INTRODUCTION
Polychaete annelids are among the most common members of benthic communities in the world's oceans. Their biomass can comprise more than 50% of the total biomass of benthic invertebrates in some areas of soft bottom (Knox, Reference Knox, Reish and Fauchald1977). Dense populations of tubicolous polychaetes may transform the microtopography of the seabed surface with their tube structures or holes from tube openings. They transport large amounts of surface materials and oxygen into deeper sediment layers (bioturbation), creating new living space for other organisms by changing physical and chemical properties of the sediment (Bolam & Fernandes, Reference Bolam and Fernandes2002; Mermillod-Blondin, Reference Mermillod-Blondin2011; Kristensen et al., Reference Kristensen, Penha-Lopes, Delefosse, Valdemarsen, Quintana and Banta2012).
Many polychaetes are active tube builders (McIntosh, Reference McIntosh1894; Defretin, Reference Defretin1971). According to Dudgeon (Reference Dudgeon and Wotton1994), the structures built by aquatic animals may be classified as burrows (in the substrate, sometimes with reinforced walls), tubes (fixed to the substrate) or cases (portable). Polychaete tubes can be similarly classified as tubes, cases, or mere burrows.
All Serpulidae, a few species of Cirratulidae, and a single species of Sabellidae produce calcareous tubes (Vinn, Reference Vinn2009). Some polychaetes secrete organic tubes without any exogenous material, e.g. certain Chaetopteridae (Barnes, Reference Barnes1965) and Onuphidae (Budaeva et al., Reference Budaeva, Pyataeva and Meißner2014). Other polychaetes make agglutinated tubes from sand grains and other particles cemented with specific secretions from epithelial glands, or line the walls of their burrows with layers of secretion (Defretin, Reference Defretin1971). In agglutinated tubes, besides the layer containing exogenous materials there is an inner sheath (Merz, Reference Merz2015; Shcherbakova & Tzetlin, Reference Shcherbakova and Tzetlin2016), deposited as overlapping layers or series of fibres, the orientation of which changes from one layer to the next. Such a plywood- or fabric-like structure helps explain the robustness of polychaete tubes (Merz, Reference Merz2015).
Sandcastle and honeycomb worms (Phragmatopoma and some species of Sabellaria, Sabellariidae) are gregarious and may build reefs in subtidal and intertidal zones. They construct solid tubes of sand grains cemented with foam-like organic material, in Phragmatopoma also including some fibres (Zhao et al., Reference Zhao, Sun, Stewart and Waite2005; Flammang & Lambert, Reference Flammang and Lambert2008; Dean et al., Reference Dean, Welch, Brandt, Tauer, Maciolek and Blake2009; Merz, Reference Merz2015). Such structures, together with the visco-elastic properties of the cement, make their tubes resistant to waves (Le Cam et al., Reference Le Cam, Fournier, Etienne and Couden2011). The underwater glue of Phragmatopoma has been a model for designing biomimetic analogues to use in human bone repair (Stewart et al., Reference Stewart, Ransom and Hlady2011).
Ice cream cone worms (Pectinariidae) are solitary, subsurface, head down deposit feeders in shallow waters, moving through the sediment with their tubes (portable cases; Desroy et al., Reference Desroy, Olivier, Retière, Reish and Qian1997). Pectinaria gouldii constructs fragile, short, conical tubes with one layer of tablet-like grains; the cement is foam-like with cells of uniform size, and the inner lining is fabric-like (Dean et al., Reference Dean, Welch, Brandt, Tauer, Maciolek and Blake2009; Merz, Reference Merz2015). Owenia spp. (Oweniidae) are another, dissimilar, example of case-bearers. They burrow through the sandy sediment in their long, cylindrical, flexible tubes covered by imbricating sand grains and shell fragments (Watson, Reference Watson1901; Noffke et al., Reference Noffke, Hertweck, Kröncke and Wehrmann2009).
We have studied the fine structure of the tubes of three species of Terebellidae (Shcherbakova & Tzetlin, Reference Shcherbakova and Tzetlin2016). In these tubes, the agglutinated layer consists of sand grains either wrapped in organic films forming cells (Pista elongata, P. flexuosa) or submerged into amorphous organic cement (Amphitrite figulus). Tubes of Pista spp. are resilient and tear-proof. They can be attached to stones or shelly ground in areas subject to fast currents (P. elongata) or flattened, regularly sinuous, lying freely on the surface of semi-liquid mud (P. flexuosa). The agglutinated layer is thick and brittle in the U-shaped tubes (burrows sensu Dudgeon) of A. figulus found within silty sand sediment.
