INTRODUCTION
Hydrozoans are common and widespread invertebrates on shallow water. Yet, as they are mostly represented by species with small body dimensions and cryptic life habits, their study is neglected and the knowledge on their biodiversity is far from satisfactory. In general, accumulated knowledge is geographically concentrated, with some areas almost unknown. This is particularly true for the Brazilian coast, in which just a few localities of its south-eastern region are relatively well-known (Migotto et al., Reference Migotto2002; Marques et al., Reference Margulis and Karlsen2003; Migotto & Marques, Reference Migotto, Marques, Morandini and da Silveira2006). New species of hydroids continue to be described every year worldwide, even in relatively well-known regions, with the discovery of inconspicuous specimens and re-evaluation of morphological variation.
Among hydrozoans, the poorly-known family Hydrocorynidae Rees, Reference Rees, Hand and Mills1957 (Cnidaria: Hydrozoa: ‘Anthoathecata’) comprises two valid genera (Kubota, Reference Kramp1988; Mangin, Reference Leloup1991): Hydrocoryne Stechow, 1907, and Samuraia Mangin, Reference Leloup1991. The three species of the group so far described are restricted to Pacific waters (Kubota, Reference Kramp1988; Mangin, Reference Leloup1991), with only one unidentified record (Hydrocoryne sp.) for the Atlantic Ocean (Wedler & Larson, Reference Walker1986).
A hydroid belonging to the family Hydrocorynidae was never reported in the southern hemisphere, including the Atlantic Ocean. Species such as Hydrocoryne, which are inconspicuous and difficult to collect individually by hand, are usually found after examining substrata—larger organisms such as algae and invertebrates with hard skeletons, and pebbles and biogenic nodules—in the laboratory. Nevertheless, delicate specimens usually get damaged during or after collecting procedures (e.g. manual, trawling, dredging or grabbing techniques) and their soft parts may shrink, degrade or be resorbed, making their remains indistinguishable or simply hard or impossible to see, even under a microscope. However, when pieces of substrata such as pebbles, broken shells and calcareous nodules are kept in the laboratory under favourable conditions, the shrunk polyps or the regressed or dormant tissues may become active or give rise to new polyps. Many species of hydroids were described employing intentionally this technique or just by chance, as was the species described here.
Distinction between species of the genus Hydrocoryne is based on the medusa stage, thus development and life cycle observations are essential for identification. In rearing the species, we gathered data of not only its sexual phase but also of an unusual mode of asexual reproduction within Hydrozoa—longitudinal fission.
Asexual reproduction is a poorly known reproductive trait in several cnidarian groups. Although hydrozoans present a wide variety of asexual processes, fission seems restricted to a few species, and may be considered a rare phenomenon within the Hydrozoa (Shostak, Reference Schulz1993). Longitudinal fission, in particular, is reported for a few hydromedusae (e.g. Stretch & King, Reference Stepanjants1980) and hydropolyps (Shostak, Reference Schulz1993; Bouillon et al., Reference Bouillon, Medel, Pagès, Gili, Boero and Gravili2004).
The uniqueness of the process of longitudinal fission among the hydrozoans highlights the importance of the new finding, which may shed light on the understanding of the evolutionary pathways in Cnidaria and animals. Therefore, the goal of this study is to describe a new species of Hydrocorynidae, from the tropical waters of the south-eastern Brazilian coast, and its unusual mode of asexual reproduction.
MATERIALS AND METHODS
The hydroids were found growing on rhodoliths of coralline algae (called ‘living stones or rocks’ by Brazilian aquarists) in an aquarium in August 2005. Rhodoliths were collected from the Guarapari county region (20°40′S 040°29′W), state of Espírito Santo, Brazil (Figure 1). Certainty concerning the original location of the species was possible because the aquarium contained only artificial seawater and recently collected rhodoliths from the same place. Two colonies were isolated from the substratum and transferred to a small glass aquarium (500 ml) with constant air bubbling.

Fig. 1. Map of Brazil, showing the type locality (Guarapari) of Hydrocoryne iemanja sp. nov.
