INTRODUCTION
There is a long tradition of researchers striving to predict what might happen in the future. Until recently, most research related to biodiversity and conservation has been performed in an unsystematic manner (Sutherland & Woodroof, Reference Sutherland and Woodroof2009; Sutherland et al., Reference Sutherland, Freckleton, Godfray, Beissinger, Benton, Cameron, Carmel, Coomes, Coulson, Emmerson, Hails, Hays, Hodgson, Hutchings, Johnson, Jones, Keeling, Kokko, Kunin, Lambin, Lewis, Malhi, Mieszkowska, Milner-Gulland, Norris, Phillimore, Purves, Reid, Reuman, Thompson, Travis, Turnbull, Wardle and Wiegand2013). However, an assessment of future research opportunities, knowledge gaps and new areas of science coonstitutes the first step in identifying and communicating hypotheses and insights for the future (Rands et al., Reference Rands, Adams, Bennun, Butchsrt, Clements, Coomes, Entwistle, Hodge, Kapos, Scharlemann, Sutherland and Vira2010; Sutherland et al., Reference Sutherland, Clout, Côté, Daszak, Depledge, Fellman, Fleishman, Garthwaite, Gibbons and De Lurio2010). Prognoses for marine ecosystems are becoming increasingly important, because of the threats that have been emerging in recent decades and which require urgent scientific attention. These threats include global climate change, ocean warming, sea level rise, biodiversity loss, overfishing, ocean acidification and expanding hypoxia (Pauly, Reference Pauly, Payne, Lipinski, Clarke and Roeleveld1998; Pauly et al., Reference Pauly, Christensen, Dalsgaard, Froese and Torres1998, Reference Pauly, Alder, Bennett, Christensen, Tyedmers and Watson2003; Orr et al., Reference Orr, Fabry, Aumont, Bopp, Doney, Feely, Gnanadesikan, Gruber, Ishida, Joos, Key, Lindsay, Maier-Reimer, Matear, Monfray, Mouchet, Najjar, Plattner, Rodgers, Sabine, Sarmiento, Schlitzer, Slater, Totterdell, Weirig, Yamanaka and Yool2005; Rockstrom et al., Reference Rockstrom, Steffen, Noone, Persson, Chapin, Lambin, Lenton, Scheffer, Folke, Schellnhuber, Nykvist, de Wit, Hughes, van der Leeuw, Rodhe, Sorlin, Snyder, Costanza, Svedin, Falkenmark, Karlberg, Corell, Fabry, Hansen, Walker, Liverman, Richardson, Crutzen and Foley2009; Turner et al., Reference Turner, Bindschadler, Convey, di Prisco, Fahrbach, Gutt, Hodgson, Mayewski and Summerhayes2009). Addressing these challenges asks for greater synergy between research, management and policy, and it will be important to inform researchers and funding agencies as to where their efforts might best be focused.
Cephalopods (Mollusca: Cephalopoda) are widely recognized as playing a pivotal role in many marine ecosystems, both as predators and prey (Clarke, Reference Clarke1996; Piatkowski et al., Reference Piatkowski, Pierce and Morais da Cunha2001; Boyle & Rodhouse, Reference Boyle and Rodhouse2005). Furthermore, cephalopod fisheries have been increasing steadily in recent decades and it is likely that more species will be commercially exploited in the future (FAO, 2005). As marine biologists whose research is focused on cephalopods, our aims include a better understanding of cephalopod biology and ecology and the role of these organisms in marine ecosystems, identifying patterns and mechanisms, quantifying changes at different scales, recognizing problems and testing potential solutions (e.g. related to conservation, fisheries management and aquaculture). Except for nautiluses, cephalopods have a short lifespan, rapid growth and semelparous maturation patterns (Boyle & Rodhouse, Reference Boyle and Rodhouse2005). These life cycle traits may have positive or negative effects on cephalopod species in relation to environmental change, as cephalopods can be both sensitive (in terms of rapid response) and resilient (in terms of recovery) to phenomena such as overfishing or climate variability and change (Pecl & Jackson, Reference Pecl and Jackson2008; Rosa & Seibel, Reference Rosa and Seibel2008; André et al., Reference André, Haddon and Pecl2010; Pierce et al., Reference Pierce, Belcari, Bustamante, Challier, Cherel, Gonzales, Guerra, Jereb, Kouéta, Lefkaditou, Moreno, Pereira, Piatkowski, Pita, Robin, Roel, Santos, Santurtún, Seixas, Shaw, Smith, Stowasser, Valavanis, Villanueva, Wang, Wangvoralak, Weis and Zumholz2010; Hoving et al., Reference Hoving, Gilly, Markaida, Benoit-Bird, West-Brown, Daniel, Field, Paressenti, Liu and Campos2013; Rodhouse, Reference Rodhouse2013). However, exactly how these, and other, phenomena affect cephalopods is not yet fully understood. Therefore, a broad discussion of these issues can be valuable in providing guidance for future directions of cephalopod research.
The 2013 World Malacological Congress, held 21–28 July in Ponta Delgada, Azores, Portugal, brought together a number of cephalopod experts to participate in a symposium focusing on the role of cephalopods in the world's oceans. The symposium was held in honour of the late Malcolm R. Clarke, FRS (1930–2013), and was endorsed by the Cephalopod International Advisory Council (CIAC). Malcolm Clarke himself had initiated the CIAC in 1981, and the council was officially founded two years later as an international forum to encourage research on cephalopods, promote international collaboration in cephalopod science, and to provide an official body to answer the increasing number of questions about cephalopods, particularly those related to cephalopod fisheries (Hochberg & Hatfield, Reference Hochberg and Hatfield2002). In this paper, cephalopod experts, including present and former members of CIAC, working in specific fields and at different organizational scales, ranging from a species perspective to the ecosystem level, discuss some of the challenges that cephalopod research will face in the future. The individual sections provide brief reviews of topics in cephalopod research that deserve further attention.
NEW WAYS TO ASCERTAIN THE TROPHIC ROLE AND FOOD WEB LINKS OF CEPHALOPODS
Studying cephalopods in the world's oceans using top predators as biological samplers: where are we heading? (José C. Xavier)
Knowledge about cephalopods, particularly those from oceanic waters that are not commercially caught, largely originates from analyses of stomach contents collected from their natural predators, such as toothed whales, seals, seabirds, sharks and teleost fish (Clarke, Reference Clarke1996). This is, because current methods for direct sampling, especially of oceanic squid, are still inefficient (Clarke, Reference Clarke1977; Xavier et al., Reference Xavier, Clarke, Magalhães, Stowasser, Blanco and Cherel2007; Hoving et al., Reference Hoving, Perez, Bolstad, Braid, Evans, Fuchs, Judkins, Kelly, Marian, Nakajima, Piatkowski, Reid, Vecchione and Xavier2014). Therefore, an essential tool in the study of cephalopod remains found in predator stomachs is the identification and measurement of their chitinized upper and lower beaks (Clarke, Reference Clarke1986; Cherel et al., Reference Cherel, Duhamel and Gasco2004; Xavier & Cherel, Reference Xavier and Cherel2009; Xavier et al., Reference Xavier, Phillips and Cherel2011), and, to a lesser extent, the morphological and molecular analysis of soft tissues in case these should still be available (Pierce & Boyle, Reference Pierce and Boyle1991; Barrett et al., Reference Barrett, Camphusysen, Anker-Nilssen, Chardine, Furness, Garthe, Hüppop, Leopold, Montevecchi and Veit2007; Karnovsky et al., Reference Karnovsky, Hobson and Iverson2012).
However, the analysis of hard tissues can be biased. For instance, a recent study showed that the ratio of upper to lower beaks in diet samples from top predators varied significantly during one year as well as between years. This bias was larger in some cephalopod species than in others, resulting in the underestimation of the relative importance of some species in data derived from this approach (Xavier et al., Reference Xavier, Phillips and Cherel2011). This can result in an under- or over-estimation of relative cephalopod abundance and suggests that it is essential to count both (i.e. lower and upper) beaks in stomach content analyses. Furthermore, in instances where there is a consistent bias (>30%), all beaks should be identified, and the higher quantity of beak type should be considered to reconstruct the cephalopod component of the diet by mass (Santos et al., Reference Santos, Clarke and Pierce2001; Xavier et al., Reference Xavier, Phillips and Cherel2011).
In samples collected from predators that tend to retain material, it is of importance to separate old and fresh material during the initial sorting process in order to obtain a qualitative assessment of the degree of erosion of the material as well (Piatkowski & Pütz, Reference Piatkowski and Pütz1994; Cherel et al., Reference Cherel, Weimerskirch and Trouve2000; Xavier et al., Reference Xavier, Croxall and Cresswell2005). These components can then be analysed separately, as required, and the results compared. In general, more effort should be put into describing upper beak morphology to aid identification (Clarke, Reference Clarke1962; Imber, Reference Imber1978; Pérez-Gándaras, Reference Pérez-Gándaras1983; Wolff, Reference Wolff1984; Kubodera & Furuhashi, Reference Kubodera and Furuhashi1987; Lu & Ickeringill, Reference Lu and Ickeringill2002; Xavier & Cherel, Reference Xavier and Cherel2009), to measuring upper beaks in diets, and to developing regressions or allometric equations for estimating cephalopod mass based on both lower and upper beak measurements. Indeed, for numerous species no allometric equations are yet available, which is why scientists have to rely on equations from closely related species. In addition, various allometric equations were produced based on a limited number and size range of cephalopod specimens. Therefore, more material must be collected, particularly from cephalopod natural predators or by research, as well as commercial fishing vessels.
