastie and Tibshirani1990). The open-source statistical programming language R v. 2.6.2 (http://cran.r-project.org/), and the MGCV library within R were used (Wood, Reference Wood2001). Given that data were coming from different sources, types of effort are difficult to compare, and therefore a model based on presence and ‘pseudo-absence’ was used. A GAM with a Tweedie distribution and logit link function was used. The parameter p chosen for the Tweedie distribution, through inspection of the generalized cross validation (GCV) score, an approximation to the Akaike information criterion (AIC) (Wood, Reference Wood2000), was 1.1, very close to a Poisson distribution but with some over-dispersion. The model used a gamma = 1.4, as recommended by Wood (Reference Wood2006), to prevent overfitting.
All the cetaceans’ sightings were used as ‘sampling stations’. The presence dataset included sightings of killer whales obtained by the different platforms. We used all the other cetacean species in the study area as ‘pseudo-absences’. In these locations, we assumed that an observer was effectively searching whales as other species were sighted, but killer whales were not detected. The general structure of the model was:
 $$E\lpar p_i \rpar =\exp \left[{\theta _0+\sum\limits_k {\,f_k \lpar z_{ik} \rpar } } \right].$$
$$E\lpar p_i \rpar =\exp \left[{\theta _0+\sum\limits_k {\,f_k \lpar z_{ik} \rpar } } \right].$$where p i is the probability to find killer whales, in the ith sampling station, θ0 is the intercept, f k are smoothed functions of the explanatory covariates and z ik is the value of the kth explanatory covariate in the ith sampling station. The environmental variables used in this study were depth, obtained from ETOPO2 (Amante & Eakins, Reference Amante and Eakins2009), its derivate slope and aspect obtained with the R library SDMtools (VanDerWal et al., Reference VanDerWal, Shoo and Januchowski2010), sea surface temperature (SST) and chlorophyll-a concentration (Chla) obtained from satellite images of MODIS (Carder et al., Reference Carder, Chen, Lee, Hawes and Cannizzaro2003). Spatial (geographical) covariates, i.e. latitude and longitude, were also used as potential proxies for other unavailable or unknown features affecting whales’ distribution. Model selection was done using three diagnostic indicators: (a) the GCV; (b) the percentage of deviance explained; and (c) the probability that each variable was included in the model by chance. The decision to include/drop a term from the model was adopted following the criteria proposed by Wood (Reference Wood2001).
Environmental predictive models
The best GAM models were used to generate predicted probability values of presence and pseudo-absence on a grid of 2 × 2 km in the study area, which were plotted using ArcMap 10.0. In order to obtain the 95% confidence interval of the predictions, 1000 bootstraps with replacement were run for each model, and a prediction was obtained for each bootstrap iteration. The 95% confidence intervals were obtained from the bootstrap process of the models predictions of killer whales in the study area and were also plotted in to assess the precision of the predictions in every point of the study area.
RESULTS
Between 2002 and 2012, 322 sightings of killer whales and 10,952 sightings of other cetaceans were recorded in the study area. We created two models for the presence of killer whales in the area (spring and summer). A total of 44 sightings of killer whales and 3746 of other cetaceans were recorded in spring. During this season the best model included two covariates (see Table 1): depth and longitude, both highly significant, and explaining 50.4% of the deviance. Killer whales presence was expected between 0 and 950 m depth showing a linear increasing pattern towards shallower waters and between 8.5° and 4°W (see Figure 2) with an equally higher presence for this longitude range and a decrease for the rest of the area, with a higher coefficient of variation (see Figure 4).

Fig. 2. Smoothed functions for the selected predictive covariates in the model of probability of presence of killer whales in the study area in spring. Continuous line represents the point estimate and grey areas represent ±ISE. The small ticks on the X axis represent the samples. (A) Bathymetry; (B) longitude.
Table 1. Detection-function model fits with the generalized cross validation (GCV) score and ΔGCV between models and Akaike information criterion (AIC) and ΔAIC between models, during the spring period. Only covariates showing significant relationship are shown.