The variability of tube morphology and composition among different species, genera or sometimes families, suggests that such data could inform polychaete taxonomy, increase understanding of polychaete phylogeny, and assist in the identification of fossil worms (e.g. see Vinn & Luque, Reference Vinn and Luque2013).
Different genera of the family Maldanidae build different tubes (both tubes s.str. and burrows sensu Dudgeon), and that stimulated us to undertake this study. As in other polychaetes, the tube structure in Maldanidae seems to correlate with their lifestyle. Maldanids inhabit soft and mixed sediments, and their tubes are characteristic of the bottom type in which they live. All maldanids are tube-dwelling, their reproduction and the development of lecithotrophic larvae also take place inside the tubes (Wilson, Reference Wilson1983; Rouse, Reference Rouse1992). However, their tube structure and biology are still little known (Jumars et al., Reference Jumars, Dorgan and Lindsay2015). The tube shape is variable; tubes may be U-, J- or Y-shaped, twisted, vertical or slanted, with one or both ends opening at the water–sediment interface (Mangum, Reference Mangum1964; Hughes, Reference Hughes1979; Wilson, Reference Wilson1983; see Discussion). Computed tomography (CT) scanning has revealed that the buried tubes of Maldane sarsi are surrounded by stacks of consolidated mud discs (Dufour et al., Reference Dufour, White, Desrosiers and Juniper2008). The fine structure of the inner tube lining was illustrated in N. lokii (Kongsrud & Rapp, Reference Kongsrud and Rapp2012) and Clymenella torquata (Merz, Reference Merz2015).
We examined the fine structure of tubes in several genera and species of Maldanidae. The following structural components of the tubes are recognized (terminology after Shcherbakova & Tzetlin, Reference Shcherbakova and Tzetlin2016): (1) inner sheath (inner cylinder); (2) agglutinated layer consisting of sediment particles bound with cement, i.e. organic material secreted from specialized glands and becoming fibrous, filmy or foamy when consolidated; (3) outer layer (outer organic layer) present in some genera.
MATERIALS AND METHODS
The fine structure of the tubes was studied in six species of five genera: Axiothella catenata (Malmgren, 1865), Maldane sarsi Malmgren, 1865, Nicomache lumbricalis (Fabricius, 1780), N. minor Arwidsson, 1906, Praxillella praetermissa (Malmgren, 1865) and Rhodine gracilior Tauber, 1879. A total of 181 specimens were examined (Table 1). Most of the material was collected in the summers of 2011–2015 near the Nikolai Pertzov White Sea Biological Station (WSBS) using a Sigsbee trawl, Ocean 0.1 m2 grab and diving techniques. Some specimens of M. sarsi, N. lumbricalis and A. catenata were obtained from bottom samples collected in the Barents Sea. Dried tubes of M. sarsi from the Sea of Japan were received courtesy of Irina Ekimova and the Vostok Marine Biological Station (MBS) diver team.
Table 1. Collected material.
While removing tubes from the sediment, we tried to keep them intact and preserve their shape. Before SEM, the tubes from which the worms were removed were rinsed in distilled water, fixed in 4% formaldehyde, rinsed once again, and put into 70% ethanol (some tubes were fixed in 96% ethanol). The tubes were fractured to examine the fracture faces, as well as the inner and outer surface. Then the tubes were dried in a Hitachi HCP-2 critical point dryer, coated with Аu/Pd using an Eiko IB-3 ion coater, and scanned under CamScan S-2 and JEOL JSM-100 microscopes. Every tube underwent the same preparation and investigation protocols. The outer surface, inner sheath and tube fracture faces were examined under a series of magnifications.
RESULTS
Nicomache lumbricalis (Figure 1A–F)
This species was collected at depths of 30–100 m on silty sediments with stones, subject to low currents. The tubes, up to 10–12 cm long and 2–5 mm in diameter, were made of fine sand and coarse silt, and usually orange coloured due to impregnation by iron and calcium salts over time. They were attached to cobbles and irregularly curved, or twisted around themselves forming a ball 2–3 cm in diameter. The tubes were hard, non-elastic, and broke apart when bent (except for the inner sheath). The tube wall thickness was about 800–1200 µm.

Fig. 1. Nicomache lumbricalis (Fabricius, 1780), tube: (A) longitudinal fracture face of tube; (B) sand grains and inner sheath; (C, D) 3D cement network; (E) inner surface of inner sheath; (F) agglutinated layer and outer layer. 3Dcn, 3D cement network; AL, agglutinated layer; FIS, fibres of inner sheath; IS, inner sheath; OL, outer layer; SG, sand grain. Scale bars: A, 300 µm; B, 30 µm; C, 5 µm; D, 10 µm; E, 2 µm; F, 3 µm.