Malcolm Clarke emphasized the importance of additional ship time devoted to cephalopod research, as well as the need for the development of better capture methods (Xavier et al., Reference Xavier, Clarke, Magalhães, Stowasser, Blanco and Cherel2007; Hoving et al., Reference Hoving, Perez, Bolstad, Braid, Evans, Fuchs, Judkins, Kelly, Marian, Nakajima, Piatkowski, Reid, Vecchione and Xavier2014). Many cephalopods are fast-swimming organisms and therefore it is usually only the small or less-mobile specimens that are captured (Clarke, Reference Clarke1977). This dilemma still holds true, despite a long history of sampling. In order to maximize the success rate of capturing bigger specimens, larger nets and modified net gear (e.g. underwater lights) have been developed to attract cephalopods into the nets (Clarke & Pascoe, Reference Clarke and Pascoe1997, Reference Clarke and Pascoe1998; Clarke, Reference Clarke2006). However, new techniques are required to enhance the catch ratio of poorly-known cephalopod species in the world's oceans in order to complement the work already being carried out on the feeding and foraging ecology of cephalopod predators.
Stable isotopes, hard tissues and the trophic ecology of cephalopods (Yves Cherel)
Stable isotopes (δ13C and δ15N) have recently emerged as new efficient intrinsic markers of the trophic ecology of cephalopods (Jackson et al., Reference Jackson, Bustamante, Cherel, Fulton, Grist, Jackson, Nichols, Pethybridge, Phillips, Ward and Xavier2007), and pioneer investigations (Takai et al., Reference Takai, Onaka, Ikeda, Yatsu, Kidokoro and Sakamoto2000; Cherel & Hobson, Reference Cherel and Hobson2005) have lead to a steady increase in the use of the method over the last 10 years (Navarro et al., Reference Navarro, Coll, Somes and Olson2013). In this section, attention will be paid to the most recent findings, methodological issues and perspectives on the use of these tools on hard tissues of cephalopods.
In contrast to soft tissues (e.g. mantle), hard tissues (i.e. beaks, gladii, statoliths and eye lenses) are metabolically inactive structures that grow continuously by accretion of new molecules with no turnover after synthesis. Consequently, these structures retain molecules laid down throughout the lives of cephalopods, and their δ13C and δ15N values thus integrate the feeding ecology of individuals over their lifetime. Indeed, various parts of hard tissues have different isotopic signatures. For example, δ13C and δ15N values of the tip of the wings and anterior tip of the gladius (i.e. the most recently synthesized parts of lower beaks and gladii, respectively) integrate the feeding ecology prior to capture (Cherel & Hobson, Reference Cherel and Hobson2005; Hobson & Cherel, Reference Hobson and Cherel2006; Cherel et al., Reference Cherel, Fontaine, Jackson, Jackson and Richard2009a). Gladii have the advantage over beaks that their growth increments are larger, better defined and easier to sample along the longitudinal proostracum axis (Cherel et al., Reference Cherel, Fontaine, Jackson, Jackson and Richard2009a). Furthermore, in the most recent part of the gladius, assuming increments are daily, a day-by-day picture can also be established which can directly be related to body size (as gladii length is approximately the same as the dorsal mantle length; Graham Pierce, unpublished data).
Stable isotopes from hard structures have two practical advantages and one methodological disadvantage. Firstly, measuring the isotopic signature of serially sampled beaks and gladii presents the unique opportunity to reconstruct the foraging history of individuals. For example, δ15N profiles of beaks from Architeuthis dux suggest an ontogenetic shift early in life (Guerra et al., Reference Guerra, Rodriguez-Navarro, González, Romanek, Alvarez-Lloret and Pierce2010), and sequential isotopic values along gladii of Dosidicus gigas highlight contrasted individual foraging strategies (Ruiz-Cooley et al., Reference Ruiz-Cooley, Villa and Gould2010; Lorrain et al., Reference Lorrain, Arguelles, Alegre, Bertrand, Munaron, Richard and Cherel2011). In the same way, the only published investigation on concentric eye lens layers reveals variations in δ13C and δ15N values at fine temporal scales, indicating substantial variability in squid feeding patterns (Hunsicker et al., Reference Hunsicker, Essington, Aydin and Ishida2010a). Secondly, the combination of the stable isotope techniques with the use of predators as biological samplers, and cephalopod identification using external features of accumulated beaks in predators' stomachs (Clarke, Reference Clarke1986; Xavier & Cherel, Reference Xavier and Cherel2009) allows information to be gathered on poorly known species. This method has already revealed new trophic relationships and migration patterns together with the trophic structure of deep-sea cephalopod assemblages (Cherel & Hobson, Reference Cherel and Hobson2005; Cherel et al., Reference Cherel, Ridoux, Spitz and Richard2009b).
However, a main problem with using δ13C and δ15N values of hard structures is that biological interpretation is confused by differences in biochemical composition between hard and soft tissues. Beaks and gladii contain not only protein but also chitin (Hunt & Nixon, Reference Hunt and Nixon1981; Rubin et al., Reference Rubin, Miserez and Waite2010), a modified polysaccharide that contains impoverished 15N nitrogen (Schimmelmann, Reference Schimmelmann and Gupta2011). The presence of chitin explains why hard tissues have consistently much lower δ15N values than soft tissues (Cherel et al., Reference Cherel, Fontaine, Jackson, Jackson and Richard2009a). Moreover, the ratio of chitin to protein varies within beaks, with the undarkened, darkening and darkened parts of beaks containing decreasing amounts of chitin (Rubin et al., Reference Rubin, Miserez and Waite2010). Chitin content is thus likely to be different between individual beaks (e.g. small, undarkened vs large, darkened beaks), and the gladius is richer in chitin than darkened beaks (Hunt & Nixon, Reference Hunt and Nixon1981). This particular issue is analogous to that arising from the different fractionation apparent in lipids compared to other components of soft tissues. Three different approaches enable the ‘chitin effect’ to be dealt with, namely the use of isotopic correction factors between hard and soft tissues (Hobson & Cherel, Reference Hobson and Cherel2006; Cherel et al., Reference Cherel, Fontaine, Jackson, Jackson and Richard2009a), the removal of chitin and measuring stable isotopes on amino acids. Determining the stable isotope ratios of chemically extracted proteins from hard tissues has not yet been performed, but a more promising way is to measure δ15N values of amino acids resulting from protein hydrolysis. Selecting appropriate source and trophic amino acids (e.g. phenylalanine and glutamic acid, respectively) allows quantification of both δ15N baseline levels and the trophic position of consumers relative to the baseline (i.e. the δ15N signature of source amino acids (e.g. phenylalanine) does not increase along the food chain, while that of trophic amino acids (e.g. glutamic acid) does—hence the δ15N difference between trophic and source amino acids is a direct estimation of the trophic position of an organism. This approach was recently used on cephalopod hard tissues, including cuttlefish cuttlebone (Ohkouchi et al., Reference Ohkouchi, Tsuda, Chikaraishi and Tanabe2013) and squid gladii (Ruiz-Cooley et al., Reference Ruiz-Cooley, Ballance and McCarthy2013) and has the potential to depict previously unknown trophic relationships, habitat use and migration patterns of cephalopods in marine ecosystems.
Population dynamics of cephalopods under a trophic relations (as well as age and growth) context: possible future research (Marek R. Lipinski)
The present-day population dynamics of cephalopods are still largely at the descriptive, natural-history stage. The best summaries of current knowledge were given by Boyle & Boletzky (Reference Boyle and Boletzky1996), Boyle & Rodhouse (Reference Boyle and Rodhouse2005) and Rodhouse et al. (in press).
The known data on cephalopod population dynamics that has been widely and comprehensively quantified may serve a practical purpose in the management of fishable stocks of selected species of octopods, cuttlefish and squid (Rodhouse et al., in press). This part, however, largely ignores the general theoretical framework of population dynamics in ecology (described by Turchin (Reference Turchin2003)), based on predator–prey interactions. However, there are efforts aimed at incorporating predator–prey relationships in sustainable resource management (Bowman et al., Reference Bowman, Stillwell, Michaels and Grosslein2000; Overholtz et al., Reference Overholtz, Link and Suslowicz2000, Reference Overholtz, Jacobson and Link2008; Tyrrell et al., Reference Tyrrell, Link, Moustahfid and Overholtz2008, Reference Tyrrell, Link and Moustahfid2011). Hence, predation should be considered in setting maximum sustainable yield (MSY) for a fishery, including those fisheries exploiting squid (such as Doryteuthis pealeii and Illex illecebrosus). When this is done, MSY is usually considerably smaller. Model inputs are usually based on stomach contents analysis, and actual consumption is calculated (subject to some assumptions). The underlying reasoning is that a lack of this resource in the diet of predators will be to the detriment of these predators.
