INTRODUCTION
The pelagic stingray Pteroplatytrygon violacea (Bonaparte, 1832), is known to be distributed worldwide, especially in temperate, subtropical and tropical seas (Mollet, Reference Mollet2002; Neer, Reference Neer, Camhi, Pikitch and Babcock2008). In the Mediterranean, McEachran & Capapé (Reference McEachran, Capapé, Whitehead, Bauchot, Hureau, Nielsen and Tortonese1984) noted that P. violacea occurred off the Maghreb coast and in the Tyrrhenian Sea. However, new records were further reported in other Mediterranean areas, such as the Ligurian (Orsi Relini et al., Reference Orsi Relini, Garibaldi, Digitali, Lanteri, Vacchi, La Mesa, Serena and Séret2002), Ionian and Adriatic Seas (Mavrič et al., Reference Mavrič, Jenko, Makovec and Lipej2004; Jardas et al., Reference Jardas, Pallaoro, Vrgoč, Jukić-Peladić and Dadić2008), and eastward to the eastern Basin (Golani, Reference Golani2005). Additionally, Jardas (Reference Jardas1996) listed P. violacea as a very rare species in the Adriatic Sea. At present, the pelagic stingray as are other elasmobranch species, is facing many threats throughout the world (Domingo et al., Reference Domingo, Menni and Forselledo2005), and also in the Mediterranean, where it is caught by pelagic longline fisheries and regularly discarded (Baum et al., Reference Baum, Bianchi, Domingo, Ebert, Grubbs, Mancusi, Piercy, Serena and Snelson2007).
In Mediterranean waters, some traits of the reproductive biology of P. violacea were studied by Hemida et al. (Reference Hemida, Seridji, Ennajar, Bradai, Collier, Guélorget and Capapé2003); similar aspects were presented for those of southern Brazil, south-western Atlantic (Ribeiro-Prado & Amorim, Reference Amorim2008). The reproductive biology of P. violacea from the eastern Pacific was based on observations of captive specimens (Mollet et al., Reference Mollet, Ezcurra and O'Sullivan2002); conversely, that of free-swimming specimens is sketchily known (Mollet, Reference Mollet2002; Mollet et al., Reference Mollet, Ezcurra and O'Sullivan2002). Neer (Reference Neer, Camhi, Pikitch and Babcock2008) presented a study of ageing in specimens from the same area.
Food composition and feeding habits of the P. violacea were up to date only poorly investigated. Information is generally known from a single studied specimen (Bigelow & Schroeder, Reference Bigelow and Schroeder1962; Scott & Tibbo, Reference Scott and Tibbo1968; Dávalos-Dehullu & Gonzáles-Navarro, Reference Dávalos-Dehullu and Gonzáles-Navarro2003) or a number of investigated specimens fewer than 20 (Wilson & Beckett, Reference Wilson and Beckett1970). Off southern Brazil, Ribeiro-Prado & Amorim (Reference Amorim2008) studied the diet of pelagic stingray on 157 specimens. In both the Mediterranean and Adriatic Sea, only Mavrič et al. (2004) provided preliminary data, available to date, on the diet of this species.
The purpose of the present paper is to give more detailed data about the feeding habits of the pelagic stingray in the Adriatic Sea, based on recent captures and its possible impact on the comestible fish fauna in the area. Such study is necessary and useful to understand the mechanism and processes which structure and influence fish assemblages. The food web structure of elasmobranch species may serve as a basis for the maintenance of trophic level balance. This study is a first step in determining prey consumption by P. violacea, which is the main information for improving fishery monitoring and management in the study area.
MATERIALS AND METHODS
Field work
Between April 2004 and October 2005, 50 sampling cruises were performed in the Gulf of Trieste and adjacent waters with pelagic trawl (Figure 1). Trawling occurred in the shallow coastal areas at a muddy and muddy–detritic bottom at a depth from 20 to 30 m. The great majority of specimens were caught in the summer period.
