INTRODUCTION
The degree of overlap in the use of available resources among closely related and sympatric elasmobranch species is variable, and interactions are associated, for example, with spatial fluctuations (e.g. Navia et al., Reference Navia, Mejía-Falla and Giraldo2007, Barausse et al., Reference Barausse, Baremore, Murie and Carlson2010; Valls et al., Reference Valls, Quetglas, Ordines and Moranta2011), ontogenetic development (e.g. Navia et al., Reference Navia, Torres, Mejía-Falla and Giraldo2011; Brickle et al., Reference Brickle, Laptikhovsky, Pompert and Bishop2003; Barausse et al., Reference Barausse, Baremore, Murie and Carlson2010; Bornatowski et al., Reference Bornatowski, Heithaus, Abilhoa and Corrêa2012) and competition (e.g. Ellis et al., Reference Ellis, Pawson and Shackley1996; Navia et al., Reference Navia, Mejía-Falla and Giraldo2007; Treloar et al., Reference Treloar, Laurenson and Stevens2007). Food partitioning between species is a common strategy for avoiding resource competition, which allows the coexistence of two or more sympatric species (Schoener, Reference Schoener1974).
Smaller elasmobranchs (<150 cm total length) are commonly consumed by large sharks and are considered mesopredators (Ferretti et al., Reference Ferretti, Worm, Britten, Heithaus and Lotze2010). The reduction of top predators can lead to a proliferation of mesopredators, leading to a destabilization of marine communities through trophic cascades (Myers & Worm, Reference Myers and Worm2003; Myers et al., Reference Myers, Baum, Shepherd, Powers and Peterson2007; Ritchie & Johnson, Reference Ritchie and Johnson2009; Heithaus et al., Reference Heithaus, Frid, Vaudo, Worm, Wirsing, Carrier, Musick and Heithaus2010; Navia et al., Reference Navia, Cortés and Mejía-Falla2010, Bornatowski et al., Reference Bornatowski, Navia, Braga, Abilhoa and Corrêa2014). Given the influence of mesopredator population growth on the marine food chain, understanding diet and competition levels in mesopredator elasmobranchs is fundamentally important. These dietary data facilitate construction of network trophic interaction models, which are essential for predicting the possible effects of species presence or absence in an ecosystem (Navia et al., Reference Navia, Cortés and Mejía-Falla2010; Braga et al., Reference Braga, Bornatowski and Vitule2012, Bornatowski et al., Reference Bornatowski, Navia, Braga, Abilhoa and Corrêa2014).
Batoid fish regularly occupy intermediate trophic levels (total length <4.0) in their communities (e.g. Muto et al., 2002; Mabragaña & Gilberto, Reference Mabragaña and Gilberto2007; Navia et al., Reference Navia, Mejía-Falla and Giraldo2007; Vaudo & Heithaus, Reference Vaudo and Heithaus2009; Barbini & Lucifora, Reference Barbini and Lucifora2011; Bornatowski et al., Reference Bornatowski, Robert and Costa2010; López-Gárcia et al., 2012) and they are also present in the diet of large sharks (Vaudo & Heithaus, Reference Vaudo and Heithaus2011). Therefore, batoids can be considered mesopredators that provide an important link between top predators and lower trophic levels in the marine ecosystem, and play an important role in marine ecosystem dynamics (Vaudo & Heithaus, Reference Vaudo and Heithaus2011).
The presence of batoid fish is common in fisheries along the southern Brazilian coast (Vooren & Klippel, Reference Vooren and Klippel2005; Costa & Chaves, Reference Costa and Chaves2006) which target commercially important species such as Paralichthys spp., Micropogonias furnieri, Genidens barbus and Cynoscion spp. In order to evaluate the competition between species of batoids regularly caught by artisanal fisheries in southern Brazil (Costa & Chaves, Reference Costa and Chaves2006; Bornatowski et al., Reference Bornatowski, Abilhoa and Charvet-Almeida2009), the present study aimed to analyse and compare the diet of the four batoid species, Rhinobatos percellens, Zapteryx brevirositrs, Rioraja agassizi and Rhinoptera bonasus. The results will provide useful information on the trophic ecology of captured species along the southern Brazilian coast, thus helping us to explain the coexistence and the role played by these mesopredator batoids on the marine food webs in this area.