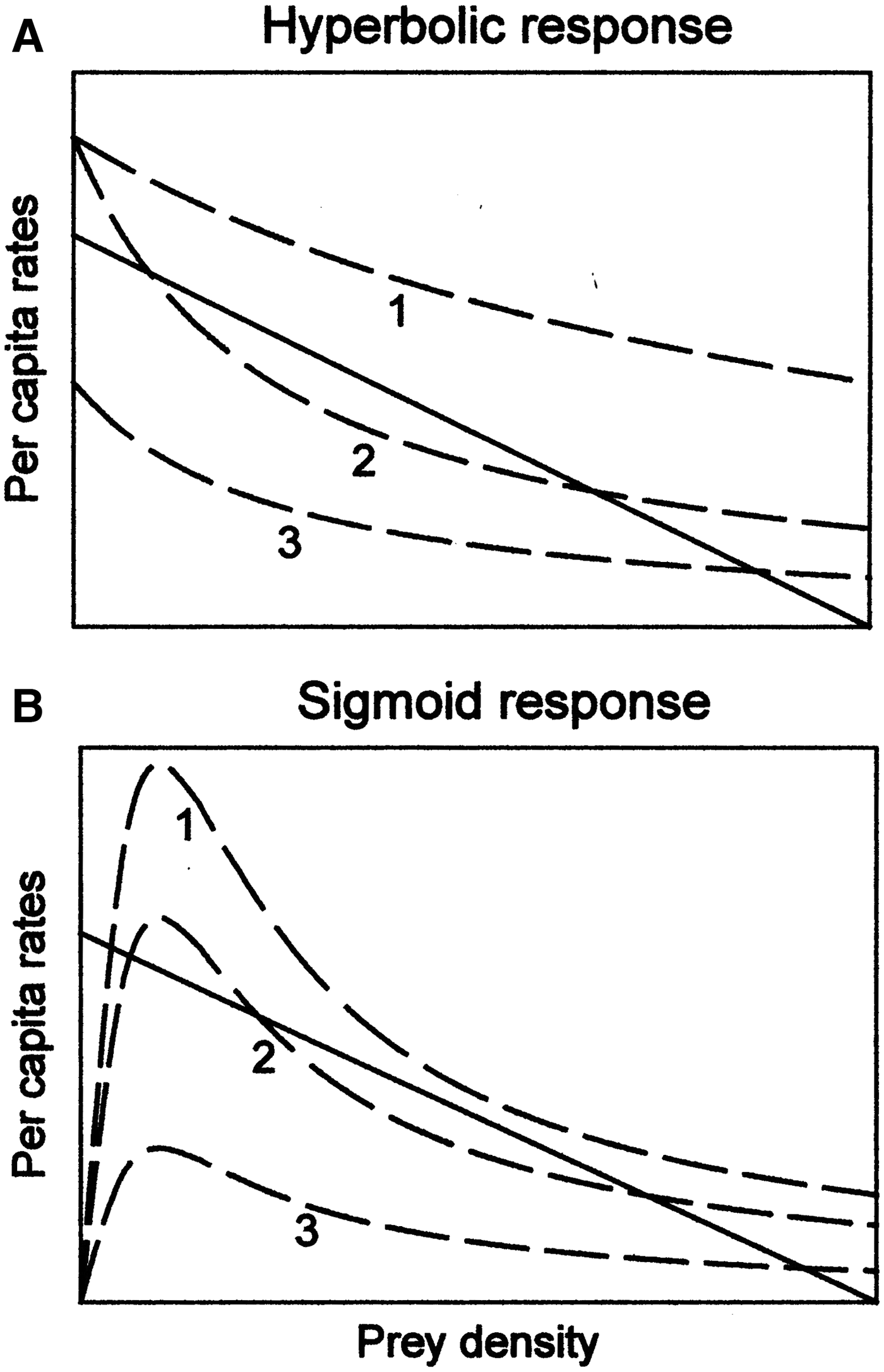
However, this may not correspond to reality. This approach usually assumes either a specialist character of these predators, or at best, a hyperbolic response of a generalist predator, according to a model where
with N: population density; r: per capita rate of population change; k: carrying capacity (logistic); d: half-saturation constant (hyperbolic); g: total killing rate by generalist predators; h: half saturation constant (sigmoid) (Turchin, Reference Turchin2003). However, cephalopods are opportunistic generalists both as predators and in turn are preyed upon by generalist predators themselves (preyed upon by fish, birds, mammals and cephalopods), giving a sigmoid response to predation according to the model
These two scenarios are illustrated in Figure 1, where solid lines represent per capita growth rates of the prey population in the absence of predators, whilst dashed curves represent per capita death rate of prey as a result of predation. Numbers correspond to the specific cases (out of many possible). In the hyperbolic scenario (Figure 1A), case 1 refers to the total extinction of prey as a result of predation. Case 2 is the situation where only very high density of prey secures the end equilibrium (and hence the survival of prey). Case 3 ends with survival of the prey population regardless what happens, therefore predators have a minimum impact upon their prey. In the sigmoid scenario (Figure 1B), case 1 refers to an equilibrium where prey densities are low (survival is probable); case 2 represents three equilibria and therefore the final result depends upon the initial conditions, but all of them are likely to be stable (survival of prey is probable in most situations); and in case 3 equilibrium is reached at high prey densities, therefore survival is even better than in case 1.

Fig. 1. Different per capita growth rates of prey, according to the presence or absence of predators: (A) hyperbolic response; (B) sigmoid response. See the main text for explanation. Modified from Turchin (Reference Turchin2003), with copyright permission from Princeton University Press.
Given the above, future work should apply theoretical ecology models to real cephalopod populations, and only then should feed into well-intentioned resource management. This is not happening as of yet, simply because it is a complicated task. Cephalopods will require the development of a ‘multi-opportunistic links model’ compatible with other findings of theoretical ecology. This model may be useful for fisheries management only if the required parameters can be obtained or assessed in practice.
A second case study considered here is on cephalopod age, ageing, longevity and growth from a population dynamics perspective. This field also has a background in theoretical ecology (Turchin, Reference Turchin2003), with its emphasis on changing ages, different average longevity (and ultimately, growth parameters) with change between subsequent generations, and on the influence these changes may have on oscillating numbers of individuals in a population. Here, in contrast to the field of predator–prey relationships, theoretical ecology feeds into practical applications (Quinn & Deriso, Reference Quinn and Deriso1999), although the focus is somewhat different. Cephalopods, however, have not yet been the subjects of thorough studies in this discipline. This is, because it is felt that some fundamental problems in understanding population structuring have not yet been resolved. There is a relative abundance of age data, but a paucity of studies using these data to model population structure based either on generations (for theoretical purposes) or to construct suitable keys (e.g. age–length) for stock assessment and management analyses.
Therefore, there is a need for new research and more data. Firstly, there is a requirement for physiological studies on the interpretation of age marks (mostly biomineralization studies) to construct true instead of biased validation procedures. Secondly, no one has so far adequately addressed Daniel Pauly's paradox regarding the metabolic limitation of squid growth (Pauly, Reference Pauly, Payne, Lipinski, Clarke and Roeleveld1998): according to him, large squid cannot grow quickly due to their energetic requirements, which goes against the age readings of squid statoliths (supported by aquarium observations), which in turn support the inference that large squid do grow quickly. However, a good start to reconciling these contradictory data was made by O'Dor & Hoar (Reference O'Dor and Hoar2000). Thirdly, studies of cephalopod growth are required, that will combine a theoretical ecology approach (suffering at the moment from an assumption of non-overlapping generation cycles) (Turchin, Reference Turchin2003), a wealth of matrix models (Quinn & Deriso, Reference Quinn and Deriso1999), and a solid physiological basis (which is lacking at the moment). It is to be hoped that the state of the art, presently fragmented into these three areas (Arkhipkin & Roa-Ureta, Reference Arkhipkin and Roa-Ureta2005; André et al., Reference André, Grist, Semmens, Pecl and Segawa2009; Keyl et al., Reference Keyl, Arguelles and Tafur2011; Semmens et al., Reference Semmens, Doubleday, Hoyle and Pecl2011; Zavala et al., Reference Zavala, Ferreri and Keyl2012), will improve in the future.
NEW APPROACHES TO THE STUDY OF CEPHALOPOD MORPHOLOGY (ELIZABETH K. SHEA, ALEXANDER ZIEGLER)
Comparative morphology is an essential, yet increasingly rare specialty in organismic biology. The slow pace of work for detailed analysis and the current lack of a centralized repository for morphological data contribute to the widely-cited ‘taxonomic impediment’ that contemporary biodiversity research is facing (Crisci, Reference Crisci2006; de Carvalho et al., Reference de Carvalho, Bockmann, Amorim, Brandao, de Vivo, de Figueiredo, Britski, de Pinna, Menezes, Marques, Papavero, Cancello, Crisci, McEachran, Schelly, Lundberg, Gill, Britz, Wheeler, Stiassny, Parenti, Page, Wheeler, Faivovich, Vari, Grande, Humphries, DeSalle, Ebach and Nelson2007). Due to the lack of open access to structural data, molecular methods (e.g. barcoding) are rapidly supplanting morphology in systematics and taxonomy research, resulting in a one-sided discussion about invertebrate relationships and evolution. Rejuvenating morphological research through the development of online repositories for morphological data will provide new avenues of inquiry that would contribute substantively to systematic and taxonomy research. In addition, morphology would become a more accessible contributor to large interdisciplinary research initiatives such as the Census of Marine Life (Decker & O'Dor, Reference Decker and O'Dor2003) or major online compilations of organismic data such as the Encyclopedia of Life (Wilson, Reference Wilson2003). In this section, we highlight several new and developing digital techniques that have the potential to expedite morphological work, and which could encourage a shift in focus from data acquisition to data analysis, consequently resulting in a more rapid and regular research output in cephalopod morphological research.