The hydroids were reared in the laboratory at the Departamento de Zoologia of the Universidade de São Paulo. The colonies were kept in natural seawater from the São Sebastião Channel. The aquarium was kept under a natural daylight regime at room temperature (20–25°C). The seawater of the cultures was changed every week and the polyps were fed every other day with Artemia nauplii (method adapted from Jarms et al., Reference Hyman2002).
After the medusae were released from the polyp, they were kept in glass Erlenmeyer flasks of different sizes (250 and 500 ml) with gentle air bubbling to provide water current (see a fuller description of the method in Stampar et al., Reference Smothers, von Dohlen, Smith and Spall2006). Seawater was changed three times per week, and Artemia nauplii and gonad macerate of the clam Perna perna (Linnaeus, 1767) were offered daily as food. The medusae were also kept under natural light and room temperature at 20–25°C.
Morphological, morphometric, and cnidome studies were recorded from fresh and preserved material. Measurements were carried out under a dissection microscope. Preserved and fresh materials were used in squash preparations, with distilled water and saliva, for cnidome observations under interference-contrast light microscopy (cf. Migotto, Reference Marques and Lamas1996). The terminology for the cnidome is that of Weill (Reference Wedler and Larson1930, Reference Weill1934) and Mariscal (Reference Mangin1974).
Type material was deposited in the cnidarian collection of the Museu de Zoologia da Universidade de São Paulo (MZUSP).
RESULTS
AMENDED DESCRIPTION
Colonies unbranched, hydranths monomorphic spindle-shaped not well demarcated from hydrocaulus, oral tentacles capitate, long, hollow, in 5–6 close sets of whorls around conical hypostome, hydrocaulus long, highly extensible, naked, with thickened mesolamella issuing from hard hydrorhizal stolonal plate; medusa buds in clusters on basal part of hydrocaulus. Medusae bell-shaped; with or without gastric peduncle; four marginal tentacles with scattered cnidae and median knob; clasping tentacular bulbs with ocelli; manubrium broadly flask-shaped or tubular, quadrate or cruciform in cross-section; mouth cruciform, with or without cnidocyst clusters; gonads interradial without longitudinal groove, almost totally surrounding manubrium.
REMARKS
In a review of the capitate hydroids, Petersen (Reference Pérez1990) established the suborder Sphaerocorynida including two superfamilies, Sphaerocorynoidea (with three families: Paragotoeidae Ralph, 1959, Sphaerocorynidae Prévot, 1959 and Zancleopsidae, Bouillon, 1978) and Hydrocorynoidea (encompassing exclusively the family Hydrocorynidae). The genus Paragotoea Kramp, 1942 is considered presently among the Corymorphidae Allman, 1872 (cf. Bouillon et al., Reference Bouillon, Medel, Pagès, Gili, Boero and Gravili2004).
The family Hydrocorynidae was proposed by Rees (Reference Rees, Hand and Mills1957) to accommodate the Japanese hydroid Hydrocoryne miurensis Stechow, 1907. Polyps of this species are notable for their length when fully expanded, reaching up to 6 cm long or more (Rees et al., Reference Raikova1976). Nevertheless, the polyps have quite simple morphologies, and the systematics of the genus is based on differences of the medusae stage (cf. Kramp, Reference Jarms, Morandini and da Silveira1961). The family comprises only two genera (Hydrocoryne Stechow, 1907 and Samuraia Mangin, Reference Leloup1991) and three species have been described so far (Hydrocoryne bodegensis Rees, Hand & Mills, Reference Raikova1976; Hydrocoryne miurensis and Samuraia tabularasa Mangin, Reference Leloup1991) (see Rees et al., Reference Raikova1976; Mangin, Reference Leloup1991). In addition, Hydrocoryne sp., possibly representing a new species, is referred to Puerto Rico by Wedler & Larson (Reference Walker1986: 79) and Panama by Calder & Kirkendale (Reference Calder and Kirkendale2005: 481). The genera are differentiated by the production and release of medusae (as in Hydrocoryne) or eumedusoids that are either retained or not on the polyps (as in Samuraia) (Kubota, Reference Kramp1988; Mangin, Reference Leloup1991).