MATERIALS AND METHODS
Observations of fish landings were conducted at the artisanal fishing communities of the central coast of the State of Paraná, southern Brazil (from 25°49′S 48°31′W to 25°36″S 48°20′W) (Figure 1) from April 2010 to March 2012. Mesh sizes used by the gillnet fishery include 7, 9, 11, 16 and 18 cm stretch mesh. Gillnets (no pre-established sizes) were set a maximum of 20 km from the coast in water depths up to 20 m.

Fig. 1. Central coast of the State of Paraná, southern Brazil. Black star represents where the sampling was conducted.
For R. percellens and Z. brevirostris the total length (TL, cm) was recorded, while for R. agassizi and R. bonasus total disc width (DW, cm) was recorded for each individual. Stomachs were removed, fixed in 10% formalin and subsequently analysed in the laboratory. The food items were separated, identified to the lowest possible taxonomic level, counted, and weighed. We used the main prey to compare diet between species (i.e. fish, Dendrobranchiata, Caridea, Gammaridae, Brachyura, Cephalochordata, Ophiuoroidea, Gastropoda and Bivalvia).
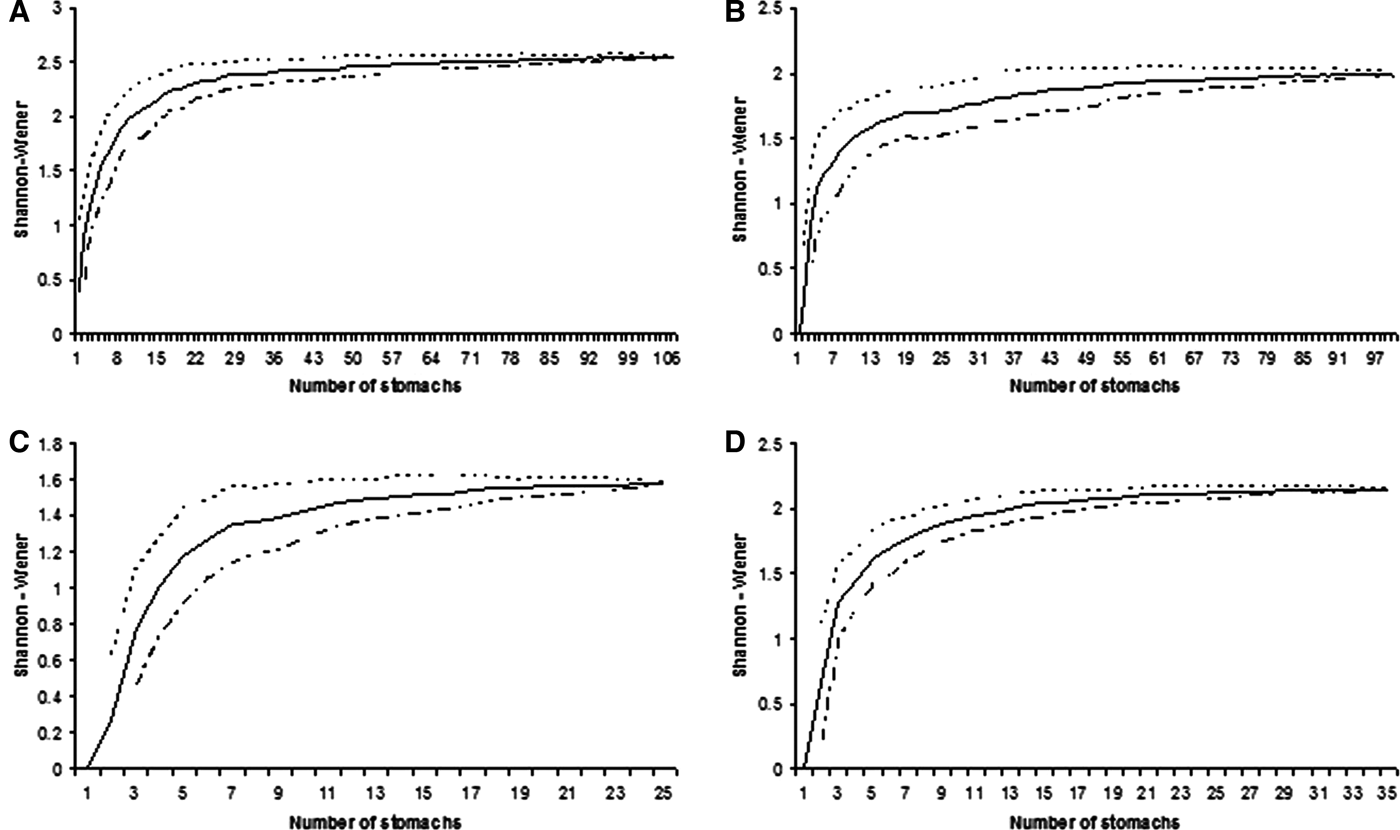
In order to investigate the effect of sample size in estimating the batoids' diets (Ferry & Cailliet, Reference Ferry, Caillet, MacKinlay and Shearer1996; Cortés, Reference Cortés1997), cumulative prey curves were created. A cumulative prey curve was constructed using the Shannon–Wiener method to evaluate whether the number of sampled stomachs was sufficient to describe the diversity of diet of four batoid species, and the samples were randomized 50 times with the routine ‘sample-based rarefaction’ using the EstimateS 7.5 software (Colwell, Reference Colwell2005). Sample size was considered sufficient if the curves visually reached an asymptote (Magurran, Reference Magurran2004).