Cephalopod beak identification is notoriously difficult, but an in-depth understanding of beak morphology is critical to stomach content analyses, as well as for an understanding of predator–prey dynamics (Clarke, Reference Clarke1986; Xavier & Cherel, Reference Xavier and Cherel2009). Three-dimensional (3D) anaglyph images constitute an alternative to complex line drawings or photographs (Xavier & Cherel, Reference Xavier and Cherel2009). Richard E. Young is in the process of building a collection of such images, archiving them on the Tree of Life Web Project website (http://www.tolweb.org/notes/?note_id=4541). The upper and lower beaks of over 140 species from all major clades have so far been analysed (Young, Reference Young2009). In addition, new hybrid approaches such as rotational scanning electron microscopy (SEM) could be used to image minuscule morphological features such as statoliths, sucker dentition or cartilaginous strips and tubercules at very high resolutions and in 3D (Cheung et al., Reference Cheung, Brunke, Akkari, Souza and Pape2013).
In addition, robotic microscopy systems developed for applications in pathology permit rapid digitization of histological sections on a large scale and at high resolutions (Al-Janabi et al., Reference Al-Janabi, Huisman and Van Diest2012). Such systems could be used to digitize and catalogue histological data on cephalopod neuroanatomy, such as, for example, the John Z. Young slide collection deposited at the National Museum of Natural History (Washington, DC, USA). The resulting tomographic image stacks can be aligned using semi-automatic and automatic algorithms (Eliceiri et al., Reference Eliceiri, Berthold, Goldberg, Ibanez, Manjunath, Martone, Murphy, Peng, Plant, Roysam, Stuurman, Swedlow, Tomancak and Carpenter2012), and can subsequently be made accessible as full 3D datasets in online repositories. These image stacks would then become a baseline of information that permits directly connecting past research (Young, Reference Young1971) to present compilations (Nixon & Young, Reference Nixon and Young2003), as well as future studies.
Furthermore, non-invasive scanning techniques such as magnetic resonance imaging (MRI), computed tomography (CT), or micro-computed tomography (μCT) now allow analysing whole specimens from the millimetre to the metre scale (Walter et al., Reference Walter, Shattuck, Baldock, Bastin, Carpenter, Duce, Ellenberg, Fraser, Hamilton, Pieper, Ragan, Schneider, Tomancak and Heriche2010). Following dataset acquisition, specialized (but often open source) software can be used to virtually dissect the scanned specimen in real-time and in 3D (Ziegler & Menze, Reference Ziegler, Menze, Zander and Mosterman2013). While MRI is particularly suitable for soft tissue imaging (Ziegler et al., Reference Ziegler, Kunth, Mueller, Bock, Pohmann, Schröder, Faber and Giribet2011a), the X-ray-based techniques CT and μCT constitute the methods of choice for hard part imaging (Ziegler et al., Reference Ziegler, Ogurreck, Steinke, Beckmann, Prohaska and Ziegler2010). However, specimen state (in vivo or ex vivo), scanning medium (e.g. air, ethanol, formalin, water), scanning time (minutes to hours), dataset resolution (nm to μm), as well as scanning cost per specimen (up to many hundreds of US$) may vary considerably and primarily depend on the system used.
Due to the dominance of soft tissues, cephalopods constitute suitable candidates for MRI scanning (Ziegler et al., Reference Ziegler, Kunth, Mueller, Bock, Pohmann, Schröder, Faber and Giribet2011a). For example, 3D MRI datasets can be used to visualize internal organs in their natural context (Figure 2A, B). In contrast, mineralized tissues present in cephalopods (e.g. eye lenses, beaks, statoliths, shells) can be rapidly visualized using CT or μCT (Figure 2C–E). However, whole specimen staining using electron-dense elements such as iodine or tungsten (Metscher, Reference Metscher2009; Kerbl et al., Reference Kerbl, Handschuh, Nödl, Metscher, Walzl and Wanninger2013) also allows analysing soft tissues of smaller cephalopod specimens using μCT (Figure 2F, G).

Fig. 2. Analysis of cephalopod specimens using non-invasive imaging techniques. The two- and three-dimensional visualizations shown here are based on a magnetic resonance imaging dataset of a whole museum wet specimen of Bathypolypus arcticus (A, B), a micro-computed tomography (μCT) dataset of the dry shell of Spirula spirula (C–E), and a μCT dataset of a tungsten-stained wet specimen of Idiosepius pygmaeus (F, G) (dataset courtesy of Brian D. Metscher).
Current online projects such as The Digital Fish Library (Berquist et al., Reference Berquist, Gledhill, Peterson, Doan, Baxter, Yopak, Kang, Walker, Hastings and Frank2012) or The Digital Morphology website (http://digimorph.org/) provide a good starting point for the web-based hosting of morphological data and constitute potential infrastructures for future efforts in cephalopod research. In addition, dissemination of complex biological structures is still carried out primarily in the form of two-dimensional publications (Ziegler et al., Reference Ziegler, Mietchen, Faber, von Hausen, Schöbel, Sellerer and Ziegler2011b), although interactive 3D models based, for example, on the ubiquitous portable document format (PDF) have been integrated into electronic publications already for several years (Ruthensteiner & Heß, Reference Ruthensteiner and Heß2008; Kumar et al., Reference Kumar, Ziegler, Grahn, Hee and Ziegler2010). Nonetheless, continued development of such approaches is required in order to adapt them, for example, to mobile devices. Furthermore, 3D printing is poised to become an important tool in the communication of complex biological structures, whether in research or in teaching (Kelley et al., Reference Kelley, Farhoud, Meyerand, Nelson, Ramirez, Dempsey, Wolf, Alexander and Davidson2007; Ziegler & Menze, Reference Ziegler, Menze, Zander and Mosterman2013).
In general, digital morphological techniques permit shifting the workload from data acquisition to data analysis, which will open new avenues of research both across and within cephalopod species. Previously collected, well-identified, and data-rich museum specimens could form the backbone of a large-scale, non-invasive scanning program (Ziegler, Reference Ziegler2012). Apart from developing a collection of 3D datasets that can be accessed in the form of a digital museum collection, the novel, high-throughput scanning techniques described above provide new opportunities for a variety of cephalopod specimens. For example, scanning of bulk-collected, commercially-trawled cephalopods could be employed to answer long-standing questions of character variation within species (Vecchione et al., Reference Vecchione, Shea, Bussarawit, Anderson, Alexeyev, Lu, Okutani, Roeleveld, Chotiyaputta, Roper, Jorgensen and Sukramongkol2005). Reared cephalopods such as Sepia officinalis could be used for in vivo experiments, where images taken before and after a stimulus would be required. Furthermore, specimens too valuable for dissection (e.g. holotypes) can now be scanned with virtually no impact on the specimen and be made fully accessible online in 3D.
Cephalopods constitute a small-enough class of molluscs that an effort to digitally scan one representative from each genus or species would constitute a realistic goal, and one that should be pursued in parallel to molecular barcoding (Strugnell & Lindgren, Reference Strugnell and Lindgren2007). A concise, user-friendly, widely-disseminated, morphological infrastructure that parallels ongoing efforts to barcode all cephalopod species would render cephalopods not just a group with multiple model organisms, but also a model clade for systematic and taxonomy research.
CHALLENGES IN CEPHALOPOD CULTURE (ROGER VILLANUEVA, ERICA A. G. VIDAL)
Experimental approaches have been an important tool for understanding fundamental principles of cephalopod life cycles, physiology and behaviour, thus providing the basis for pilot commercial culture of some species. A recent publication summarizes modern culture techniques used for the most common cephalopod species (Iglesias et al., Reference Iglesias, Fuentes and Villanueva2014). Another publication focuses on four species which are highlighted as cephalopod culture models for which there are comprehensive data available, primarly because they are frequently used by researchers around the world, namely Sepia officinalis, Sepioteuthis lessoniana, Octopus maya and O. vulgaris (Vidal et al., Reference Vidal, Villanueva, Andrade, Gleadall, Iglesias, Koueta, Rosas, Segawa, Albertin, Caamal-Monsreal, Chimal, Edsinger-Gonzales, Franco-Santos, Gallardo, Grasse, Le Pabic, Pascual, Roumbedakis and Wood2014). These four species show versatile characteristics for culture, such as fast growth and high food conversion rates. In addition, these species mate and spawn in captivity, laying eggs that, with the exception of O. vulgaris, produce large hatchlings. These biological features make them suitable candidates as experimental laboratory animals with a potential for aquaculture. However, nearly all zootechnical aspects related to the culture of these species still require improvement and need to be adapted for closely related species from different geographical regions.