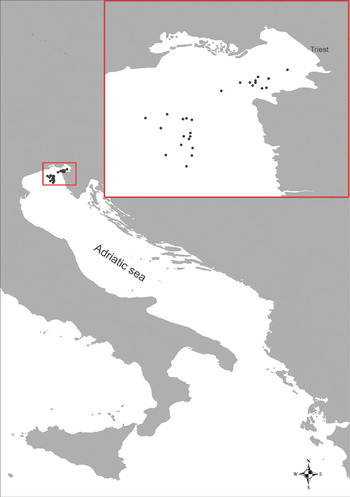
Fig. 1. Map of the studied area with the sampling stations (black dots) in the northern Adriatic Sea.
Among other by-catch, 84 specimens of the pelagic stingray were captured. Immediately after capture the specimens were frozen and stored in deep freeze chambers of the fishing enterprise Delamaris in Izola (Slovenia).
Biometry and diet analysis
Unfrozen specimens were photographed and sexed. The collected stingrays were measured to the nearest millimetre following Jardas (Reference Jardas1996) and weighed. All measured specimens were discriminated on the basis of sex and age. Juveniles were considered those not yet sexually matured males and females, which were smaller than 420 mm and 450 mm of the disc width, respectively (sensu Hemida et al., Reference Hemida, Seridji, Ennajar, Bradai, Collier, Guélorget and Capapé2003). After measurement specimens were dissected to obtain stomachs, which were weighed before and after emptying. The isolated food items from stomachs were preserved in 5% formaldehyde solution. Prey items in each stomach were sorted and determined to the lowest possible taxonomic level. In certain cases, when the prey remains in stomachs were not a whole item, the prey count was based on the number of different typical parts such as beaks for cephalopods, carapaces of crustaceans and whole vertebral columns for fish. Wet weights were measured to the nearest g.
Data analysis
To study and describe diet composition, we calculated: the vacuity index (VI = number of empty stomachs/total number of stomachs × 100), relative frequency of occurrence (%F = number of stomachs containing prey i/total number of filled stomachs × 100), relative numerical abundance (%N = number of prey i/total number of prey × 100) and relative gravimetric composition (%W = weight of prey i/total weight of all prey × 100). The index of relative importance (IRI) of Pinkas et al. (Reference Pinkas, Oliphant and Iverson1971), as modified by Hacunda (Reference Hacunda1981): IRI = %F × (%N + %W). This index, that integrates the three previous percentages, allows an interpretation much more real for food by minimizing the skews caused by each one of these percentages.
In order to determine the different categories of food, this has been regrouped according to the classification proposed by Rosecchi & Nouaze (Reference Rosecchi and Nouaze1987). Prey species were stored in decreasing order according to their relative IRI (%IRI = IRI of prey i/ΣIRI of all prey × 100) contribution and then cumulative %IRI was calculated. In this order, the %IRI of first prey were gradually added to obtain 50% or more, these items are main food; this calculation is pursued until another 25% or more is obtained, these items are called secondary food; the other items are accidental food.
The trophic level for any consumer species i is (Pauly et al., Reference Pauly, Froese, Sa-a, Palomares, Christensen and Rius2000; Pauly & Christensen, Reference Pauly, Christensen, Pauly and Froese2000; Pauly & Palomares, Reference Pauly, Palomares and Briand2000):
where TROPH j is the fractional trophic level of prey j, DC ij represents the fraction of j in the diet of i.
The TROPH and standard errors (SE) of pelagic stingray in the study area were calculated using TrophLab (Pauly et al., Reference Pauly, Froese, Sa-a, Palomares, Christensen and Rius2000); a stand-alone Microsoft Access routine for estimating trophic levels, downloadable from www.fishbase.org. The relationship between TROPH and the mid-point of each length-class considered here was quantified using the following equation (Cortés, Reference Cortés1999):
where TROPHL∞ is the asymptotic TROPH and K is the rate at which TROPHL∞ is approached. Statistical differences (P < 0.05) in basic diet composition as a function of size and season were established by applying a Chi-square (χ2)-test (Sokal & Rohlf, Reference Sokal and Rohlf1987). For assessing the diet in relation to size of stingrays, all specimens were grouped in four size-classes (SC): I—specimens smaller than 425 mm in disc width (DW); II—specimens from 426–500 mm DW; III—specimens from 501 to 575 mm DW; and IV—specimens bigger than 576 mm DW. Dietary diversity was calculated using the Shannon–Wiener diversity index. For comparing the diet between four different size-classes we calculated the average prey weight for a single size-class by dividing the total biomass with the number of prey items. Additionally, we calculated the average meal for the size-class, by dividing the total biomass with the total number of filled stomachs.