In summer 278 sightings of killer whales and 7206 of other cetaceans were recorded. The best model included three covariates (Table 2): depth, longitude and sea surface temperature, all of them significant and explaining in total 49.60% of the deviance. Presence probability was higher at 0–950 m depth, between 8.5° and 4°W and at 19–24°C SST (see Figure 3).
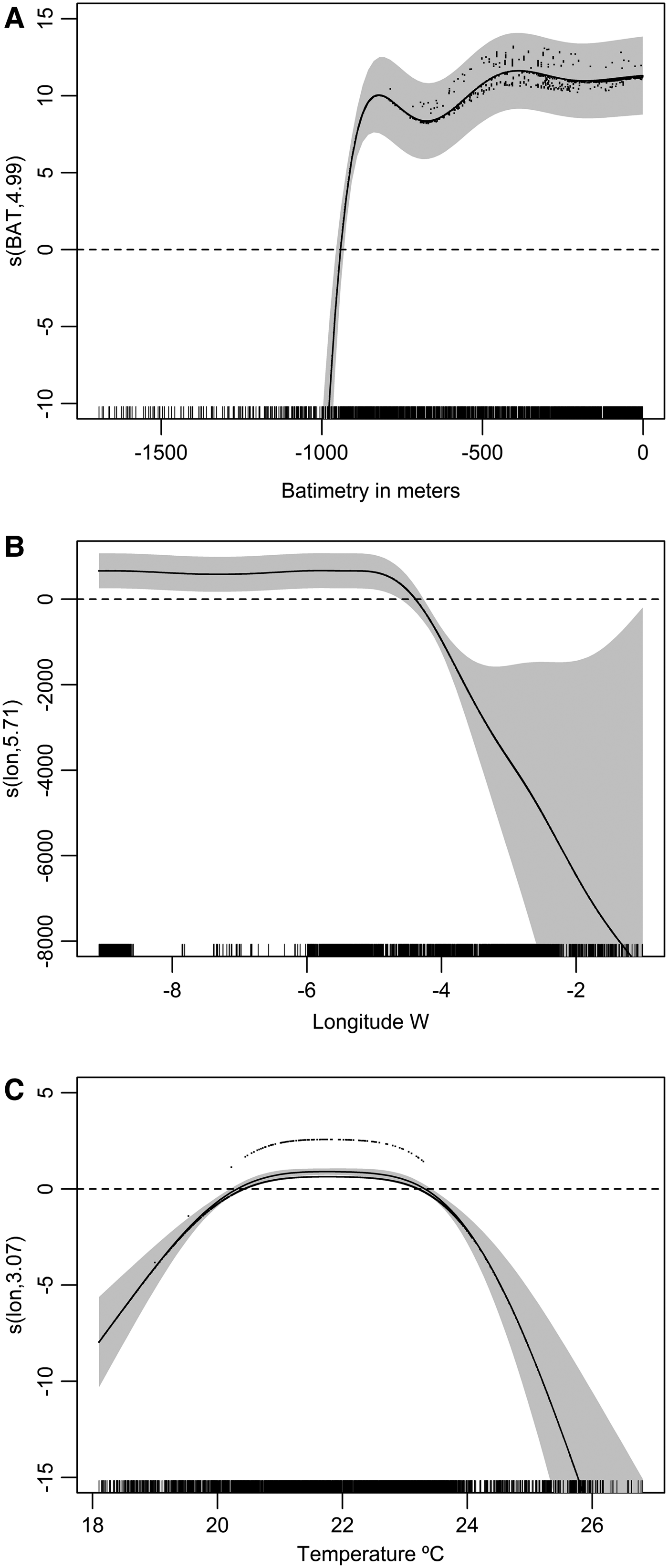
Fig. 3. Smoothed functions for the selected predictive covariates in the model of probability of presence of killer whales in the study area in summer. Continuous line represents the point estimate and grey areas represent ±ISE. The small ticks on the X axis represent the samples. (A) Bathymetry; (B) Longitude; (C) sea surface temperature.