At present, most of our knowledge about cephalopod culture techniques relies on shallow water species. This is due to the relatively easy access to this group of cephalopods, most of them with commercial interest, and to the ease of reproducing the characteristics of coastal waters in the laboratory. In contrast, techniques for the maintenance of oceanic or deep-sea cephalopods remain virtually unexplored. In particular, little experimental work has been directed towards deep-sea octopods (Wood et al., Reference Wood, Kenchington and O'Dor1998; Hunt, Reference Hunt1999), oceanic squids (O'Dor et al., Reference O'Dor, Durward and Balch1977; Bower & Sakurai, Reference Bower and Sakurai1996; Hunt, Reference Hunt1999; Bush, Reference Bush2012; Hoving & Robison, Reference Hoving and Robison2012; Villanueva et al., Reference Villanueva, Staaqf, Argüelles, Bozzano, Camarillo-Coop, Nigmatullin, Petroni, Quintana, Sakai, Sakurai, Salinas-Zavala, De Silva-Dávila, Tafur, Yamashiro and Vidal2012), or polar species (Daly & Peck, Reference Daly and Peck2000). However, as research efforts in the open ocean, the deep sea, and polar regions are bound to increase around the world in the near future, methods for the study of captured cephalopods from these regions will be needed to obtain new information on their life cycles and ecology.
High-priority research targets in cephalopod culture are the development of sustainable artificial foods and the control of reproduction (Villanueva et al., Reference Villanueva, Sykes, Vidal, Roas, Nabhitabhata, Fuentes, Iglesias, Iglesias, Fuentes and Villanueva2014). Littoral cephalopods are carnivorous and require food rich in protein to maintain their vigorous metabolism, as well as high quality lipids rich in essential fatty acids, phospholipids and cholesterol to sustain their fast growth. Recent efforts to obtain artificial foods have shown promising results (Rosas et al., Reference Rosas, Tut, Baeza, Sánchez, Sosa, Pascual, Arena, Domingues and Cuzon2008, Reference Rosas, Valero, Caamal-Monsreal, Uriarte, Farias, Gallardo, Sánchez and Domingues2013; Martínez et al., Reference Martĺnez, Gallardo, Pascual, Navarro, Sánchez, Caamal-Monsreal and Rosas2014). However, a major challenge will be to develop a sustainable artificial diet independent from fisheries products, completely formulated from plant sources, and in addition supporting good survival and growth, as is now a reality for some marine carnivorous fish (Watson et al., Reference Watson, Barrows and Place2013). The study of feeding dynamics of delicate planktonic paralarvae of cephalopods should also become a priority, because it would enable the commercial culture of octopod species such as O. vulgaris, which produce small eggs (Iglesias et al., Reference Iglesias, Sãnchez, Bersano, Carrasco, Dhont, Fuentes, Linares, Muñoz, Okumura, Roo, van der Meeren, Vidal and Villanueva2007; Villanueva & Norman, Reference Villanueva and Norman2008). For example, an adapted, enriched Artemia protocol would be desirable to feed planktonic octopods or squids—recent work is currently shedding light on this aspect (Guinot et al., Reference Guinot, Monroig, Navarro, Varó, Amat and Hontoria2013).
A further area of development required to facilitate cephalopod culture is the control of reproduction and an understanding of the effects of maternal condition on egg quality and offspring competence. Currently, egg masses are collected from the field, are obtained by spontaneous spawning in aquaria, or stem from in vitro fertilization. As cephalopods are semelparous and often have a natural spawning period restricted to a few months in the year, researchers currently need to adapt their laboratory studies, timing and experimental protocols to the natural sexual maturation period of the target species. The development of methods to accelerate or retard sexual maturation and spawning in aquaria will open new experimental possibilities and will be particularly useful to the planning and development of commercial culture. The influence of light intensity and photoperiod on sexual maturation has been studied in a few cases (Richard, Reference Richard1971; Zúñiga et al., Reference Zúñiga, Olivares and Ossadón1995) and, if extended, could open new opportunities for the control of reproduction. Furthermore, in cephalopod culture, the development of ethical guidelines that aim to reduce pain, suffering and stress are strongly encouraged and should be based on the 3Rs principle, i.e. replacement, refinement and reduction (Mather & Anderson, Reference Mather and Anderson2007; Moltschaniwskyj et al., Reference Moltschaniwskyj, Hall, Lipinski, Marian, Nishiguchi, Sakai, Shulman, Sinclair, Sinn, Staudinger, Gelderen, Villanueva and Warnke2007; Andrews et al., Reference Andrews, Darmaillacq, Dennison, Gleadall, Hawkins, Messenger, Osorio, Smith and Smith2013; Fiorito et al., Reference Fiorito, Affuso, Anderson, Basil, Bonnaud, Botta, Cole, D'Angelo, Girolamo, Dennison, Dickel, Cosmo, Cristo, Gestal, Fonseca, Grasso, Kristiansen, Kuba, Maffucci, Manciocco, Mark, Melillo, Osorio, Palumbo, Perkins, Ponte, Raspa, Shashar, Smith, Smith, Sykes, Villanueva, Tublitz, Zullo and Andrews2014).
Finally, genetic intervention has already been applied to other metazoans in culture in order to enhance production of cultured animals and to tackle challenges in culture (Hulata, Reference Hulata2001). Such an approach can be expected to have the potential for taking cephalopod culture to the next level. Important new research topics in this respect would be genomic sequencing or studies looking for genes that code for particular traits or that govern protein expression. For example, it would be interesting to identify the genes responsible for desirable broodstock features, control of sexual maturation, growth, immunology and pathology.
NEW WAYS TO RESEARCH CEPHALOPOD ADAPTATIONS AND RESPONSES TO ENVIRONMENTAL CHANGE
Cephalopods and climate change (Paul G. K. Rodhouse)
The effects of global climate change will include warming of the atmosphere and the oceans, intensification of ocean currents, more frequent and intense extreme weather events, retreat of sea ice in the polar regions, reduction in the depth of the oxygen minimum layer and reduced seawater pH (Raven et al., Reference Raven, Caldeira, Elderfield, Hoegh-Guldberg, Liss, Riebesell, Shepherd, Turley and Watson2005). These physical changes will drive changes in marine ecosystems, which are predicted to reduce biodiversity, although they will not necessarily reduce overall primary and secondary production. However, these effects will not be uniform. Currently, warming of the atmosphere is most intense in Alaska, Siberia, and the Antarctic Peninsula. In addition, warming of the ocean surface and upper layers in the vicinity of the Antarctic Peninsula has been reported by Meredith & King (Reference Meredith and King2005).
Because cephalopods are poikilotherms, they could be expected to physiologically respond to ocean warming. Warming will increase growth rate (subject to food availability and sufficient water oxygen), shorten lifespan, and increase turnover, which in turn might drive changes in life history parameters (Pecl & Jackson, Reference Pecl and Jackson2008). This will only happen if the species do not shift their distribution in response to warming in order to remain within their present thermal environment. However, there is evidence that some species expand their distribution when facing a warmer environment (Zeidberg & Robison, Reference Zeidberg and Robison2007; Golikov et al., Reference Golikov, Sabirov, Lubin and Jørgensen2013).
Furthermore, many cephalopods, especially the oegopsid squids, produce planktonic paralarvae, which, by definition, are transported by ocean currents and have been shown in some species to be dependent on mesoscale structuring in the ocean to complete their planktonic phase (Bakun & Csirke, Reference Bakun and Csirke1998; Dawe et al., Reference Dawe, Colbourne and Drinkwater2000). Such species are likely to be affected by changes in oceanic circulation, the effects of which may be positive or negative. For example, small changes in large-scale circulation are unlikely to affect Antarctic squid, but changes in mesoscale oceanography may have a significant impact (Rodhouse, Reference Rodhouse2013).
Extreme local events such as storms or basin-scale events such as the El Niño Southern Oscillation or North Atlantic Oscillation, which are predicted to be intensified by global climate change, will influence changes in populations (Hoving et al., Reference Hoving, Gilly, Markaida, Benoit-Bird, West-Brown, Daniel, Field, Paressenti, Liu and Campos2013). Basin-scale events are known to drive variability in the recruitment and abundance of species, including Illex argentinus (Waluda et al., Reference Waluda, Trathan and Rodhouse1999), I. illecebrosus (Dawe et al., Reference Dawe, Colbourne and Drinkwater2000) and Dosidicus gigas (Waluda et al., Reference Waluda, Yamashiro and Rodhouse2006). Intensification of such events might be deleterious and/or advantageous to these species, but there are currently no models which can predict likely outcomes.
In the polar regions, changes in sea ice may cause changes in the distribution of some species, but there are no species known to be dependent on sea ice as, for instance, is the Antarctic krill Euphausia superba (Murphy et al., Reference Murphy, Watkins, Trathan, Reid, Meredith, Thorpe, Johnston, Clarke, Tarling, Collins, Forcada, Shreeve, Atkinson, Korb, Whitehouse, Ward, Rodhouse, Enderlein, Hirst, Martin, Hill, Staniland, Pond, Briggs, Cunningham and Fleming2007; Constable et al., in press; Xavier & Peck, in press). In these high latitudes, changes in ocean ecology driven by retreating sea ice may have a greater effect on cephalopod populations than the direct effect of ice retreat.