The importance of various prey taxa to the batoids' diets, was assessed by calculating the index of relative importance (IRI; Pinkas et al., Reference Pinkas, Oliphant and Iverson1971):
where %N is the number of a given prey type as a percentage of the total number of prey; %W is the weight of a given prey type as a percentage of the total weight of prey; and % FO is the percentage of frequency of occurrence of each prey type (Hyslop, Reference Hyslop1980). The IRI values were standardized in percentage values according to Cortés (Reference Cortés1997):
Diet niche breadth was estimated using Levin's (Bi): Bi = 1/ΣP 2j, where P j is the fraction by IRI of each food in the diet j(ΣP j = 1) (Krebs, Reference Krebs1999). The values were standardized (B A) so that it ranges from 0 to 1 by using the equation B A = (Bi−1)/(N−1), where N is the number of classes (Krebs, Reference Krebs1999). Low values indicate diets dominated by few prey items (specialist predators) while higher values indicate generalist diets.
Niche overlap was calculated with the IRI of each prey using the Pianka index with EcoSim 7.72 software (Gotelli & Entsminger, Reference Gotelli and Entsminger2005). Overlap was considered biologically significant when values exceed 0.60 (Zares & Rand, Reference Zares and Rand1971). The overlaps found were compared with a distribution of expected values based on simulations (1000 repetitions) of a null model to evaluate the statistical significance of estimated overlaps. Observed values were considered statistically different from the null distribution values if they were higher or lower than 95% of the simulated indices (Gotelli & Graves, Reference Gotelli and Graves1996). Lower values suggest differences in diets or resource partitioning, while higher values suggest similar diets or strong resource competition.
The standardized trophic levels of batoids were calculated using the trophic index (TR), proposed by Cortés (Reference Cortés1999):
where TRj is the trophic level of each prey taxon j (see Cortés, Reference Cortés1999) and Pj is the proportion of each prey taxa in the diet based on %IRI values.
To test for variation between the diets of batoid species, a similarity matrix with the transformed estimated contribution values of food items, based on weight of preys, was then generated using the Bray–Curtis similarity coefficient. Diet similarity was analysed with non-metric multidimensional scaling analysis (nMDS). Data were then investigated using one-way analysis of similarity (ANOSIM), with individuals as samples and species as factor. This test was used to verify similarities (distance) within defined groups (factors = species) against similarities between groups and also calculates the statistic ρ = R, which varies between −1 and +1 (Clarke & Gorley, Reference Clarke and Gorley2006). The significance (P values) was assessed using a random permutation test 999 times and R was calculated for each total permutation. In the context of this study, the R value of zero represents the null hypothesis (there are no differences between our factor groups or subset samples—stomach), which means that similarities within and between samples are the same; and R value of 1 indicates that the subset samples (stomachs) within species (factors) were totally similar among themselves and dissimilar between the levels of each factor. Similarity of percentages (SIMPER) was used to estimate the contribution of each prey category to species differences in diets. The analyses were performed using the software PRIMER v.6 (Clarke & Gorley, Reference Clarke and Gorley2006).