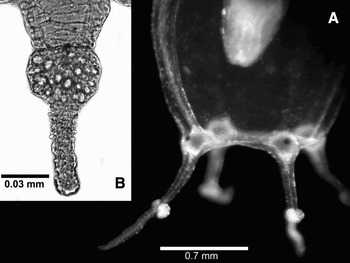
Fig. 2. Hydrocoryne iemanja sp. nov. (A) Basal half of a medusa with tentacles; (B) detail of the tentacle knob and contracted tentacle, note that in the tentacle there are nematocysts only in the knob.

Fig. 3. Hydrocoryne iemanja, sp. nov. (A) Polyp colony base with hard plate and five zooids partly laid on the bottom; (B) detail of a zooid with gonophores at the basal region of hydrocaulus; (C–D) zooid undergoing longitudinal fission; (E) hard plate formation at middle of hydrocaulus; (F) reproductive branch arising from the hydrocaulus, with stalked medusae in different stages of development.

Fig. 4. Hydrocoryne iemanja, sp. nov. (A) Newly released medusa; (B) newly released medusa just after feeding with Artemia; (C) mature male medusa (7 days old); (D) mature female medusa (7 days old), showing eggs on the sides of the manubrium; (E) detail of the manubrium of a female medusa, showing eggs; (F) detail of the umbrellar margin of a newly released medusa, showing a tentacle bulb with nematocysts and an ocellus.

Fig. 5. Asexual reproduction of polyp of Hydrocoryne iemanja, sp. nov. Diagram showing the sequence of a zooid undergoing longitudinal fission from its oral tip (A), branching (B), forming a hard plate at the basal part of hydrocaulus (C), and the new, asexually produced zooid (D).

Fig. 6. Hydrocoryne iemanja, sp. nov. Diagram showing the sequence of stages in the life cycle of the species: polyp; polyp with budding medusae arising from hydrocaulus; young medusa; mature male and female medusae; and planula larva. Drawings are not to scale.

Fig. 7. Hydrocoryne iemanja, sp. nov. Cnidome (A–D polyp tentacle; E–J polyp hydrocaulus; O–P young medusa; K–N and Q–R mature medusa). (A–B) Undischarged and discharged heterotrichous microbasic euryteles with inclusion; (C–D) undischarged and discharged stenoteles; (E–F) undischarged and discharged heterotrichous microbasic euryteles; (G–H) undischarged and discharged small stenoteles; (I–J) undischarged discharged large stenoteles; (K–L) undischarged and discharged small stenoteles; (M–N) undischarged discharged large stenoteles; (O–P) undischarged and discharged holotrichous isorhizae (from exumbrella of recently released medusae); (Q–R) undischarged and discharged microbasic mastigophores; (S–T) undischarged and discharged desmonemes. Scale bar = 15 µm.
TYPE MATERIAL
Holotype: female medusa from polyp culture, 0.9 mm high, 0.7 mm wide, preserved in 4% formaldehyde solution in seawater (Guarapari, state of Espírito Santo, Brazil, 20°40′S 040°29′W), reared in the laboratory for 7 days, 12 September 2005 [MZUSP 1972].
Paratypes: one 7-day-old male medusa, preserved in 4% formaldehyde solution in seawater (same locality as holotype), 12 September 2005 [MZUSP 1973]; five recently released medusae, preserved in 70% ethanol (same locality as holotype), 5 September 2005 [MZUSP 1974]; two polyps reared in the laboratory, preserved in 4% formaldehyde solution in seawater, 5 January 2005 [MZUSP 1975].
ETYMOLOGY
The specific name iemanja is derived from the name of the queen of the waters and seas (Iemanjá in Portuguese) of the African–Brazilian religions, derived from the Yoruba language expression Yèyé ọmọ ẹjá (‘mother which sons are little fishes’). Iemanjá is the daughter of the God or Goddess of the Sea (Olóòkun), and also the name attributed by the Yoruba ethnic nation in Nigeria of a river (Yemọja) (Verger, Reference Weill1981; Walker, Reference Tardent1990).