At least two cephalopod species, D. gigas and Vampyroteuthis infernalis, are associated with the oxygen minimum layer, where they descend to during daylight (Robison et al., Reference Robison, Reisenbichler, Hunt and Haddock2003; Rosa & Seibel, Reference Rosa and Seibel2008; Hoving & Robison, Reference Hoving and Robison2012). These two species are physiologically adapted to survive the low oxygen tension of the oxygen minimum layer, and probably enjoy the selective advantage of avoiding active water-breathing predators in this zone. Depending on how widespread this habit is among pelagic cephalopods, changes in the oxygen minimum layer associated with global climate change will have effects on other species (Bograd et al., Reference Bograd, Castro, Di Lorenzo, Palacios, Bailey and Gilly2008; Stramma et al., Reference Stramma, Johnson, Sprintall and Mohrholz2008; Keeling et al., Reference Keeling, Körtzinger and Gruber2010; Gilly et al., Reference Gilly, Beman, Litvin and Robison2013).
Furthermore, all cephalopods possess calcareous statoliths, while some possess larger mineralized structures such as an external shell (e.g. nautiluses) or an internal shell (e.g. cuttlefish). Although there is some evidence that cuttlefish are pre-adapted to ocean acidification (Gutowska et al., Reference Gutowska, Pörtner and Melzner2008), there is still a need for more data on the effects of reduced ocean pH on cephalopods.
Cephalopods evolved from an ancestral mollusc in the Cambrian. They have survived major extinction events at the end of the Palaeozic and at the end of the Mesozoic, and have thrived in spite of competition from fish (Packard, Reference Packard1972; Rodhouse, Reference Rodhouse2013). Although some cephalopod groups such as ammonites and belemnites became extinct in geological time, the coleoids have survived and radiated. Their life history traits have adapted them for ecological opportunism and provide them with the potential to quickly evolve in response to new selection pressures (Murphy et al., Reference Murphy, Rodhouse and Nolan1994; Murphy & Rodhouse, Reference Murphy and Rodhouse1999; Hoving et al., Reference Hoving, Gilly, Markaida, Benoit-Bird, West-Brown, Daniel, Field, Paressenti, Liu and Campos2013). There is, therefore, reason to believe that these characteristics will enable cephalopods to evolve under global climate change, enabling them to avoid becoming extinct, and ultimately giving rise to new forms adapted to a new ‘greenhouse world’.
Physiological adaptations of cephalopods to environmental change (Rui Rosa)
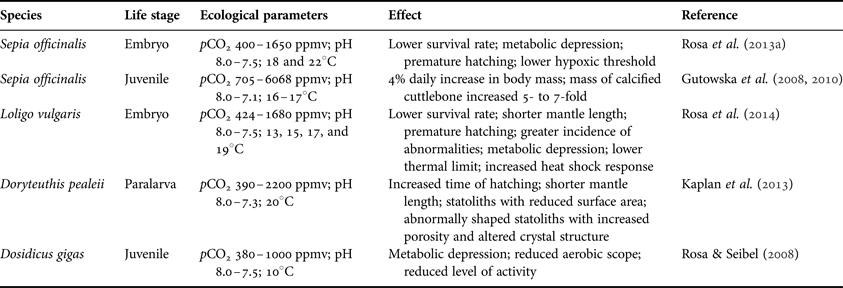
Coastal marine ecosystems are warming at a higher rate than most other ecosystems (MacKenzie & Schiedek, Reference MacKenzie and Schiedek2007). Because many coastal organisms already live close to their thermal tolerance limits (Helmuth et al., Reference Helmuth, Mieszkowska and Hawkins2006), ocean warming is expected to negatively impact their performance and survival. Cephalopods are some of the most adaptable marine organisms, capable of adjusting their biology (and life cycles) according to the prevailing environmental conditions (Boyle & Rodhouse, Reference Boyle and Rodhouse2005; Hoving et al., Reference Hoving, Gilly, Markaida, Benoit-Bird, West-Brown, Daniel, Field, Paressenti, Liu and Campos2013). Yet, although their short lifespans and great life history plasticity allow them to respond rapidly to new climate regimes, ocean warming may cause serious biological impairments to the more vulnerable early ontogenetic stages, namely shorter embryonic periods and an increased likelihood of premature hatching (Rosa et al., Reference Rosa, Pimentel, Boavida-Portugal, Teixeira, Trübenbach and Diniz2012b, Reference Rosa, Trübenbach, Pimentel, Boavida-Portugal, Faleiro, Baptista, Dionĺsio, Calado, Pörtner and Repolho2014). Future changes in ocean chemistry are also expected to pose particular problems. Cephalopods possess statoliths that may be reduced and abnormally shaped (with increased porosity) under hypercapnia (Kaplan et al., Reference Kaplan, Mooney, McCorkle and Cohen2013). It is also noteworthy, that along with the rise of pCO2 in the embryo (combined with a drop in pH and pO2), the current record of oxygen tension below critical pO2 values reveals that the harsh (i.e. hypoxic and hypercapnic) conditions inside cephalopod egg capsules are expected to be magnified in the future (Rosa et al., Reference Rosa, Trübenbach, Repolho, Pimentel, Faleiro, Boavida-Portugal, Dionĺsio, Leal, Calado and Pörtner2013a). Such environmental conditions may promote untimely hatching and smaller post-hatching body sizes (Table 1), thus challenging survival and fitness.
Table 1. Responses of different cephalopod life stages to ocean acidification.

In the last few decades, marine hypoxia has become a major ecological concern (Diaz & Rosenberg, Reference Diaz and Rosenberg2008). Surprisingly, some squids that were thought to be driven from hypoxic areas due to anatomical and physiological constraints (e.g. Dosidicus gigas) instead seem to benefit from expanding hypoxia (Rosa et al., Reference Rosa, Yamashiro, Markaida, Rodhouse, Waluda, Salinas, Keyl, O'Dor, Stewart, Gilly, Rosa, Pierce and O'Dor2013b). Nonetheless, the synergistic impact of these climate-related factors (i.e. hypoxia, global warming and ocean acidification) is expected to compress the habitable night-time depth range of these vertically migrating squid species due to unfavourable high temperature and decreasing pH at the ocean surface (Rosa & Seibel, Reference Rosa and Seibel2008).
At macroecological scales, a species distribution model (SDM) linked to the field of conservation physiology may help to explore future changes in the global patterns of cephalopod diversity. However, the reliability of SDM-based predictions needs to be improved, because models often lack a physiological underpinning and rely on assumptions that may be unrealistic under global climate change. For instance, additional information on the limits of thermal tolerance (e.g. maximum critical temperature (CTMax), lethal temperature at which 50% of the sample population dies (LT50)) will improve our ability to predict the effects of climate change on the present distribution patterns of cephalopods (Rosa et al., Reference Rosa, Dierssen, Gonzalez and Seibel2008a, Reference Rosa, Dierssen, Gonzalez and Seibelb, Reference Rosa, Gonzalez, Dierssen and Seibel2012a).
FUTURE CEPHALOPOD RESEARCH IN GENETICS (JAN M. STRUGNELL)
The volume of research that contains cephalopod genetic sequences has increased markedly over the last 20 years, in particular thanks to the decreasing costs of molecular sequencing. Prices are now sufficiently low for sequencing to become an attractive research tool for scientists representing a range of disciplines, including fisheries science, systematics, or neuroscience and developmental biology. The next exciting wave of genetic research on cephalopods is approaching as the first cephalopod genomes are being sequenced. Genome sequencing of at least ten cephalopod species is currently underway, representing a broad range of taxonomic groups, including Octopus vulgaris, O. bimaculoides, Hapalochlaena maculosa, Sepia officinalis, Doryteuthis pealeii, Euprymna scolopes, Idiosepius paradoxus, I. notoides, Architeuthis dux and Nautilus pompilius (Albertin et al., Reference Albertin, Bonnaud, Brown, Crookes-Goodson, da Fonseca, Di Cristo, Dilkes, Edsinger-Gonzales, Freeman, Hanlon, Koenig, Lindgren, Martindale, Minx, Moroz, Nödl, Nyholm, Ogura, Pungor, Rosenthal, Schwarz, Shigeno, Strugnell, Wollesen, Zhang and Ragsdale2012). Obtaining high-quality whole genome sequences of cephalopods will stimulate new inquiries by providing a wide range of research opportunities in which a reference genome is required, as well as in the interpretation of the genomes themselves.
However, the sequencing of cephalopod genomes is not without its challenges, and early work has shown cephalopod genomes to be large and to contain many repeated regions, making sequence assembly difficult (Albertin et al., Reference Albertin, Bonnaud, Brown, Crookes-Goodson, da Fonseca, Di Cristo, Dilkes, Edsinger-Gonzales, Freeman, Hanlon, Koenig, Lindgren, Martindale, Minx, Moroz, Nödl, Nyholm, Ogura, Pungor, Rosenthal, Schwarz, Shigeno, Strugnell, Wollesen, Zhang and Ragsdale2012). In addition, at least one whole genome duplication event has been suggested to have occurred during the evolution of the Cephalopoda (Hallinan & Lindberg, Reference Hallinan and Lindberg2011), which may further complicate assembly. Nonetheless, important lessons in sequencing whole molluscan genomes have been learned through sequencing of the few whole molluscan genomes that exist to date (i.e. Lottia, Aplysia and Biomphalaria). But, although best-practice methods of sequencing and assembly are being implemented (Albertin et al., Reference Albertin, Bonnaud, Brown, Crookes-Goodson, da Fonseca, Di Cristo, Dilkes, Edsinger-Gonzales, Freeman, Hanlon, Koenig, Lindgren, Martindale, Minx, Moroz, Nödl, Nyholm, Ogura, Pungor, Rosenthal, Schwarz, Shigeno, Strugnell, Wollesen, Zhang and Ragsdale2012), the task will not be trivial.