RESULTS
A total of 369 individuals were collected and studied. Sizes ranged from 45.0–97.0 cm TL (mean 77.0; standard deviation (SD) ±12.4) in R. percellens, from 35.0–55.0 cm TL (mean 45.1; SD ±3.8) in Z. brevirostris, from 24.9–34.0 cm DW (mean 29.3; SD ±3.5) in R. agassizi, and from 38.0–95.0 cm DW (mean 62.7; SD ±20.0) in R. bonasus. Of a total of 369 stomachs collected, 265 stomachs (71.8%) contained prey. From 138 examined stomachs of R. percellens, 108 (79.3%) contained prey and 30 (21.6%) were empty. For Z. brevirostris, of 116 stomachs 99 (85.3%) contained prey and 17 (14.7%) were empty. From 53 stomachs of R. agassizi, 34 (64.1%) contained prey and 19 (35.9%) were empty. Finally, from 51 stomachs of R. bonasus, 24 (47.1%) contained prey and 27 (52.9%) were empty (Table 1). The cumulative prey curves based on diversity of preys reached an asymptote for all four species indicating the sample size was satisfactory to describe the general diets of batoids.
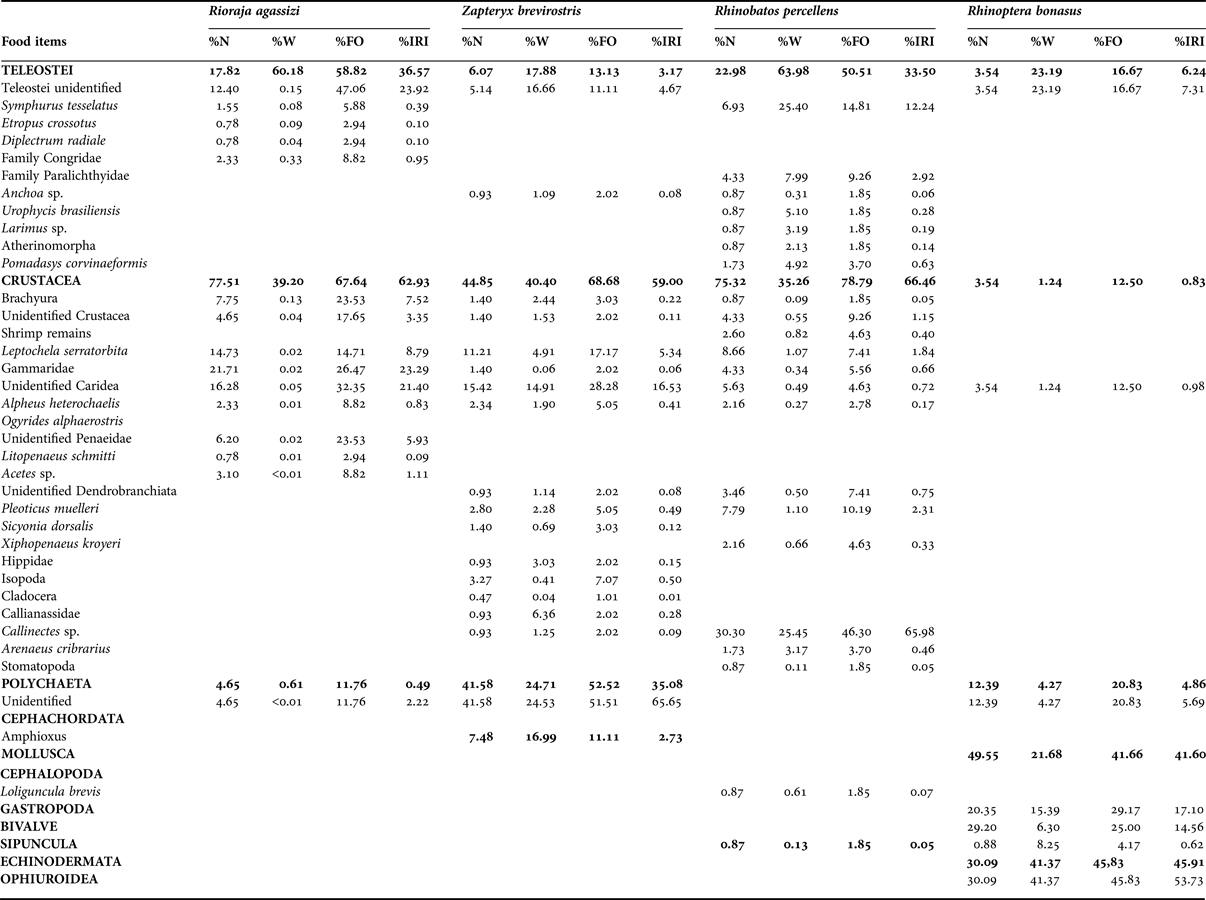
Table 1. Diet composition of four batoid species caught along the Paraná coast, southern Brazil expressed in percentage by number (%N), weight (%W), frequency of occurrence (%FO), and percentage of the index of relative importance of food (% IRI).