The inner sheath (Figure 1A, B), 9–11 µm in thickness, consisted of fine organic fibres about 0.5 µm in diameter arranged in many layers, each with a different fibre orientation. The fibres partially merged and formed a fabric-like structure. The variable orientation of fibres provided the inner sheath with tensile strength (Figure 1E). The outer surface of the inner sheath comprises of numerous fibres, together forming a 3D network or open-cell foam. This cement filled the gaps between the sediment grains and attached them to the inner sheath. On the surface of the grains, cement fibres formed films and left traces of their attachment (Figure 1C, D). In one specimen from the White Sea, the agglutinated layer consisted of two sublayers, the inner of coarse silt (40–60 µm), and the outer of fine sand (60–140 µm) with individual grains of coarse sand (up to 2 mm in diameter; Figure 1A). The tubes were covered with a thin outer layer, which resembled a fibrous lace rather than the fabric of the inner sheath (Figure 1F).
Nicomache minor (Figures 2A–E & 3)
This species was collected at depths of 5–35 m on silty sediments with stones. The tubes, up to 10 cm long and 3.2 mm in diameter, were made of fine sand grains (110–170 µm). They were attached on one side to cobbles and small boulders (5–25 cm in diameter) that were partially embedded into the sediment. Sometimes the tubes bound stones together, if these were close to each other. Some tubes bifurcated, formed superstructures or even mazes. The tubes were usually light grey, tan, or sometimes orange coloured if impregnated with iron salts. The walls were rather thick (800–1200 µm); the side attached to the stone was flattened, but the lumen was rounded in cross-section.

Fig. 2. Nicomache minor Arwidsson, 1906, tube: (A) longitudinal fracture face of tube; (B) inner sheath and sand grains; (C) inner surface of inner sheath; (D) sand grains fastened with cement fibres forming 3D network; (E) outer layer covering agglutinated sand grains. BF, bands of fibres; FAL, fibres of agglutinated layer; FIS, fibres of inner sheath; IS, inner sheath; OL, outer layer; SG, sand grain. Scale bars: A, 100 µm; B, C, 10 µm; D, E, 30 µm.

Fig. 3. Nicomache minor Arwidsson, 1906, schematic section of tube wall. 3DCN, 3D cement network; IS, inner sheath; OL, outer layer; SG, sand grain.
The inner sheath (Figure 2A, B), 1.4–1.8 µm thick, consisted of fibres 0.2–0.4 µm in diameter, applied layer by layer in several directions and forming a fine mesh with gaps under 1 µm. Also, some fibres covered the inner surface without any visible order, curving at different angles (Figure 2C).
Sand grains in the agglutinated layer connected to each other and to the inner sheath with a 3D network of cement fibres (Figures 2D & 3). These fibres merged into bands attached to the inner sheath (Figure 2B), and formed a plexus rather than films on the surface of the grains. The tubes were covered with a thin outer layer, which resembled a fibrous lace rather than the fabric of the inner sheath (Figure 2E).
Maldane sarsi (Figures 4A–F & 5)
This species was collected at depths of 20–100 m from silty sediments subject to low currents. The tubes were soft and easily deformed or torn, generally 5–8 cm long, up to 5.4 mm in diameter, usually grey, straight, buried vertically in the sediment. The tube walls were 600–1100 µm thick.