RESULTS
Overall diet analysis
Of the 84 examined stomachs of the pelagic stingray, only 4 were empty (VI = 4.76%). The diet consisted of two main taxonomic groups such as teleost fish and cephalopods (Table 1). Other groups such as crustaceans were represented with proportion lower than 1% in terms of number, biomass or index of relative importance. In the stomachs analysed 11 species of teleost fish, 2 species of cephalopods and 2 species of crustaceans were found. Bony fish were by far the most important food category in terms of abundance (95.94%), weight (79.55%) and IRI (95.55%). The fish encountered in the stomachs of stingrays were mainly comestible teleost species such as anchovy Engraulis encrasicolus, pilchard Sardina pilchardus, gilt sardine Sardinella aurita, hake Merluccius merluccius, Atlantic horse mackerel Trachurus trachurus and red band fish Cepola macrophthalma. Among other teleost species, seahorses Hippocampus hippocampus and H. guttulatus, black goby Gobius niger and brown comber Serranus hepatus were encountered in the diet. The preferential prey species was the anchovy, as shown by all three diet indices (%N = 74.27, %W = 55.86 and %F = 73.42) and %IRI (86.95%). The second most important fish species in the diet was C. macrophthalma (%N = 6.66, %W = 5.73, %F = 40.51 and %IRI = 4.33). All other fish species were more or less only occasionally preyed by the stingray.
Table 1. Diet of the pelagic stingray, Pteroplatytrygon violacea in the studied area.

Cephalopods were alternative prey species in the diet of pelagic stingray. Cuttlefish Sepia officinalis and Sepia sp. were preyed rather regularly and represented the second most important food item with IRI of 4.33%. Other species, such as squid Loligo vulgaris were present in negligible proportions. Among crustaceans two species were found, Squilla mantis and Liocarcinus depurator, both in very low proportions (%IRI < 0.1).
Diet related to sex
Of the 84 specimens 20 were males and 64 females. The VI was 5.00% and 6.25% for males and females, respectively; no significant difference was observed between them (χ2 = 0.78, P = 0.74, df = 1) (Figure 2). Conversely, there was a clear difference in diet composition between sexes, especially in terms of anchovies (χ2 = 61.078, P < 0.0001), red band fish (χ2 = 16.156, P < 0.0001) and cuttlefish (χ2 = 8.350, P < 0.0004). Females tended to selectively prey anchovies (%IRI = 90.52), while their proportion in males was much lower (%IRI = 37.47). Males tended to prey also C. rubescens (%IRI = 19.88), Sepia sp. (%IRI = 18.81), and, in smaller number, Gobius niger (%IRI = 1.74).

Fig. 2. Diet composition of males and females of the pelagic stingray in the northern Adriatic Sea.
Diet differences between juveniles and adults
Of the 84 specimens, 18 were juveniles and 66 adults (Figure 3). All stomachs of adult specimens were full, while the VI of juveniles was 27.28%, so VI significantly varied between adults and juveniles (χ2 = 372.09, P < 0.0001, df = 1). Adults preyed significantly more anchovies than juveniles (%IRI = 87.72% versus 73.52%, respectively; χ2 = 6.451, P < 0.01), while the difference in preying cuttlefish and red band fish was not statistically significant.
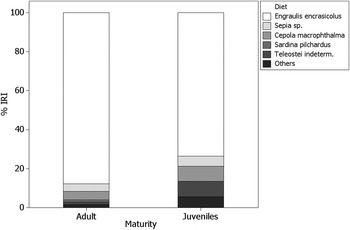
Fig. 3. Diet composition of adult and juveniles of the pelagic stingray in the northern Adriatic Sea.