In addition, annotation of cephalopod genomes will likely prove to be a significant challenge as well. Part of the annotation process for a novel genome typically involves de novo gene prediction, a task that is known to be difficult and error-prone (Albertin et al., Reference Albertin, Bonnaud, Brown, Crookes-Goodson, da Fonseca, Di Cristo, Dilkes, Edsinger-Gonzales, Freeman, Hanlon, Koenig, Lindgren, Martindale, Minx, Moroz, Nödl, Nyholm, Ogura, Pungor, Rosenthal, Schwarz, Shigeno, Strugnell, Wollesen, Zhang and Ragsdale2012; Yandell & Ence, Reference Yandell and Ence2012). Large taxonomic distances exist between cephalopods and taxa with well-annotated animal genomes, which will increase the difficulties of annotation. Therefore, the sequencing of corresponding transcriptome data will be essential to supplement any de novo predictions, because it definitively identifies regions of the genome that are transcribed, and thus can help to identify boundaries between genes through differences in transcript abundance.
Despite these initial difficulties, the sequencing of the first cephalopod genome holds great promise for improving our understanding of the evolution and function of this fascinating group of marine organisms. Completely sequenced genomes will provide researchers with the ability to thoroughly study the function of different genes and also to investigate evolutionary relationships, not only within cephalopods, but also more broadly within molluscs and lophotrochozoans. In addition, whole genomic data of cephalopods will open up fields of research that have to date largely been unavailable or subject to only a handful of studies. Such research areas include epigenetic modification, RNA editing and microRNAs (Albertin et al., Reference Albertin, Bonnaud, Brown, Crookes-Goodson, da Fonseca, Di Cristo, Dilkes, Edsinger-Gonzales, Freeman, Hanlon, Koenig, Lindgren, Martindale, Minx, Moroz, Nödl, Nyholm, Ogura, Pungor, Rosenthal, Schwarz, Shigeno, Strugnell, Wollesen, Zhang and Ragsdale2012).
The development of a cephalopod model organism (possibly Idiosepius due to its small size) will allow focused studies of the development of the cephalopod body plan. This will facilitate investigation and understanding of many morphological features characteristic of cephalopods that are commonly suggested to be ‘vertebrate-like’, such as complex eyes, well-developed brains and highly differentiated vascular and neuroendocrine systems. As such, research of the evolution and development of these features, facilitated by whole-genome data, may not only provide further insight into cephalopod evolution, but also into the evolution of man (depending on whether the similarity to vertebrate structures is superficial or based on genuine homology).
CHALLENGES IN CEPHALOPOD FISHERIES AND CONSERVATION
The future trends in cephalopod fisheries (Graham J. Pierce)
Historically, cephalopod fisheries have been less important in the north-east Atlantic compared to much of the rest of the world (Caddy & Rodhouse, Reference Caddy and Rodhouse1998; Hunsicker et al., Reference Hunsicker, Essington, Watson and Sumaila2010b), despite a strong tradition of cephalopod consumption in southern Europe. However, a combination of declines in other fishery resources has led to an increase in directed cephalopod fishing as well as increased attention from fishers, national governments, and fisheries organizations such as the International Council for the Exploration of the Sea. In Europe, therefore, the short-term trend is likely to be an increased effort in cephalopod fishing, extending exploitation to currently under- or unexploited species, coupled with novel implementation of formal stock assessment and regulated fishing policies. However, it is fairly unlikely that existing stocks can absorb a substantial increase in fishing pressure (Royer et al., Reference Royer, Peries and Robin2002) and past experience shows that the unpredictable nature of cephalopod abundance tends to discourage commercial fishery interests (Young et al., Reference Young, Pierce, Stowasser, Santos, Wang, Boyle, Shaw, Bailey, Tuck and Collins2006).
These remarks can be generalized to world cephalopod fisheries in the sense that landings have been increasing (at least until around 2005), new species have assumed high importance (notably Dosidicus gigas in the eastern Pacific) and evidence is already being seen of overexploitation in some areas (Pierce & Portela, Reference Pierce, Portela, Iglesias, Fuentes and Villanueva2014). A key issue will be understanding the rise (and fall) of important cephalopod fisheries, especially those of ommastrephid squids such as Todarodes pacificus, Illex argentinus and D. gigas. While we suspect that environmental sensitivity is one key to understanding population trajectories, effects of overexploitation may at least partially explain some of the spectacular crashes like that of the T. sagittatus fishery off Norway in the mid-1980s. As suggested above, global climate change may have a range of impacts on cephalopod populations and may result in a shift in the relative importance of fisheries and environment in controlling population dynamics.
Cephalopod culture, especially for Octopus spp. (Iglesias et al., Reference Iglesias, Fuentes and Villanueva2014), may help to fill the growing demand for cephalopods in Europe and its export markets. Relevant recent developments in cephalopod culture include in vitro fertilization (Villanueva et al., Reference Villanueva, Quintana, Petroni and Bozzano2011). Nonetheless, artisanal fisheries will remain important, and are increasingly in need of assessment and management that is appropriate to the small scale of the fisheries and the particular biological features of the resource species. However, perhaps the biggest question mark concerns whether exploitation of deep-sea cephalopod resources is capable of expansion. Malcolm Clarke, among other cephalopod scientists, suggested that there are vast resources of oceanic squids in the world. His assessment was based on the estimated amount of food needed to sustain the world's sperm whale population (Clarke, Reference Clarke1996; Santos et al., Reference Santos, Clarke and Pierce2001). This potential resource presents an enticing opportunity for fisheries, but others have cast doubt on the large abundance of such species. In addition, a practical challenge relates to palatability, although fishery companies are currently developing processing methods for ammonium-rich squid tissues to permit their marketing as food products.
Fisheries management and governance in Europe is currently undergoing a revolution, with the implementation of an integrated ecosystem assessment and management approach as part of the reform of the Common Fisheries Policy, while at the same time looking ahead to a future integrated marine management, in which fisheries are simply one of many relevant sectors. The move towards an ecosystem approach to fisheries is of course not unique to Europe. However, the steep increase in data requirements (compared to single species assessments) presents a real obstacle, especially in a period of economic recession; thus, alternative approaches based on indicators and expert judgement are also likely to be needed. In this context, the Marine Strategy Framework Directive (MSFD) of the European Union is relevant, as it focuses on the development of indicators of ocean health. At least in the United Kingdom, there are plans to develop cephalopod indicators for the MSFD. As a final note, cephalopod waste from fishery processing, and cephalopod species of lesser interest for human consumption, may be increasingly used in animal feedstuffs, fertilizers (Fetter et al., Reference Fetter, Brown and Amador2013), or other industrial products such as pharmaceuticals.
Cephalopod conservation (A. Louise Allcock)
Assessing the conservation status of a wide range of cephalopod taxa reveals just how little is known about many species. Studies carried out for the International Union for the Conservation of Nature (IUCN) Red List, focusing on different higher cephalopod taxa (e.g. Sepiida, Oegopsida, Cirrata), have found that between about 50 and 75% of species in these higher taxa are ‘Data Deficient’ (Kemp et al., Reference Kemp, Peters, Allcock, Carpenter, Obura, Polidoro, Richman, Collen, Böhm, Kemp and Baillie2012). Many species are known from just a few specimens, so that little is known about their biology and ecology. In some cases, we can conclude that species meet the IUCN category of ‘Least Concern’ simply because their very wide geographical distribution and high fecundity with planktonic dispersal means that they are unlikely to be impacted across their entire distribution range, despite the possible existence of local threats, so the lack of data is actually under-reported.
In particular, data are lacking for cirrate octopods. These cephalopods are potentially long-lived, are slow to reach maturity and have low fecundity (Collins & Villanueva, Reference Collins and Villanueva2006). Opisthoteuthis, the most shallow cirrate genus, is characterized by a close association with the benthos, and is therefore the genus most affected by commercial deep sea trawling. Opisthoteuthis chathamensis was considered ‘Nationally Critical’ on the New Zealand Red List (Freeman et al., Reference Freeman, Marshall, Ahyong, Wing and Hitchmough2010) and Collins & Villanueva (Reference Collins and Villanueva2006) suggested that populations of other species may already have declined as a result of deep sea trawling. However, a lack of specific population data and information on fisheries impacts will likely prevent many potentially vulnerable species being listed in a category other than ‘Data Deficient’. Therefore, one of the future challenges for cephalopod biologists is to improve the quality and consistency of population estimates for all cephalopod species, particularly those subjected to direct or indirect anthropogenic impacts, including fishing.