Table 2. Detection-function model fits with generalized cross validation (GCV) score and ΔGCV between models and Akaike information criterion (AIC) and ΔAIC between models and the deviation explained for each model, during the summer period. Only covariates showing significant relationship are shown.

The spring model prediction map shows two important areas for the presence of killer whales, one in the eastern part of the Gulf of Cadiz in shallow Spanish and Moroccan waters and another one in the south of Portugal (Figure 4). While the variance is very low for the Gulf of Cadiz, it is high in the south of Portugal (Figure 4).

Fig. 4. Predicted density surface map of killer whales in the area from spatial modelling in spring by bootstraps: (A) the inferior 95% CI of the predicted model at southern Iberian Peninsula; (B) the inferior 95% CI of the predicted model in the Strait of Gibraltar; (C) best predicted model at southern Iberian Peninsula; (D) best predicted model in the Strait of Gibraltar; (E) the superior 95% CI of the predicted model at southern Iberian Peninsula; (F) the superior 95% CI of the predicted model in the Strait of Gibraltar.
The summer model prediction map shows a high presence only in the western-central part of the Strait of Gibraltar (Figure 5), with little variance in the prediction (Figure 5).

Fig. 5. Predicted density surface map of killer whales in the area from spatial modelling in summer by bootstraps: (A) the inferior 95% CI of the predicted model at southern Iberian Peninsula; (B) the inferior 95% CI of the predicted model in the Strait of Gibraltar; (C) best predicted model at southern Iberian Peninsula; (D) best predicted model in the Strait of Gibraltar; (E) the superior 95% CI of the predicted model at southern Iberian Peninsula; (F) the superior 95% CI of the predicted model in the Strait of Gibraltar.
DISCUSSION
This study highlights the importance of collaborative datasets to understand the distribution of animals with low encounter rates. The kind of datasets we have used are statistically difficult to deal with, due to the differences in surveying conditions, but these data are of great importance in understanding which environmental variables are responsible for the spatial distribution of this species. Here we have solved the heterogeneity of effort from different datasets by using other cetacean species’ presence as a proxy for killer whale absence. Recent studies have highlighted several methods for selection of pseudo-absence, including: random (Stockwell, Reference Stockwell1999); random with geographical-weighted exclusion (Hirzel et al., Reference Hirzel, Helfer and Metral2001), random with environmentally-weighted exclusion (Zaniewski et al., Reference Zaniewski, Lehmann and Overton2002) and locations that have been visited (i.e. occurrences for other species, as in our case) but where the target species was not recorded (Elith & Leathwick, Reference Elith and Leathwick2007). The benefits of each technique have been discussed previously (Lütolf et al., Reference Lütolf, Kienast and Guisan2006; Phillips & Dudík, Reference Phillips and Dudík2008), and are outside the scope of this study. Although the chosen approach is not ideal, in our opinion it is suitable due to the high amount of sightings of other cetacean species in the study area, and the non-availability of homogeneous effort.
We have demonstrated clear and predictable influences of environmental and geographical factors on the distribution of killer whales in this region. The results support previous studies showing that killer whales are encountered in shallow waters of the south-western part of the Strait of Gibraltar in summer (de Stephanis et al., Reference de Stephanis, Cornulier, Verborgh, Salazar Sierra, Pérez Gimeno and Guinet2008a). The distribution pattern of the species seems to be closely linked to the bathymetric structure within the area. Previous studies have shown that water depth can be a significant factor in determining the distribution of marine top predators, (e.g. Selzer & Payne (Reference Selzer and Payne1988), de Stephanis et al. (Reference de Stephanis, Cornulier, Verborgh, Salazar Sierra, Pérez Gimeno and Guinet2008a) and Schneider (Reference Schneider2008) have suggested that the importance of water depth is related to prey availability.