Diet and niche breadth
Rhinobatos percellens had a specialized diet (BA = 0.05), consisting predominantly of blue crabs Callinectes sp. (66% IRI). The diet was supplemented by teleost fish, with Symphurus tesselatus (12% IRI) and Paralichthyidae (3% IRI) being the most abundant (Table 1). Zapteryx brevirostris also had a very specialized diet (BA = 0.07), feeding primarily on Polycheata (65% IRI), followed by Caridea shrimp (22% IRI). The diet was supplemented with amphioxus (6% IRI), unidentified fish (4% IRI), and Dendrobranchiata shrimp (1% IRI) (Table 1). For Rhinoptera bonasus, Ophiuroidea was the predominant prey item (54% IRI), followed by Gastropoda (17% IRI) and Bivalvia (14% IRI) (Table 1). Its niche breadth value was also low (BA = 0.07), indicating a specialized diet. Rioraja agassizi, in contrast, had the greatest niche breadth (BA = 0.33), with a varied diet divided among items: fish (24% IRI), Gammaridae (23% IRI), Caridea shrimp (21% IRI) and Dendrobranchiata shrimp (6% IRI) (Table 1). Even with a niche breadth higher (BA = 0.33) than the other species, R. agassizi can still be considered as specialized species.
Feeding similarities, overlap and trophic level
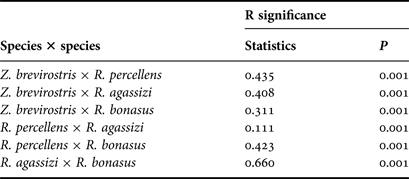
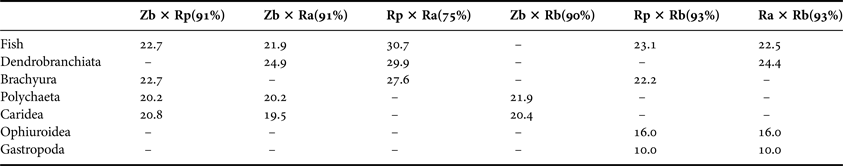
Significant differences were observed among species based on ANOSIM (R global = 0.356; P = 0.001; Figure 3). All paired analyses (species × species) were also significantly different (P < 0.01) (Table 2). On the other hand, nMDS analysis suggests that there is a marginal overlap between R. percellens and R. agassizi (Figure 3), however this difference was not confirmed in ANOSIM (Table 2). SIMPER analysis indicated that the dissimilarities between species were greater than 75% (Table 3). The most-observed prey items that contributed to the dissimilarity were Polychaeta (48.5%) and Caridea (45.6%) in Z. brevirostris, Brachyura (48.7%) and fish (46.7%) in R. percellens, Dendrobranchiata (53.5%) and fish (42.3%) in R. agassizi, and Ophiuroidea (48.2%), Gastropoda (18.2%), Bivalvia (13.5%), Polychaeta (9.3%), and fish (9.2%) in R. bonasus.