Diet of different size groups
Pelagic stingrays were segregated in four size-classes: I, II, III and IV, according to their disc width in order to check the differences in diet. The VI of classes I and II were 25.00% and 3.70%, while all stomachs of the largest specimens included in classes III and IV contained food and/or remains of food. Significant differences were observed between the VI of size-class I and other size-classes (χ2 = 18.4, P < 0.0001) (Table 2), but not among other size-classes. The anchovy is still the most important feeding category; however, its proportion in the diet was much bigger in bigger size-classes (χ2, P < 0.0001). On the contrary, the proportion of the red banded ribbonfish was decreasing with the increase in size of pelagic stingray. The diet diversity is broader in the size-class I (H′ = 0.77) and is more or less decreasing with the increasing size in other size-classes (II, H′ = 0.54; III, H′ = 0.39 and IV, H = 0.42), showing the obvious specialization of the pelagic stingray for anchovies. There was also an increasing trend in average meal (Σtotal biomass/filled stomachs), from I to IV (114.9 g, 127.65 g, 240.54 g and 285.60 g, respectively). The same was also recorded for the mean number of prey items in stomachs which increased with the size-class (4.22, 6.96, 14.12 and 15.45). The average meal in relation to the predator size was rather the same in different size-classes (6.88%, 4.71%, 4.95% and 4.23%, respectively). The calculated TROPH value of pelagic stingrays was almost identical for all studied size-classes—4.50; confirming that the pelagic stingray is a top predator of pelagic fish species.
Table 2. Comparison of diet among four size-classes of pelagic stingrays.

DISCUSSION
The VI of Pteroplatytrygon violacea reached low values for the total sample (4.76%) suggesting that the species is an active and voracious feeder. No significant differences appeared in the VI between sexes, but conversely, significant difference appeared between juvenile and adult VI (27.88% versus 0%). Additionally, a similar pattern was observed related to size, the smallest specimens having the biggest VI (25.00% versus 4–0%). This is due to ontogenic changes in feeding habits, an almost universal phenomenon in fish, especially in elasmobranch species due to increase of swimming speed, movements patterns, experience with preys and improved ability of larger specimens to capture prey items (Wetherbee & Cortés, Reference Wetherbee, Cortés, Carrier, Musick and Heithaus2004). Additionally, Heithaus (Reference Heithaus, Carrier, Musick and Heithaus2004) noted that small elasmobranch specimens generally inhabit productive areas in the early stages of their life. Such productive areas are the object of intraspecific and interspecific intense competition pressure for food. Such a pattern could also explain a lower VI for smaller pelagic stingrays. Off southern Brazil, Ribeiro-Prado & Amorim (Reference Amorim2008) noted that of the 157 stomachs analysed, 99 (63%) were empty and 58 (33%) presented some contents. These differences could be due to prey availability in the biological environment; however, capture technique may also influence contents in stomachs. The pelagic stingrays from southern Brazil were caught by longlines, and generally specimens with empty stomachs are attracted by baits. Additionally, captured specimens could stay a period prior to being handled, and the preys they consumed prior to being caught were more or less completely digested (see Wetherbee & Cortés, 2008).
Our data showed that the prey size was correlated with the size of predator. Bigger P. violacea were thus preying bigger and heavier preys. As mentioned in the Introduction there are only limited available data on the diet of this species in other areas. The staple foods of the pelagic stingray in the waters off Brazil were amphipods, decapods, teleosts and pteropods (Pinheiro Véras et al., Reference Pinheiro Véras, Vaske Júnior, Vieira Hazin, Lessa, Travassos, Travassos Tolotti and Martins Barbosa2009). The majority of prey measured between 1 and 40 mm in total length and were represented by small crustaceans and planktonic molluscs such as pteropods and heteropods. The difference between the data reported by Pinheiro Véras et al. (Reference Pinheiro Véras, Vaske Júnior, Vieira Hazin, Lessa, Travassos, Travassos Tolotti and Martins Barbosa2009) and those included in the present study reflect the difference between the living environments of both populations. The first one is obviously opportunistically hunting in the open waters of the Atlantic Ocean, while the second one is more or less selectively preying pelagic and occasionally also benthic fish in a very shallow environment like the Gulf of Trieste.