Taxonomic issues may also prevent the actual vulnerability of a species from being reflected in its conservation assessment. Recent dramatic declines in the size of the Sepia apama population in the upper Spencer Gulf (South Australia) have been well documented (Hall, Reference Hall2008, Reference Hall2010), but attempts to have this population listed as ‘Critically Endangered’ under Australia's Environment Protection and Biodiversity Conservation Act 1999 failed (Anonymous, 2011), apparently because the population had not been formally described as a distinct species, despite little evidence of it inter-breeding with other populations (Anonymous, 2011). However, a temporary localized ban on fishing was enacted in 2013. Sepia apama was assessed as ‘Near Threatened’ on the IUCN Red List (Barratt & Allcock, Reference Barratt and Allcock2012), but this assessment considered the whole range of the species, as is normal practice. The IUCN assessment notes that ‘If the population in the upper Spencer Gulf is shown to be a separate species then the Spencer Gulf species would be assessed as Endangered.’
Conservation efforts for Nautilus are similarly hindered. The slow growth and low fecundity of nautiluses (Dunstan et al., Reference Dunstan, Ward and Marshall2011) make them vulnerable to fishing pressure and several overfished populations have crashed (Dunstan et al., Reference Dunstan, Alanis and Marshall2010). The very wide distribution range reported for N. pompilius suggests that threats are likely to be local, until one considers recent genetic data. For example, molecular phylogenetic work (Bonacum et al., Reference Bonacum, Landman, Mapes, White, White and Irlam2011; Sinclair et al., Reference Sinclair, Newman, Vianna, Williams and Aspden2011; Williams et al., Reference Williams, Newman and Sinclair2012) indicates that N. pompilius comprises several distinct phylogenetic species. This suggests that the impact of fisheries is far more likely to lead to species extinctions than previously thought. However, descriptions of individual species within the N. pompilius species complex and accurate information on the range of these species are required if conservation listings are to reflect the perceived vulnerability to anthropogenic impacts. Therefore, ensuring that all cephalopod species are accurately described, and that species complexes and cryptic species are distinguished, constitutes an essential future challenge for cephalopod conservation.
DISCUSSION
Cephalopods will continue to attract scientific interest, particularly in the fields of physiology, genetics, ecology and fisheries. Furthermore, the traditional scientific disciplines of taxonomy and morphology are currently being rejuvenated by the application of new technologies. Studies on cephalopods will continue to range from the organismic level (e.g. physiology, behaviour), to the species level (e.g. taxonomy, systematics, population dynamics, distribution, abundance), and finally to the ecosystem level (e.g. fisheries, biodiversity, conservation). In addition, new cephalopod research is emerging on issues such as global climate change and ocean acidification or habitat and food-web modelling.
Cephalopods constitute an important trophic link between the lower levels of food webs and top predators (Young et al., Reference Young, Olson and Rodhouse2013). About 800 species of extant cephalopods have been described, but we only have sufficient data to understand the life history (e.g. distribution, habitat, feeding ecology, reproductive biology) for approximately 60 species (Jereb & Roper, Reference Jereb and Roper2005, Reference Jereb and Roper2010; Jereb et al., Reference Jereb, Roper, Norman and Finn2014). Therefore, taxonomists and geneticists must increasingly work together to ensure that specimen data uploaded to databases are based on correctly identified specimens. The combination of molecular genetics, DNA barcoding, and digital morphological techniques offers new ways to resolve numerous outstanding issues in cephalopod taxonomy and evolution. In this context, an increase in molecular work is of particular importance, because the lack of transcriptomic and genomic information, for example, has limited advances in neurobiology research, where cephalopods act as model organisms (Zhang et al., Reference Zhang, Mao, Huang, Qu, Chen, Ding, Hong and Sun2012).
Cephalopods have several interesting traits, which make them suitable model organisms for broad evolutionary research. For instance, they have one of the largest size ranges of any metazoan class and could, therefore, become model species for studying metazoan growth and metabolism. Furthermore, cephalopods show a remarkable diversity of life history traits, and a better understanding of evolutionary relationships among cephalopods would help to determine the plasticity of these traits or could reveal simple switches between individual strategies. In addition, because of the presence of mineralized structures or the planktonic early life stages, most cephalopod species may be highly sensitive to global climate change and/or ocean acidification, because of the presence of mineralized structures or the planktonic early life stages. Hence, cephalopods should be increasingly used as model organisms to predict the effects of global warming on ocean life (Hanlon et al., Reference Hanlon, Bidwell and Tait1989; Rodhouse, Reference Rodhouse2013).
In addition, a quantitative PCR approach should finally allow reliable identification of cephalopod species as prey. Also, because top predators are still a major source of information on cephalopods, novel techniques in trophic research such as the analysis of stable isotopes, DNA, or fatty acids as well as 3D imaging will complement the data obtained by conventional means (Jarman et al., Reference Jarman, Deagle and Gales2004; Barrett et al., Reference Barrett, Camphusysen, Anker-Nilssen, Chardine, Furness, Garthe, Hüppop, Leopold, Montevecchi and Veit2007; Karnovsky et al., Reference Karnovsky, Hobson and Iverson2012). These latter techniques have suffered due to a decline in taxonomists actually able to perform this type of work (Pearson et al., Reference Pearson, Hamilton and Erwin2011). Furthermore, the use of ecological tracers, especially fine-scale analyses of tracer molecules within informative structures, such as statoliths, beaks or shells, will offer new insights into stock structuring and individual life history (Cherel & Hobson, Reference Cherel and Hobson2005; Cherel et al., Reference Cherel, Fontaine, Jackson, Jackson and Richard2009a; Ramos & Gonzalez-Solis, Reference Ramos and Gonzalez-Solis2012). In addition, recent improvements in specimen tagging now allow studying movements of cuttlefish and squid (Gilly et al., Reference Gilly, Markaida, Baxter, Block, Boustany, Zeidberg, Reisenbichler, Robison, Bazzino and Salinas2006; Semmens et al., Reference Semmens2007; Wearmouth et al., Reference Wearmouth, Durkin, Bloor, McHugh, Rundle and Sims2013). If tag weight could be further reduced and some attachment issues resolved, this approach might be extendible to smaller cephalopod species or earlier developmental stages.
Future research should certainly also focus on the ecology of cephalopod species, particularly for those species with immediate commercial fishery interest. As a result of the increasing international capacity to explore deeper environments, deep-sea cephalopods will attract the attention of fisheries and research. For example, the increasing amount of deep sea imagery calls for creative solutions to compiling and using such data. Advanced and more complete morphological data will improve our ability to identify specimens based on photographic records alone. For the well-known commercial cephalopod species, long-term monitoring and the establishment of marine protected areas will be the primary focus of discussion in cephalopod conservation. Furthermore, the usage of the continental shelf slope, deep sea, and oceanic areas by numerous pelagic predators and cephalopods is a further area that will receive attention from conservationists (Harris et al., Reference Harris, Haward, Jabour and Woehler2007; Game et al., Reference Game, Grantham, Hobday, Pressey, Lombard, Beckley, Gjerde, Bustamante, Possingham and Richardson2009; Tancell et al., Reference Tancell, Phillips, Xavier, Tarling and Sutherland2012). In order to catch fast-swimming cephalopods, efforts should be channelled into the use of more efficient nets that allow catching sub-adult or adult stages of the larger species. Incorporating such research foci into major multidisciplinary projects could become essential for success in obtaining funding.
At present, the effects of global climate change, linked with acidification, warming, and expanding hypoxia, perhaps represent the biggest threat to certain species of cephalopods, but also constitute a challenge to researchers, policymakers, and society at large. From a scientific point of view, one of the greatest challenges in this discipline will be to discriminate between the effects of global climate change and fisheries on cephalopod populations. In this regard, experimentation has always been an important approach to resolving open questions in cephalopod research. From a technological perspective, cephalopod culture should be further developed to meet challenges such as the development of sustainable artificial foods or the control of reproduction and genetic manipulation. In addition, the successful maintenance of deep-sea and oceanic cephalopods in captivity would be a major step forward to understanding their life cycles and would contribute to assessing the potential impact of fisheries targeted at other species in their habitats. Such research efforts would also constitute an important contribution to cephalopod conservation efforts (Hoving et al., Reference Hoving, Perez, Bolstad, Braid, Evans, Fuchs, Judkins, Kelly, Marian, Nakajima, Piatkowski, Reid, Vecchione and Xavier2014).
Finally, collaboration, in particular between scientific disciplines, is essential for tackling some of the big scientific challenges the world is currently facing. Early career scientists, such as the CIAC Young Researchers group, should make ample use of novel, digital approaches to networking, communication and collaboration. Social media, along with digital repositories as well as new data and research sharing protocols, will continue to facilitate international and interdisciplinary research on cephalopods and related scientific areas. Furthermore, education and outreach initiatives are bound to follow suit, resulting in the increased dissemination of cephalopod science to a wider audience.
ACKNOWLEDGEMENTS
We thank António M. de Frias Martins, past President of the Unitas Malacologica and Peter Marko, President of the American Malacological Society for organizing the 2013 World Congress of Malacology, and the Cephalopod International Advisory Committee for endorsing a symposium held in honour of Malcolm R. Clarke. In particular, we would like to thank the many professional staff from the University of the Azores for their hospitality, organization, troubleshooting and warm welcome to the Azores. We also thank Malcolm Clarke's widow, Dorothy, his daughter Zoë, José N. Gomes-Pereira and numerous colleagues and friends of Malcolm's from around the world for joining us at Ponta Delgada. We are grateful to Lyndsey Claro (Princeton University Press) for granting copyright permissions.