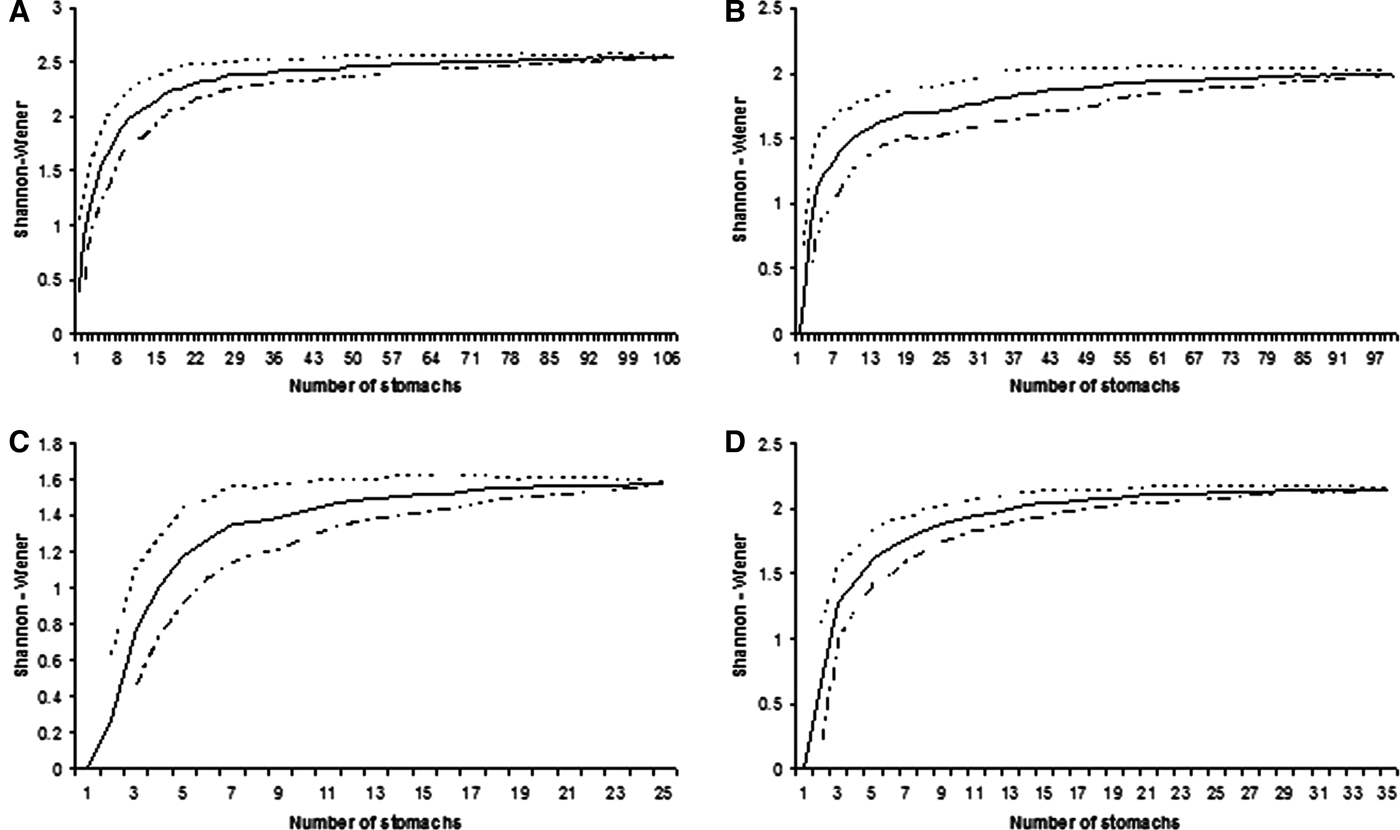
Fig. 2. Cumulative average (solid line) and standard deviation (dotted lines) of Shannon–Wiener diversity index for samples of studied batoid species in southern Brazil: (A) Rhinobatos percellens; (B) Zapteryx brevirostris; (C) Rhinoptera bonasus; (D) Rioraja agassizi.

Fig. 3. Non-metric multidimensional scaling between the four studied species of batoids in southern Brazil.
Table 2. Analysis of similarities pairwise tests for all four species of batoids on the southern coast of Brazil.

Table 3. Items that contributed most to the dissimilarity between groups (> 10%). Dissimilarity percentages are expressed in bold in the column headings. Zb, Zapteryx brevirostris; Rp, Rhinobatos percellens; Ra, Rioraja agassiz i, Rb, Rhinoptera bonasus.

There was no feeding overlap among species (Table 4). The trophic levels of the batoids in this study are <4.0, placing them in intermediate trophic levels. The values were 3.4, 3.5, 3.6, and 3.7 for R. bonasus, Z. brevirostris, R. percellens, and R. agassizi, respectively.
Table 4. Feeding overlap using the Pianka index for all four species of batoids on the southern coast of Brazil.

DISCUSSION
In this study, R. percellens fed mainly on blue crabs Callinectes sp., and, to a lesser extent, on benthic teleost fish (e.g. flatfish). A study conducted more to the south of the Paraná coast found a similar diet for R. percellens, which ate shrimps, Brachyura crustaceans and teleost fish (Bornatowski et al., Reference Bornatowski, Robert and Costa2010). However, studies in estuarine regions have found R. percellens diet to lack significant quantities of Brachyura crustaceans and teleost fish (Carmo et al., unpublished data). It is possible that R. percellens present ontogenetic variation in their diet, because specimens studied by Carmo et al. (unpublished data) were smaller than those analysed in this paper and in comparison to animals analysed in the border region between Paraná and Santa Catarina (Bornatowski et al., Reference Bornatowski, Robert and Costa2010). However, other hypotheses are that individuals use different food resources in areas occupied by other species, thereby avoiding interspecific competition or also could be due to different food availability in different locations.