Atlantic bluefin tuna (ABFT) live in pelagic ecosystems of the entire North Atlantic and its adjacent seas, primarily the Mediterranean Sea (Fromentin & Powers, Reference Fromentin and Powers2005). Some tuna born in the Mediterranean Sea, leave and undertake trophic migrations within the eastern Atlantic Ocean up to age 4 or 5 years, at which point they reach sexual maturity and return to the Mediterranean Sea to spawn in spring (Aloncle, Reference Aloncle1964b; Dicenta & Piccinetti, Reference Dicenta and Piccinetti1978; Cort, Reference Cort1990; Mather et al., Reference Mather, Mason and Jones1995). Then, ABFT return to the Atlantic Ocean in summer crossing the Strait of Gibraltar in a trophic migration (Sella, Reference Sella1929; Rodríguez-Roda, Reference Rodríguez-Roda1964). The different predicted spatial distributions of killer whales in spring and summer match the spawning and trophic migrations of ABFT through the study area.
The spring model predicts killer whales presence in shallow waters close to the west coast of the study area, where they would be capable of hunting schools of ABFT entering the Mediterranean, using the endurance–exhaustion hunting technique described in the Gulf of Cadiz (<100 m depth) by Guinet et al. (Reference Guinet, Domenici, de Stephanis, Barrett-Lennard, Ford and Verborgh2007). Killer whales are known to dive to depths no more than 300 m (Bowers & Henderson, Reference Bowers and Henderson1972). Hunting in shallow waters can prevent ABFT from taking refuge at depths (Guinet et al., Reference Guinet, Domenici, de Stephanis, Barrett-Lennard, Ford and Verborgh2007).
In addition to depth and longitude, temperature had a significant influence on the presence probability of killer whales during summer, restricting their distribution area to the Strait of Gibraltar. Quílez-Badia et al. (Reference Quílez-Badia, Cermeño and Tudela2012) showed that ABFT were crossing the Strait in water temperatures between 19 and 24°C while swimming to the Atlantic Ocean, which matches the temperature range predicting the presence of killer whales in the Strait of Gibraltar in summer in this study. Deep dives have been reported through the use of tags on ABFT while they are crossing the Strait of Gibraltar (Wilson & Block, Reference Wilson and Block2009), suggesting: (a) that they may forage on squid and fish inhabiting deep Mediterranean outflow waters; (b) that maybe these dives were related to predator avoidance by the killer whales; or (c) that the dives were related to thermoregulation and energetic saving associated with avoiding ocean currents. Since 1995, Spanish and Moroccan fisheries have been established and developed near sea mounts in the Strait of Gibraltar during the western tuna migration (Srour, Reference Srour1994; de la Serna et al., Reference de la Serna, Alot, Majuelos and Rioja2004). Killer whales have been observed among fishing boats at least since 1999 in the central part of the Strait (de Stephanis et al., Reference de Stephanis, Cornulier, Verborgh, Salazar Sierra, Pérez Gimeno and Guinet2008a) either (a) depredating tuna from the long-lines or (b) actively chasing tuna in the area, in these shallower waters where ABFT are not able to avoid them by performing deep dives. The presence of ABFT long-line fisheries, the shallow waters of the Strait, as well as the bottleneck that represents the Strait of Gibraltar for the migration of the ABFT would explain the high predictability of the species in the area both in summer and spring.