DIAGNOSIS
Hydrocoryne species with hydrorhiza embedded in chitinous dark-brown hard-plate with minute spines; asexual reproduction by longitudinal fission, and hydrorhizal plate arising from column. Tentacle of medusa filiform, except for a large, adaxial nematocyst cluster on its middle (Figure 2).
DESCRIPTION
Colonies with up to 7 sessile polyps (usually 2–3) (Figure 3A), arising from hydrorhiza embedded in chitinous hard dark-brown plate up to 2.5 cm in diameter, with minute spines (Figure 3A). Living polyps 7.0–8.5 cm high, 1–2 mm wide, whitish (or slightly orange tinted because of Artemia diet), monomorphic, variable in shape, generally elongate, spindle-shaped; hypostome prominent, nipple-shaped when relaxed, proboscis-like when extended, up to 2 mm high. Capitate tentacles (Figure 3B–D) up to 20 in number, 0.2–0.4 mm long, in 4–5 closely arranged whorls at suboral region; capitulum 0.08–0.12 mm in diameter. Stalked medusa buds on lateral branches, closely and alternately arranged at basal third of hydrocaulus; up to 9 lateral branches per hydranth and up to 7 medusa buds on each branch (Figure 3F). Asexual reproduction by longitudinal fission and production of a chitinous hard plate at basal 1/3 of parental hydrocaulus; fission process starts from oral end towards new hard plate; polyp becomes free and starts a new colony (Figures 3C–E & 5A–D). Nematocysts (Figure 7A–J): tentacles—heterotrichous microbasic euryteles with inclusion 13.7–14.7 × 4.9–5.8 µm (N = 10, mean = 14.4 × 5.19 µm, SD±0.47 × 0.47 µm), and stenoteles 14.7–16.6 × 9.8–10.7 µm (N = 10, mean = 15.4 × 9.9 µm, SD±0.9 × 0.41 µm); column—heterotrichous microbasic euryteles 10.7–12.7 × 4.9–6.8 µm (N = 10, mean = 11.5 × 5.9 µm, SD±0.77 × 0.55 µm), smaller stenoteles 11.7–14.7 × 5.8–8.8 µm (N = 10, mean = 12.9 × 7.4 µm, SD±1.2 × 1.0 µm), and larger stenoteles 17.6–19.6 × 11.7–13.7 µm (N = 5, mean = 18.6 × 13.1 µm, SD±0.98 × 0.87 µm).
Newly-released medusa is released in immature condition, transparent, slightly flattened on top, higher than wide (0.57 mm high; 0.51 mm in diameter) (Figure 4A–B). Mesoglea thin, without apical process and apical canal. Exumbrella with scattered nematocyst clusters; 3–4 nematocysts per cluster. Radial canals four, simple and straight; circular canal narrow. Velum narrow; velar opening ~1/3 of total diameter at margin (0.32 mm). Four perradial marginal bulbs, each with a short tentacle (0.16 mm long, 0.032 mm wide) and a large abaxial red ocellus (Figure 4A–B & F). Tentacles hollow, filiform, except for a large, adaxial nematocyst cluster on its middle. Manubrium quadratic, 0.24 mm high, with simple mouth; peduncle lacking. Gonad rudiments not visible on release. Nematocysts (Figure 7K–T): tentacles—smaller stenoteles 6.8–9.8 × 4.9–5.8 µm (N = 10, mean = 8.2 × 4.9 µm, SD±0.82 × 0.3 µm) with shaft length 9.8 µm, spines length 4.9 µm, and larger stenoteles 12.7–15.6 × 8.8–10.7 µm (N = 10, mean = 14.5 × 9.9 µm, SD±0.77 × 0.61 µm) with shaft length 11.7 µm, spines length 5.8 µm; tentacular bulbs—microbasic mastigophores 9.8–10.7 × 8.8–9.8 µm (N = 10, mean = 10.3 × 9.2 µm, SD±0.5 × 0.5 µm) and desmonemes 6.8–7.8 × 3.9–5.8 (N = 10, mean = 7.4 × 4.9 µm, SD±0.5 × 0.46 µm); exumbrella–holotrichous isorhizae in scattered pads with 2–6 nematocysts 7.8–9.8 × 4.9 µm (N = 10, mean = 8.4 × 4.9 µm, SD±0.68 × 0 µm).