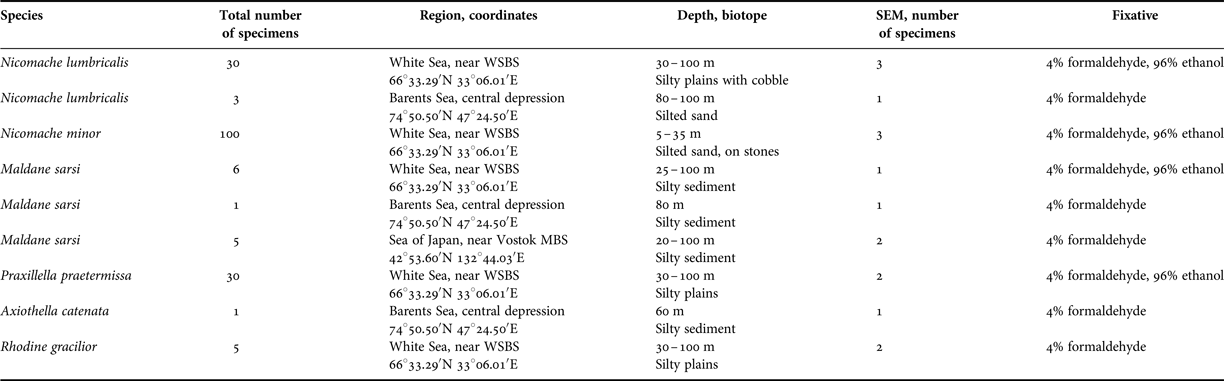
Fig. 4. Maldane sarsi Malmgren, 1865, tube: (A) longitudinal fracture face of tube; (B) inner surface of inner sheath; (C) outer side of inner sheath and cemented sediment; (D) mud half-rings covered with fabric of fibres; (E) mud half-rings containing cemented sediment particles; (F) transverse fracture face of tube. CS, cemented sediment; FHR, radial fibres inside half-ring; FIS, fibres of inner sheath; HR, mud half-rings; IS, inner sheath; WHR, wrapped half-ring. Scale bars: A, 300 µm; B, 1 µm; C–E, 3 µm; F, 10 µm.
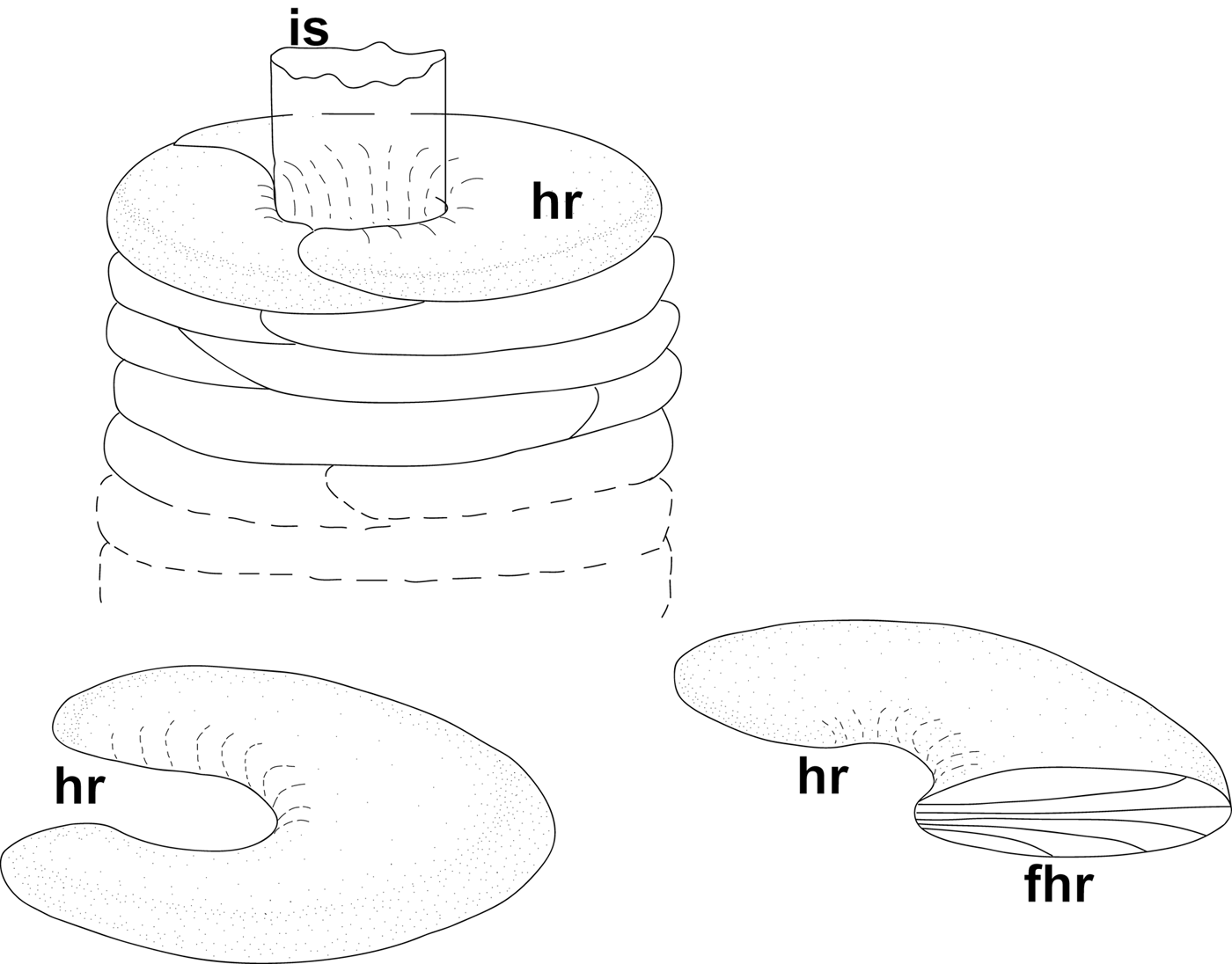
Fig. 5. Maldane sarsi Malmgren, 1865, general scheme of tube structure. Abbreviations: FHR, radial fibres inside half-ring; HR, mud half-rings; IS, inner sheath.