In the Alboran Sea a large effort has been made for the study of cetaceans, but only four sightings of killer whales have been recorded in the area in 10 years (Figure 1), and none of the selected models have identified any important area for killer whales in that area in this study (Figures 4 & 5). The Alboran Sea is characterized by a narrow, steep shelf (5–10 km) (Ercilla et al., Reference Ercilla, Alonso and Baraza1992; Hernández-Molina, Reference Hernández-Molina1993; Hernández-Molina et al., Reference Hernández-Molina, Somoza, Rey and Pomar1994) compared to the wide shelf (30–40 km) of the Gulf of Cadiz (Baraza et al., Reference Baraza, Ercilla and Nelson1999). This difference could be crucial for the presence of killer whales in the area, as they seem to prefer hunting in shallow areas (Guinet et al., Reference Guinet, Domenici, de Stephanis, Barrett-Lennard, Ford and Verborgh2007). Therefore, it seems better for the killer whales to wait for ABFT near the shallow sea mounts of the Strait of Gibraltar and adjacent waters. In southern Portugal (Algarve), there are few sightings of killer whales (Figure 1), but the spring model predicts an important area near Faro (Figure 4). This area used to be one of the main places for Portuguese tuna trap nets (Ravier & Fromentin, Reference Ravier and Fromentin2001), therefore it could be a suitable area for killer whale endurance–exhaustion hunting (Guinet et al., Reference Guinet, Domenici, de Stephanis, Barrett-Lennard, Ford and Verborgh2007). Little effort was available in the Algarve, and the model prediction shows a great variance in the area (Figure 4), that could be due to the low availability to ‘pseudo-absences’ in the models (Figure 1). Therefore, this area should be further surveyed to improve the spring model prediction. No cetacean survey has been made in Moroccan waters; however, the spring model has identified the North Atlantic coast of Morocco as an important area. Further studies should focus on refining habitat predictions and examining relationships between killer whale distributions and environmental correlations over the years and in areas not so well studied as Moroccan and Portuguese coasts and offshore waters of the southern Iberian Peninsula. Scarce sightings (both of killer whales and other species) were recorded in autumn and winter, which did not allow modelling of their presence during these periods of the year.
Conservation of the eastern Atlantic stock of killer whale prey is essential for the future of both endangered predator and prey in the area, as ABFT has been overfished (Collette et al., Reference Collette, Amotin, Boustanu, Carpenter, De Oliveira Leite, Di Natale, Die, Fox, Fredou, Graves, Viera Hazin, Hinton, Juan Jorda, Schratwieser, Texeira Lessa, Pires Ferreira Travassos and Uozumi2012) and Iberian killer whales have a highly specialized diet (García-Tiscar, Reference García-Tiscar2009). The fecundity of another population of killer whales in the eastern Pacific Ocean has been strongly related to the abundance of their prey, the Chinook salmon, where a decrease in salmon populations caused senescence in the whales and affected their fecundity rates (Ward et al., Reference Ward, Holmes and Balcomb2009). For that reason, any decrease in the abundance of ABFT could set the Iberian population of killer whales at greater risk. Therefore, important areas for southern Iberian killer whales require adequate protection, such as the creation of an exclusion zone where activities that may disrupt their hunting technique, such as whale watching, military exercises or sport fishing, are prohibited in spring in the eastern areas of the Gulf of Cadiz (both in Spain and Morocco). The conservation of this killer whale population distributed across national borders will only be achieved through collaboration and coordination between the neighbouring countries Spain, Portugal and Morocco, and the implementation of a joint action plan to protect the habitat.
ACKNOWLEDGEMENTS
We would like to specially thank Alnitak for supplying their sighting data-base. Thanks are due to the Consejería de Agricultura, Pesca y Medio Ambiente of the Junta de Andalucía and the Agencia de Medio Ambiente y Agua of the Junta de Andalucía, especially to Manuel Fernández Casado, Diego Moreno, Agustin Barrajón, Maria del Carmen Arroyo, Julio de la Rosa, Jose Miguel Remón, Eduardo Fernández Tabales and María Soledad Vivas. Thanks to Rebecca Bringley, Teo Todorov, Javier Elorriaga, Rafael García, José María Carceles and Clive Finlayson for supplying killer whale sightings. João Nuno Gonçalves, Bruno Claro, CIRCE volunteers and research assistants, helped in the fieldwork of CIRCE and the EBD-CSIC. Thanks are also due to the IFAW for providing the software Logger 2000.
FINANCIAL SUPPORT
This work was funded by the Loro Parque Foundation, CEPSA, Ministerio de Medio Ambiente, Fundación Biodiversidad, Life Indemares (LIFE 07 NAT.E.000732) and LIFE ‘Conservación de Cetáceos y tortugas de Murcia y Andalucía’ (LIFE 02 NAT/E/8610), the ‘Plan Nacional I + D + I’ of the Spanish Ministerio de Economía y Competitividad. De Stephanis was supported by the Ministerio de Economía y Competitividad through the ‘Subprograma Juan de la Cierva’.