In contrast to the present work, a study conducted on the São Paulo coast showed that Z. brevirostris exhibited a preference for Decapoda, followed by Polychaeta, Amphipoda, Isopoda and Cumacea (Soares et al., Reference Soares, Rossi-Wongtschowski, Álvares, Muto and Gasalla1992; Marion et al., Reference Marion, Vaske-Junior, Gadig and Martins2011). In another study on the coast of Argentina, Amphipoda, Polychaeta and Cephalochordata were the most abundant items in the diet (Barbini et al., Reference Barbini, Lucifora and Hozbor2011), corroborating the results of the present study mainly by the considerable presence of Cephalochordata. As both regions are located further south in the Atlantic, it is possible that variation in prey abundance occurs, thus forcing this species to feed on different items exhibiting different behaviour between regions to avoid competition among species.
Studies conducted with R. agassizi on the coast of São Paulo have revealed a diet consisting mainly of crustaceans, teleost fish and Polychaeta (Soares et al., 1999; Muto et al., Reference Muto, Soares and Goitein2001). On the coast of Argentina, crustaceans were also the most abundant items (shrimps, crabs and amphipods), followed by teleost fish (Barbini et al., Reference Barbini, Lucifora and Hozbor2011). These results support the analysis of the present study, where crustaceans and fish have similar importance in the diet of R. agassizi.
Other studies have suggested that R. bonasus feeds on Mollusca, influencing the abundance of these prey and affecting the commercial bivalve industry (Orth Reference Orth1975; Kraeuter & Castagna, Reference Kraeuter and Castagna1980; Smith & Merriner, Reference Smith and Merriner1985; Peterson et al., Reference Peterson, Fodrie, Summerson and Powers2001). However, some studies have shown that high consumption of molluscs is not common to all locations where the species is observed. In the present study, R bonasus demonstrated a preference for Echinodermata (Ophiuroidea), followed by gastropods and bivalves. In a study conducted in the Gulf of Mexico, polychaete worms dominated the stomach contents of R. bonasus, with low numbers of bivalve molluscs (Craig et al., 2010). In an estuarine area of Port Charlotte, Florida, USA, another study found high consumption of crustaceans by R. bonasus, followed by polychaete worms and, finally, bivalve molluscs (Collins et al., Reference Collins, Heupel and Motta2007). The high consumption of Ophiuroidea by R. bonasus in the present study was also reported for a congener, R. steindachneri, on the Colombian coast (Navarro-González et al., Reference Navarro-González, Bohórquez-Herrera, Navia and Cruz-Escalona2012).
The body pattern of batoids enables predation on benthic organisms (Moyle & Cech Jr, Reference Moyle and Cech1982) because these animals have a ventral mouth with high mandibular protrusion, making them capable of feeding on prey associated with the bottom. They also exhibit a high degree of interspecific variation in jaw and teeth morphology, showing different feeding performance and thereby allowing a widely varied diet (Goiten et al., Reference Goiten, Torres and Signorini1998; Wilga & Motta, Reference Wilga and Motta1998; Dean et al., Reference Dean, Bizzarro and Summers2007). In fact, besides the anti-predatory tactics, abundance of prey in the environment, and interspecific competition, food ingestion is highly dependent on the morphology and behaviour of the predator (Moyle & Cech Jr, Reference Moyle and Cech1982; Heithaus Reference Heithaus, Carrier, Musick and Heithaus2004). The dietary differences observed between the studied species seem to be related to behavioural and mechanical versatility of feeding apparatus (mouth and jaws structure) of each species (Dean et al., Reference Dean, Bizzarro and Summers2007). Rhinobatos percellens, for example, has relatively larger mouth, allowing the consumption of elusive larger prey such as blue crabs and flatfish (Dean et al., Reference Dean, Bizzarro and Summers2007). Rhinoptera bonasus, in contrast, has large dental plates and functional specialization for durophagy, making possible the consumption of hard-shelled organisms (Smith & Merriner Reference Smith and Merriner1987; Summers Reference Summers2000; Collins et al., Reference Collins, Heupel and Motta2007). Zapteryx brevirostris has a smaller mouth, allowing the consumption of small prey such as small shrimps and Polychaeta. Despite the relatively smaller mouth in a V-shape (Dean et al., Reference Dean, Bizzarro and Summers2007), R. agassizi feeds on small prey items such as Gammaridae, but also is capable of consuming larger preys such as teleost fish. Future studies on the feeding apparatus (jaw and teeth morphology) related to prey items are needed to evaluate with precision the feeding performance of batoid species.