The inner sheath was 1.0–2.4 µm thick, easily separable from the agglutinated layer, and composed of fibres 0.10–0.15 µm in diameter. The fibres on the inner surface were oriented tangential to the tube axis, and those of the underlying levels showed variable orientations (Figure 4A, B).
The agglutinated layer consisted of small sediment particles cemented into mud rings. These rings, or rather half-rings, were wrapped in a fabric-like structure similar to that of the inner sheath, but much thinner (about 0.4 µm; Figure 4D, E). The rings contained cemented sediment and detritus particles (including numerous diatoms), and also radial fibres 0.1–0.2 µm in diameter, arranged more or less perpendicular to the tube axis (Figures 4C, F & 5).
According to Dufour et al. (Reference Dufour, White, Desrosiers and Juniper2008), the ‘inner tube’ of M. sarsi, with a diameter of less than 1 cm, is surrounded by stacks of consolidated mud discs 2–6 cm in diameter that were not extracted from the sediment along with the tube. Our description refers to this ‘inner tube’.
Praxillella praetermissa (Figure 6A–D)
This species was collected at depths of 30–100 m from mainly silty sediments subject to low currents. Its fine-sand or silty tubes were up to 12 cm long and 1.8 mm in diameter, grey, sometimes branching. It was easy to break the agglutinated layer, but the inner sheath was tear-proof. The tube wall in this species was the thinnest (220–260 µm) of the species studied.

Fig. 6. Praxillella praetermissa (Malmgren, 1865), tube: (A) longitudinal fracture face of tube; (B) agglutinated layer; (C) inner sheath; (D) sand grains fastened with cement fibres. AL, agglutinated layer; FAL, fibres of agglutinated layer; IS, inner sheath; SG, sand grain. Scale bars: A, 500 µm; B, 50 µm; C, 2 µm; D, 10 µm.
The inner sheath, 0.9–1.5 µm thick, was made of interlaced fibres (0.17–0.35 µm in diameter) applied layer by layer in several directions, as seen in its inner part. The outer part of inner sheath showed no trace of fibrous structure, but numerous fibres arose from its smooth surface. These fibres fastened sand grains in the agglutinated layer (Figure 6C). These fibres anastomosed and formed a 3D network between the grains, producing a lace-like attachment net on the grain surface (Figure 6D).
The composition of the agglutinated layer depended on the surrounding sediment. Our specimens included both coarse silt and fine sand grains (20–80 µm). The spaces between larger grains were filled with unicellular algae debris, detritus and smaller sediment particles (Figure 6A, B).
Axiothella catenata (Figure 7A–F)
This species was collected in silty sediments at a depth of 60 m. The tube was made of sand and silt, about 2 mm in diameter with the wall about 450 µm thick (Figure 7A).

Fig. 7. Axiothella catenata (Malmgren, 1865), tube: (A) longitudinal fracture face of tube; (B) outer side of inner sheath, sand grains partly removed; (C) inner view of tube with inner sheath removed; (D) agglutinated layer, 3D cement network; (E) inner surface of inner sheath; (F) outer side of inner sheath. 3DCN, 3D cement network; AL, agglutinated layer; FAL, fibres of agglutinated layer; FIS, fibres of inner sheath; IS, inner sheath; SG, sand grain. Scale bars: A, 300 µm; B, C, 30 µm; D–F, 3 µm.
The inner sheath, about 0.8 µm thick, consisted of interlaced fibres 0.15–0.20 µm in diameter, applied layer by layer in several directions (Figure 7E, F). The outer surface of the inner sheath and the grains of sand and silt (30–250 µm) glued to it were covered by a thin, uneven layer of cement made of tightly interlaced fibres (0.07–0.13 µm in diameter) connected in a foam-like structure (Figure 7B–D).
Rhodine gracilior (Figures 8A–F & 9)
This species was collected at depths of 30–100 m on silty plains subject to low currents. Its elastic and tear-proof tubes, up to 1.3 mm in diameter with walls up to 350 µm thick, were sparsely covered with grains of sand (60–800 µm; Figure 8A, F) and varied in colour from yellow to dark red with black inclusions.