These ontogenic changes in diet and prey size were due to the ability of larger specimens to capture larger preys, having larger mouth and teeth, and energy requirements. Another reason could be the different foraging strategy, since small specimens were more able to find small preys buried into sediments, or collecting prey by excavation. Similar patterns were reported by Capapé (Reference Capapé1975) for the common stingray Dasyatis pastinaca (Linnaeus, 1758) and by Smith & Merriner (Reference Smith and Merriner1985) for the cownose ray Rhinoptera bonasus (Mitchill, 1815).
The pelagic stingray is predominantly preying anchovies which are forming an important portion of the diet in juveniles and adults and also in males and females. The alternative preys are cuttlefish and the red band fish. Among other species there are other pelagic comestible fish, such as S. pilchardus, S. aurita, M. merluccius, T. trachurus and cephalopods (L. vulgaris and others). The proportion of anchovies in the diet could be even underestimated, since the proportion of unidentified clupeid fish and teleosts may be composed mainly of them. The predominant proportions of anchovies and the shares of many pelagic fish species in the diet are confirming that the stingray is a pelagic dwelling species. However, the non-negligible amounts of C. macrophthalma and cuttlefish Sepia sp., together with the occasionally preyed gobies, seahorses, brown combers, mantis shrimps and crabs suggested that the P. violacea fed not only on pelagic preys but also at the bottom. Such a phenomenon could also explain the high VI values recorded in the sample for males and females, and especially for specimens included in large size-classes. Ribeiro-Prado & Amorim (Reference Amorim2008) reported similar patterns for specimens caught off southern Brazil. Previously, Nakaya (Reference Nakaya, Okamura, Amaoka and Mitani1982) suggested that P. violacea may be a benthopelagic species utilizing both benthic and pelagic habitats.
The calculated TROPH value, 4.50, showed that according to the classification of Stergiou & Karpouzi (Reference Stergiou and Karpouzi2002) the pelagic stingray is a carnivore species, predominantly piscivorous predator, feeding also on cephalopods and other preys, included at the very top of the food pyramid. A TROPH value of P. violacea is included among those estimated for elasmobranch species which generally ranged between 3.10 and 4.70 (Cortés, Reference Cortés1999), and many marine mammals, which generally ranged between 3.20 and 4.50 (Pauly et al., 1998) with members of both groups being considered as top predators. Stergiou & Karpouzi (Reference Stergiou and Karpouzi2002) assigned a trophic level of 3.70 to the relative species, the blue stingray Dasyatis chrysonota marmorata (Steindachner, 1892), referring to specimens collected by Capapé & Zaouali (Reference Capapé and Zaouali1992) in the Tunisian waters. The TROPH value of the latter species is quite high, but less than the value for P. violacea. Such difference between the two species may be explained by the fact that D. chrysonota marmorata feeds mainly on benthic preys, such as polychaetes and gastropods. Pteroplatytrygon violacea also possesses cuspidate cutting teeth in jaws of males and females, a pattern that is not found in benthic relative species belonging to the genus Dasyatis which exhibit teeth rather smooth and plate more efficient to consume molluscs, gastropods or bivalves (Capapé, Reference Capapé1975; Capapé & Zaouali, Reference Capapé and Zaouali1992). This is even more pronounced in myliobatids which have teeth fused into grinding plates forming a tesselate pattern (Capapé, Reference Capapé1977; McEachran & Capapé, Reference McEachran, Capapé, Whitehead, Bauchot, Hureau, Nielsen and Tortonese1984), a morphological adaptation to benthic life. Similarly, the tooth shape of P. violacea could explain differences in its life style and food composition and feeding habits (Pinheiro Véras et al., Reference Pinheiro Véras, Vaske Júnior, Vieira Hazin, Lessa, Travassos, Travassos Tolotti and Martins Barbosa2009).