The sharing of resources can be a facilitating mechanism in explaining the observed coexistence of species (Schoener, Reference Schoener1974; O'Shea et al., Reference O'Shea, Thums, van Keulen, Kempster and Meekan2013). In a study conducted off the coast of Tasmania, Australia, two sympatric rays coexisted in the absence of dietary overlap (Yick et al., Reference Yick, Barnett and Tracey2011). In contrast, a study of six species of rays on the Australian coast found some diet overlap, suggesting the presence of degrees of resource partitioning (Treloar et al., Reference Treloar, Laurenson and Stevens2007). On the coast of Argentina, two sympatric rays, Psammobatis normani and P. rudis, also showed dietary overlap, revealing a degree of competition for resources (Mabragaña & Gilberto, Reference Mabragaña and Gilberto2007). Despite marginal overlapping between R. percellens and R. agassizi studied here, the diets of the batoids indicate that feeding differs substantially among the four species, suggesting a partitioning of food resources available in the environment. Although results of the present study indicate feeding partitioning between the four batoid species, future studies on spatial and temporal distributions of species and the abundance of prey in the area of the present study are needed to determine the feeding strategies of each species. In addition, more comparisons with other benthic species (e.g. inshore lizardfish Synodus foetens, flatfish species, the Brazilian electric ray Narcine brasiliensis, stingrays Dasyatis spp., the Brazilian cownose ray Rhinoptera brasiliensis and others) are needed to verify the levels of competitions between all communities.
All four species had trophic levels <4.0, thus characterizing them as intermediate food chain predators (Cortés Reference Cortés1997; Ebert & Bizarro, Reference Ebert and Bizzarro2007). The study of mesopredators is extremely important because the population decline of large predators (such as by fishing pressure) can lead to substantial increases in mesopredator populations, causing cascading trophic effects within the ecosystem (Ritchie & Johnson, Reference Ritchie and Johnson2009; Heithaus et al., Reference Heithaus, Frid, Wirsing and Worm2008; Navia et al., Reference Navia, Cortés and Mejía-Falla2010). For instance, the cownose ray, R. bonasus, has been the subject of studies regarding its population impact on bivalve molluscs in the North Atlantic (e.g. Orth, Reference Orth1975; Kraeuter & Castagna, Reference Kraeuter and Castagna1980; Smith & Merriner, Reference Smith and Merriner1987; Peterson et al., Reference Peterson, Fodrie, Summerson and Powers2001). Due to a high degree of feeding specialization, R. bonasus is a candidate to be a strong interactor (Paine, Reference Paine1969), with a strong influence on cascading effects (Power et al., Reference Power, Tilman, Estes, Menge, Bond, Mills, Daily, Castilla, Lubchenco and Paine1996; Power, Reference Power1997; Myers et al., Reference Myers, Baum, Shepherd, Powers and Peterson2007).
Three of the four species analysed, R. percellens, R. agassizi and R. bonasus, are found in the stomach contents of large predators in the study area (e.g. Sphyrna lewini, Carcharias taurus, Carcharhinus obscurus, C. limbatus and Galeocerdo cuvier) (Bornatowski, unpublished data). These data reveal the importance of these batoid species as a link between the higher and lower levels of the regional marine food chain. Thus, future studies on the diet and trophic relationships between large sharks and these and other species in the area are required to understand the real role of mesopredator elasmobranchs in food chains and ecosystem resource availability.
ACKNOWLEDGEMENTS
We thank the fishermen of Ipanema, Shangri-lá and Pontal do Paraná beaches for contributing to this study, Dr Jeffrey Muehlbauer for English reviewing and Andrielli Medeiros and Felippe Abbatepaulo for help in data collection.
FINANCIAL SUPPORT
We thank the Conselho Nacional de Desenvolvimento e Pesquisa (CNPq) for research grants to HB, and the CAPES for research grants to N.W. and W.P.D.C.