Fig. 8. Rhodine gracilior Tauber, 1879, tube: (A) general view; (B) outer side of inner sheath; (C) surface of one of layers of inner sheath; (D) inner surface of inner sheath; (E) longitudinal fracture face of tube; (F) transverse fracture face of tube. AL, agglutinated layer; FIS, fibres of inner sheath; IS, inner sheath; OSIS, outer side of inner sheath; SG, sand grain. Scale bars: A, 500 µm; B, 2 µm; C, D, 1 µm; E, 20 µm; F, 100 µm.
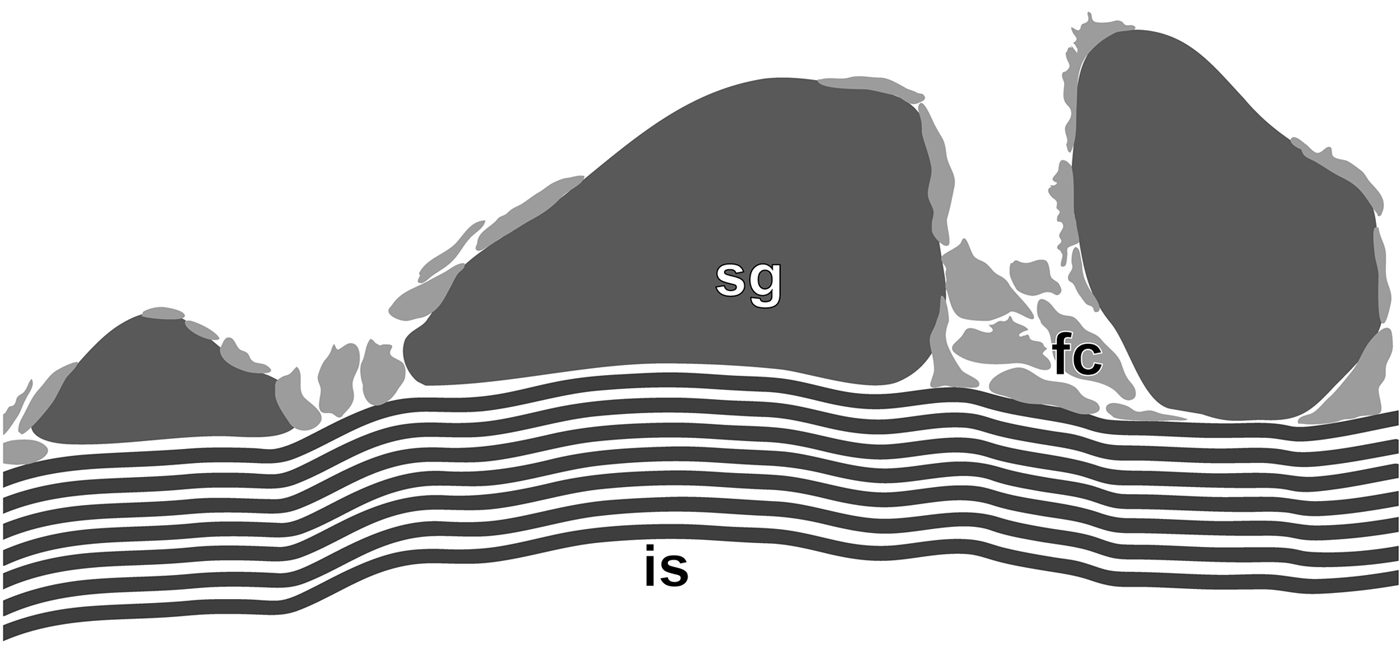
Fig. 9. Rhodine gracilior Tauber, 1879, schematic section of tube wall. FC, foamy cement; IS, inner sheath; SG, sand grain.
The inner sheath, 50–60 µm thick, was composed of 5–8 layers, each consisting of tightly bound and flattened fibres (0.1–0.2 µm in diameter). These layers were cemented with similar but more loosely arranged fibres (Figures 8B–E & 9). The outer surface of the inner sheath had numerous fibres that attached to the grains of sand; both the sheath and grains were covered by spots of foamy cement. In dried tubes grains of sand detached easily from the inner sheath (Figure 8A).