Mature medusa bell-shaped (Figure 4C–D); bell 1.2–1.4 mm high, 0.9–1.1 mm in diameter at median part; umbrellar margin diameter up to 1.2 mm. Velum large; velar opening ~1/5 of total aboral diameter. Four radial canals, ring canal present. Four perradial tentacular bulbs with one marginal tentacle and one red adaxial ocellus each (Figure 4C–D). Tentacles short, contractile, with a large, adaxial nematocyst knob at middle of tentacle. Manubrium tubular, about 1/2–4/5 of subumbrellar height, without concentrations of nematocysts on lips. Males with shorter manubrium than females (Figure 4C–D). Gonads interradial, covering nearly whole manubrium, light orange to deep orange in male specimens and deep orange to red in female living specimens (Figure 4C–D & E). Eggs (0.05–0.06 mm) visible by transparence through thin manubrial epithelium (Figure 4D–E). Nematocysts (Figure 7K–R): tentacular bulbs—microbasic mastigophores 12.7–15.6 × 9.8–11.7 µm (N = 10, mean = 13.5 × 10.9 µm, SD±1 × 0.77 µm); tentacles—smaller stenoteles 8.8–10.7 × 5.8–6.8 µm (N = 10, mean = 9.9 × 6 µm, SD±0.61 × 0.41 µm) and larger stenoteles 13.7–15.6 × 9.8–12.7 µm (N = 10, mean = 15 × 11 µm, SD±0.68 × 0.8 µm). Exumbrella without nematocysts.
LIFE CYCLE AND BIOLOGICAL DATA
The long and thin polyps of Hydrocoryne iemanja sp. nov. are very extensible and flexible. They preferably stay upwards, moving back and forth, when subjected to strong water circulation in the aquarium; not being able to maintain an erect position, they lay on the bottom in quiet water. Polyps that stayed longer periods laid on the bottom had fewer and less developed tentacles. Polyps that were overgrown by filamentous algae reduced the number and length of their tentacles, and their basal chitinous hard-plate started to produce new hydranths, functioning as a stolon-like structure.
Asexual reproduction by longitudinal fission was commonly observed (Figures 3E & 5). Although this process occurred in normal condition polyps, it was also initiated when the polyp remained lying on the bottom for extended periods or presented some kind of injury in their column.
Fission started at the oral region (Figures 3C & 5A), extending aborally (Figures 3D & 5B, C). The hypostome expanded laterally and the division proceeded along the oral–aboral axis of the polyp, resulting in a polyp with two oral ends well-defined, each with a hypostome and a half crown of tentacles, which gradually formed new tentacles, completely encircling the hypostome. At this point, the dividing polyp looked like a Y, both oral ends being able to catch food independently; their individual gastrodermal cavity merged at the point of fission, and food passed to the common cavity from either end, as could be clearly seen by transparence. Meanwhile, a new hard plate of epidermal origin and involving soft tissues gradually appeared in the middle of hydrocaulus, being globular (0.25–0.3 mm) and flexible when well developed (Figures 3E & 5C). When the fission process reached the new hard plate—which keeps its globular shape or becomes dish-like in recumbent polyps—the hydrocaulus separated from each other and the new hard plate gradually detached from the other hydrocaulus. Subsequently, one of the polyps detached from the other, becoming free and sinking to the bottom, where its hard plate attached, forming a new colony (Figure 5D). The whole fission process took about 48 hours. After detachment, the original polyp remained alive and functional, without scars on its hydrocaulus. Soon after release, the new polyp was about half the length of the remaining one, being morphologically indistinguishable from other polyps; it was able to bud off medusae and soon attained the size of fully developed polyps. Mother and daughter polyps might undertake subsequent fissions.
The species is metagenetic (Figure 6). Polyps released medusae during the period of cultivation (from August 2005 to September 2007); the medusae were easily reared through maturity. Since the first visual indication of the formation of the gonophores, it took about 24 hours for the medusae to start to be released; polyps released medusae for about a week. Medusae production was triggered by intense feeding of polyps. Newly-liberated and mature medusae exhibited positive phototaxis, swimming more intensely during light hours. Medusae became mature, with fully developed gonads (Figure 4C–D), 5–7 days after release. Eight to fourteen days after release, the medusae shed their gametes on the water column (at night), where fertilization occurred. Some planulae attached to the bottom of the culture dishes, but further development was not obtained. Medusae degenerated after spawning.
DISCUSSION
Species of the genus Hydrocoryne were recorded only in four locations hitherto: Japan, California (USA) and Russia (Peter the Great Bay, Japan Sea) (Margulis & Karlsen, Reference Mariscal, Muscatine and Lenhoff1980; Hirohito, Reference Hirano, Hirano and Yamada1988; Kubota, Reference Kramp1988), all in the North Pacific Ocean, and in the Caribbean (Wedler & Larson, Reference Walker1986; Calder & Kirkendale, Reference Calder and Kirkendale2005). Kubota (Reference Kramp1988) suggested that the validity of the species H. bodegensis and H. miurensis is doubtful, because both are sympatric or partially sympatric (see also Stepanjants, Reference Stampar, Tronolone and Morandini1994). The Caribbean Hydrocoryne sp. is only known from its polyp stage and newly-released medusae (Wedler & Larson, Reference Walker1986). Although Wedler & Larson's (1986) brief description does not mention tentacle features of the newly-released medusa, we believe that the tentacles of the Caribbean Hydrocoryne sp. is distinct from Hydrocoryne iemanja sp. nov. based on the unique nematocyst knob, which could hardly pass unnoticed. We therefore conclude that Hydrocoryne sp. from the Caribbean is not conspecific with H. iemanja sp. nov., being possibly a distinct and undescribed species, as supposed by Calder & Kirkendale (Reference Calder and Kirkendale2005).
Hydrocoryne iemanja sp. nov. represents the first record of the family Hydrocorynidae for the South Atlantic. Its known distributional range is restricted to the type locality on the south-eastern coast of Brazil. The knowledge on the hydrozoan fauna of the Brazilian coast is heterogeneous, the south-eastern region being the best-known (Migotto et al., Reference Migotto2002; Marques et al., Reference Margulis and Karlsen2003; Migotto & Marques, Reference Migotto, Marques, Morandini and da Silveira2006). This region includes the states of São Paulo, Rio de Janeiro, and Espírito Santo, the last one by far with the less known fauna among the three (cf. Marques & Lamas, Reference Marques, Morandini and Migotto2006). Specifically concerning hydroids, 72 species were recorded so far for the state, 21 anthoathecates and 51 leptothecates (Migotto et al., Reference Migotto2002; Grohmann et al., Reference Grohmann, Nogueira and da Silva2003; Marques et al., Reference Margulis and Karlsen2003).
The differences among the previously described species of Hydrocoryne are well defined, although polyps and young medusae of H. bodegensis and H. miurensis are morphologically indistinguishable from each other (see Kubota, Reference Kramp1988). Indeed, polyps of Hydrocoryne iemanja sp. nov. have fewer tentacles than the two other species of the genus, but we believe that presently, before variation may be better known, polypoid characters are not conclusive to distinguish among the congeners. As with the two other species of Hydrocoryne, the diagnostic features are present in the medusa phase. Hydrocoryne iemanja sp. nov. may be clearly set aside from the two other species by the distinct marginal tentacles of the medusae—short, with a large adaxial nematocyst cluster—an unambiguous character useful even for the identification of newly liberated medusae (Figure 2). Hydrocoryne iemanja sp. nov. also lacks nematocyst clusters on the manubrial lips of mature medusae, and its cnidome is distinct, lacking desmonemes only on the hydroid phase.