DISCUSSION
Tube shape and position
Nicomache minor and N. lumbricalis attach their tubes to stones partly embedded in the sediment, and will use both the upper and lower sides of a stone (Tzetlin & Markelova, Reference Tzetlin and Markelova1985; own observations). The tube apertures usually open at the water–sediment interface. The tubes are twisted, and in N. minor sometimes form dense aggregations inhabited by individuals of different size. These species feed from the sediment surface (Fauchald & Jumars, Reference Fauchald and Jumars1979) and use both ends of the tube, since the worms can easily turn around inside their tubes (Tzetlin & Markelova, Reference Tzetlin and Markelova1985).
The species of the other maldanid genera make their tubes within the sediment – and, following Dudgeon (Reference Dudgeon and Wotton1994), these should be classified as burrows, even those with complicated constructions as in Maldane sarsi. We collected these tubes using grabs and by trawling, so we cannot determine their positions accurately. However, information on the same or congeneric species is available in the literature. They are infaunal deposit feeders making vertical or inclined tubes, which are inhabited head downwards. Tubes of M. sarsi are vertical (Dufour et al., Reference Dufour, White, Desrosiers and Juniper2008); tubes of Axiothella catenata are slanted, often branched; tubes of Praxillella gracilis are steeper, with numerous branches (Hughes, Reference Hughes1979).
The tube shape shows both inter- and intraspecific variation. Tubes are J-shaped in Clymenella zonalis, Y-shaped in females of C. mucosa during the breeding season, and convoluted in Petaloproctus socialis (Mangum, Reference Mangum1964). Axiothella rubrocincta represents a complex of sibling species that differ inter alia in tube shape: the worms from False Bay, San Juan Island, Washington live in vertical tubes (Wilson, Reference Wilson1983), and those from Tomales Bay, California inhabit U-shaped tubes (Kudenov, Reference Kudenov1978, Reference Kudenov1982). The tube position in the sediment and sometimes the tube shape may differ even in neighbouring populations, and such variations may correlate with sediment types and underwater currents.
Tube construction
Sabellariidae, Pectinariidae, Oweniidae, Terebellidae and some other polychaetes build their permanent tubes like masons. They select particles with the mouth and head appendages and glue them to the tube edge using specialized anterior glands. The wall is then consolidated and lined with secretions from epidermal glands (Defretin, Reference Defretin1971). Some Maldanidae closely approach this category. In Nicomache minor, constructing branched tubes along the water–sediment interface, the worm applies sediment particles to the tube with its mouth, and the rate of tube extension is about 1.3 mm per hour (Tzetlin & Markelova, Reference Tzetlin and Markelova1985).
In contrast, Nereididae, some Sabellidae and several other groups may not have special tube-building organs other than epidermal glands along the body, and can easily regenerate their tubes (Bonar, Reference Bonar1972; Merz, Reference Merz2015). Maldanid genera such as Praxillella and Axiothella belong to this group. They live head down in slanted tubes open at the surface, and ingest sediment from the buried lower end of the tube forming a feeding cavity. New tube branches are built to change the feeding position, and the old ones disintegrate. As observed in A. catenata, worms without tubes burrow in sediment and quickly form new tubes by secreting from the anterior end a covering of mucus to which a layer of sediment adheres; the worm then moves forward to form the next portion of the tube, and after several movements the whole body is inside the tube (Hughes, Reference Hughes1979).
Maldane sarsi appears to be intermediate between these two categories. Its tubes are vertically oriented in the sediment, and consist of cemented mud half-rings wrapped in fibrous fabrics. These fabrics are probably deposited in the same way as the layers of the inner sheath, i.e. from glands along the body, and then are used to pack portions of sediment, which may take place in the feeding cavity and apparently requires complex movements of the worm's head.
Particle selection
Polychaetes exhibit varying degrees of selectivity in the use of particles for building tubes (see Dudgeon, Reference Dudgeon and Wotton1994). Sabellariidae, Pectinariidae, Oweniidae and some others choose particles of a certain size, shape or density. Particles selected may become increasingly larger as worms grow. Juveniles of Owenia fusiformis are extremely selective, choosing the larger, coarser particles for their tubes and sometimes preferring the heavy green hornblende mineral – they must handle tens of thousands of grains per centimetre of a tube less than 1 mm in diameter (Fager, Reference Fager1964; Noffke et al., Reference Noffke, Hertweck, Kröncke and Wehrmann2009).
Particle selection in Maldanidae has not been well studied. The tubes of some maldanid species differ significantly in their particle size from each other and from their parent sediments; e.g. Clymenella mucosa and Sabaco elongatus use smaller particles, whereas Petaloproctus socialis utilizes larger particles. The worms do not choose individual particles, they are simply able to discern patches of sediment which suit their requirements (Mangum, Reference Mangum1964), shifting the process of particle selection to larval settlement.
Likewise, the species of Maldanidae studied here differ in the average size of tube particles, though they apparently do not tend to choose particles for tube construction. In the agglutinated layer of one tube of Nicomache lumbricalis from the White Sea, we have found two sublayers with sediment particles of different sizes: coarse silt in the inner, fine and sometimes coarse sand grains in the outer. The tubes of N. minor from the White Sea were made of minerals common on the Karelian Craton (unpublished), so selection in this species appears to be based on the grain size, rather than its shape or specific gravity.
Microstructure
The tube structure in Maldanidae fits into the overall scheme of polychaete tube organization shown for terebellids (Shcherbakova & Tzetlin, Reference Shcherbakova and Tzetlin2016). An inner organic sheath covers the tube from the inside. The agglutinated layer contains sediment particles glued together with organic cement. The outer organic layer is present in Nicomache spp. All tube layers contain variously arranged and interconnected organic fibres.
In all species studied, the tube lumen is lined with an inner sheath made of fibres 0.1–0.5 µm in diameter. This sheath is deposited as successive layers of fibres, and the fibre orientation varies from one layer to the next (usually nearly parallel fibres of one layer intersect the fibres of the next layer at an acute angle). Such a structure resembling a non-woven fabric makes the inner sheath elastic and tear-proof. The fabric-like structures of the inner sheath were recorded in N. lokii (Kongsrud & Rapp, Reference Kongsrud and Rapp2012), Clymenella torquata, and many other polychaete taxa (Merz, Reference Merz2015).
In the tubes of Maldane sarsi, Praxillella praetermissa, and Rhodine gracilior the inner sheath is the most robust component, and its structure differs between the species. In M. sarsi it is composed of several levels of fibres. In P. praetermissa, the inner sheath consists of the fibrous, fabric-like inner part and the outer part, though showing no fibrous structure itself, sends numerous fibres into the agglutinated layer. In R. gracilior, the greatly developed inner sheath is tens of times thicker than in the other species (Figures 8 & 9) and contains numerous layers of fibres, providing the tube with elasticity and strength.
The agglutinated layer is likewise variously developed within the family. In R. gracilior it contains fewer large sand grains not interconnected by cement and weakly cemented to the very thick inner sheath (Figure 8F). In the other species some fibrous structures are present, either 3D networks interconnecting sediment particles (Nicomache spp., P. praetermissa, A. catenata), or fabrics enveloping portions of sediment (M. sarsi). The cement of Nicomache spp. also contains some kind of open-cell foam, with the cell walls made of fibres. The most massive agglutinated layer is found in N. lumbricalis (sometimes it contains smaller particles in the inner, and larger particles in the outer sublayer). We suppose that the tubes of Nicomache spp. are more robust than the others due to their 3D network being denser than in P. praetermissa or A. catenata, and stronger than the radial fibres and wrappings of the half-rings in M. sarsi.
The outer organic layer present in Nicomache spp. is made of fibres, visible in damaged areas; when intact parts of the layer are examined, its fibrous nature remains inconspicuous.
So, agglutinated tubes of Maldanidae are composed of an inner sheath including successive layers of fibres, the orientation of which varies from one layer to the other, an agglutinated layer with sediment particles attached to the inner sheath by fibres (often forming a 3D network or foam), and sometimes also a fibrous outer layer. In contrast to Terebellidae for example, the fibres fastening the agglutinated particles are loosely spaced and do not merge into films.
ACKNOWLEDGEMENTS
We thank the scuba divers of the White Sea Biological Station of Moscow State University (A. Semenov, G. Kolbasova, N. Neretin, A. Chava, A. Makarov, M. Simakov) for their assistance in collecting the specimens, and the scuba divers of the ‘Vostok’ Marine Biological Station of the Marine Biological Institute, for collecting specimens from the Sea of Japan. We are grateful to I. Ekimova, A. Zhadan, E. Vortsepneva and D. Shcherbakov (Moscow) for their assistance and valuable comments on the results, Max Barclay (Natural History Museum, London) for linguistic editing, and two anonymous reviewers for their insightful comments and suggestions. We are grateful to Andrew Mackie for the invaluable help in the final stage of the work on the manuscript.
FINANCIAL SUPPORT
This study was supported by the Russian Foundation for Basic Research (T.S., A.T., grant no. 16-04-00343; A.T., grant no. 15-29-02447). The SEM investigations, performed at User Facilities Center of M.V. Lomonosov Moscow State University, were supported by the Russian Science Foundation (A.T., grant no. 14-50-00029).