The presence of desmonemes in the medusae and absence in the polyp have been used to include the family Hydrocorynidae in the Corynoidea, though Petersen (Reference Pérez1990: 134–135) considered Hydrocorynidae assigned to the Sphaerocorynida because of the quadrate manubrium and interradial gonads in the medusae, and the absence of a whorl of oral tentacles in the hydroids.
We tentatively considered the longitudinal reproduction as a diagnostic character of Hydrocoryne iemanja sp. nov. However, the asexual process of longitudinal fission and production of the hard plate on the column may exist in other species of Hydrocoryne. From laboratory cultures, Kubota (Reference Kramp1988: 4) found a polyp of Hydrocoryne miurensis ‘bifurcated at the lowest portion of hydrocaulus’, but he neither recorded a new hard plate nor that the bifurcation apparently had given rise to a new, independent polyp.
The asexual reproduction observed for Hydrocoryne iemanja sp. nov. suggests that its hydroid generation may disperse and successfully colonize new and favourable habitats. However, once the hard plate of the new free-living polyp generated by asexual reproduction is negative buoyant, it tends to sink fast and, therefore, its dispersal is probably limited to the surroundings of the mother colony. The chances of survival of the new colony are probably high, because the propagules derived by longitudinal fission are large and provided with nematocysts, which probably makes them less vulnerable to predation than the sexually produced ones (planulae). Also, the new large polyp is capable to feed just after settlement, enhancing the chance to establish a new colony. Furthermore, keeping settlement near the parental colony increases the chance of the new hydroid to stay within the ecological tolerance limits of the species.
Besides the possible occurrence of longitudinal fission in other species of Hydrocoryne, the universality of this reproductive type is restricted in other cnidarians. In general, some kind of longitudinal fission occurs in polyps of a few species of Scyphozoa (e.g. Aurelia aurita and Catostylus mosaicus; Pérez, Reference Parke1920; Pitt, Reference Petersen2000), in which the fission begins at base and runs to the oral disc. This kind of reproduction also represents a common mode of vegetative proliferation among certain Anthozoa (Actiniaria, Scleractinia, Corallimorpharia and Zoanthidae) (Cairns, Reference Cairns1988; Ryland, Reference Ritchie1997; Fautin, Reference Fautin2002; Geller et al., Reference Geller, Fitzgerald and King2005), in which the fission also proceeds from the base towards the oral disc (Geller et al., Reference Geller, Fitzgerald and King2005). A detailed analysis on these processes, however, indicates that they are not homologous, even in less inclusive groups (cf. Daly et al., Reference Daly, Fautin and Cappola2003). The uniqueness of the process among the hydrozoans highlights the importance of the new finding.
The Hydrozoa has a vast repertoire of asexual processes, which usually includes budding (almost universal and plesiomorphic for the species of the group), and the formation of different types of propagules (podocysts, frustules), more restricted to some specific groups. Fission seems to be rare in Hydrozoa, being only reported in a few species (Table 1).
Table 1. Compilation of hydrozoan species where fission is already known, and taxonomic arrangements including these species.

Fissiparity (either longitudinal or transversal) is an interesting character to be investigated concerning its phylogenetic significance. Presently, a simple optimization of fissiparity on hydrozoan phylogenies is impossible, because of the lack of consistent and broad phylogenetic frameworks for the hydrozoans as a whole. However, some interesting taxonomic significance in the group may be depicted. Longitudinal fission is taxonomically scattered among Hydrozoa and, most probably, arose several independent times in its evolutionary history, while transverse fission is characteristic of somewhat taxonomically (though still considering classical taxonomy) related groups (Table 1).
The existence of transverse fission seems to be restricted to the Capitata, although this is probably not a monophyletic group (cf. Collins et al., Reference Collins, Winkelmann, Hadrys and Schierwater2005, Reference Collins, Schuchert, Marques, Jankowski, Medina and Schierwater2006). Many groups presenting transverse fission belong to the Aplanulata (a group supported by mitochondrial 16S and 28S rDNA analyses, and consisting of a putative clade uniting Hydridae with Candelabridae, Corymorphidae, and Tubulariidae; Collins et al., Reference Collins, Winkelmann, Hadrys and Schierwater2005, Reference Collins, Schuchert, Marques, Jankowski, Medina and Schierwater2006). However, as noticed by Collins et al. (Reference Collins, Schuchert, Marques, Jankowski, Medina and Schierwater2006: 111), to confirm the monophyly of Aplanulata and understand the relationship among their families, many taxa have yet to be sampled, for instance Acaulidae, Margelopsidae, Paracorynidae and Tricyclusidae.
Compilatory literature studies overlooked the process of longitudinal fission (e.g. Boero et al., Reference Boero, Bouillon, Piraino, Schmid and Hughes2002), although it has already been described. In Hydrozoa, the most similar phenomenon occurs in the model organism genus Hydra (Parke, Reference Migotto, Marques and Lewinshon1900; Hyman, 1928). However, longitudinal fission is not an ordinary type of asexual reproduction in Hydra, and apparently occurs in damaged, regenerating, or abnormal specimens (Hyman, 1928; Shostak, Reference Schulz1993). The sequence of the process is the same as we found in Hydrocoryne iemanja sp. nov., beginning at the hypostome, and requiring several days to reach the basal disc.
Polypodium hydriforme Ussow, 1887 is a specialized parasitic species of sturgeon ova (Raikova, Reference Raikova, Koltun and Stepanjants1994) and, besides its odd morphology and life cycle, has been considered by some authors as a Hydrozoa (e.g. Smothers et al., Reference Shostak1994), though some others have proposed a new class to accommodate the species (Raikova, Reference Pitt1988). The free-living, creeping, non-sexual stage of this species multiplies by longitudinal fission, but this fission begins at the aboral pole and progresses towards mouth (Raikova, Reference Raikova2002).
Cnidarians are a key early diverging lineage among metazoans and, therefore, phenomena observed in the group may be precursors to derived key characteristics in evolution of animals, including traits of reproduction and body plans (e.g. Galliot & Schmid, Reference Galliot and Schmid2002). The data shown above indicates that the longitudinal fission in Hydrocoryne iemanja sp. nov. is a process distinct from the longitudinal fission exhibited by other cnidarians. Therefore, the importance in the understanding of this asexual process may be in the investigation of regulatory genes in lower metazoans and their significance in the production of clonal organisms and evolutionary trends.
The asexual reproduction is also a divergent point to understand the evolution of polarity processes within Cnidaria (for more detailed accounts see Geller et al., Reference Geller, Fitzgerald and King2005; Collins et al., Reference Collins, Schuchert, Marques, Jankowski, Medina and Schierwater2006). The hypothesis of a new method of reproduction, the longitudinal fission in the Hydrocoryne iemanja sp. nov., may shed light on the understanding of the evolutionary pathways in Cnidaria and animals.
ACKNOWLEDGEMENTS
We thank F.L. da Silveira (IBUSP) for providing laboratory facilities and commenting on the manuscript; Ricardo Miyazaki and Augusto A. Tinfre (Água e Estilo) for providing aquarium facilities; and H.M. Pacca for the line-art drawings. A.C. Morandini was supported by a FAPESP (2003/02433-0) postdoctoral scholarship, CEPG/UFRJ–FUJB (ALV′2006 13126-1), CNPq (481399/2007-0) and FAPERJ (E-26/171.150/2006); S.N.S. was supported by CAPES MSc scholarship from ‘Programa de Pós-Graduação em Ciências Biológicas, Área Zoologia, IBUSP’; A.E.M. is supported by CNPq (300194/1994-3) and FAPESP (2006/05821-9); A.C. Marquer has financial support from CNPq (55.7333/2005-9, 490348/2006-8, 305735/2006-3), FAPESP (2003/02432-3; 2004/09961-4) and NSF (AToL-EF-0531779). The experiments herein performed (cultivation) comply with the current laws of Brazil.










