INTRODUCTION
Understanding the feeding biology of horseshoe crabs (Chelicerata: Xiphosura) is essential to identify their ecological roles within estuarine and coastal ecosystems, particularly when there is increasing evidence that populations of horseshoe crabs in Asian regions are in decline (Hsieh & Chen, Reference Hsieh, Chen, Tanacredi, Botton and Smith2015; Nelson et al., Reference Nelson, Satyanarayana, Zhong, Shaharom, Sukumaran and Chatterji2015; Kwan et al., Reference Kwan, Hsieh, Cheung and Shin2016). The feeding of American horseshoe crab Limulus polyphemus has previously been well studied using gut content examination (Botton & Shuster, Reference Botton, Shuster, Shuster, Barlow and Brockmann2003) and stable isotope analysis (Gaines et al., Reference Gaines, Carmichael, Grady and Valiela2002; Carmichael et al., Reference Carmichael, Rutecki, Annett, Gaines and Valiela2004, Reference Carmichael, Gaines, Sheller, Tong, Clapp, Valiela, Tanacredi, Botton and Smith2009). Both methods demonstrated that adult L. polyphemus feed on a mixed variety of marine invertebrates, mainly bivalves, crustaceans, polychaetes and gastropods. For juvenile L. polyphemus, Gaines et al. (Reference Gaines, Carmichael, Grady and Valiela2002) analysed the stable isotope δ13C and δ15N values of 2nd–11th instar juveniles, and suggested that their food sources shifted from mainly suspended and benthic particulate organic matter to crustaceans and polychaetes when they are large enough to feed on small benthic invertebrates. However, natural diet composition of juvenile Asian horseshoe crabs Tachypleus tridentatus, T. gigas and Carcinoscorpius rotundicauda in different growth stages is limited mainly to gut content examinations (Zhou & Morton, Reference Zhou and Morton2004). Consistent with their American counterpart, a great variety of small intertidal animals, especially insect larvae, molluscs, crustaceans, polychaetes and oligochaetes, were also found. Juvenile T. tridentatus that occupied intertidal mudflats included crustaceans, bivalves and polychaetes into their diets (Kwan et al., Reference Kwan, Chan, Cheung and Shin2015a, Reference Kwan, Cheung and Shinb). However, the types of fatty acids required by juvenile horseshoe crabs remain uncertain.
Quantitative FA signature analysis has been developed as a trophic biomarker in feeding studies of jellyfish (Cui et al., Reference Cui, Wu, Zhang and Wang2012), molluscs (Redmond et al., Reference Redmond, Magnesen, Hansen, Strand and Meier2010), fishes (Zainudin et al., Reference Zainudin, Takaomi and Razikin2016) and marine mammals (Ko et al., Reference Ko, Ju, Choi and Shin2016). Compared with a relatively limited number of stable isotopes (i.e. C, N, S and O) found in animals, the determination of over 20 different FA components available in animal tissues thus provides more useful and reliable information in tracing the trophic relationships (Kelly & Scheibling, Reference Kelly and Scheibling2011). Long-chain FAs, in general, cannot efficiently be biosynthesized in marine animals to meet developmental and physiological needs (Delaporte et al., Reference Delaporte, Soudant, Moal, Lambert, Quéré, Miner, Choquet, Paillard and Samain2003; Pernet & Tremblay, Reference Pernet and Tremblay2004); therefore, these FAs from the prey pass intact into the circulation of the predator, taken up and stored by tissues in a predictable way. To this end, specific FAs are known to be characteristic of certain animal species and can be used as useful biomarkers in tracing dietary history (Iverson et al., Reference Iverson, Field, Don Bowen and Blanchard2004). However, since benthic invertebrates are more capable of altering dietary FAs, controlled feeding studies are therefore necessary before using FAs as dietary tracers in field studies (Dalsgaard et al., Reference Dalsgaard, St. John, Kattner, Müller-Navarra and Hagen2003; Bell & Tocher, Reference Bell, Tocher, Arts, Kainz and Brett2009).
In the present study, we aimed to develop a FA analysis method to assess the diet composition of juvenile T. tridentatus through a 12-month feeding experiment. The juveniles were provided with selected feed at various growth stages according to their food sources in the nursery habitat: brine shrimp Artemia salina larvae for 2nd–3rd instars (as part of suspended particulate organic matter; Gaines et al., Reference Gaines, Carmichael, Grady and Valiela2002), A. salina, short-necked clam Ruditapes philippinarum and greasyback shrimp Metapenaeus ensis meat for 4th and larger instars (crustaceans and bivalves; Kwan et al., Reference Kwan, Cheung and Shin2015b). By comparing the proportional FA signatures of the juveniles and their potential prey, this method can be useful as an alternative approach to reflect on foraging and trophic ecology of juvenile horseshoe crabs at the natural habitat.
MATERIALS AND METHODS
Culture conditions and experimental procedures
Fertilized T. tridentatus eggs were obtained from Guangxi Institute of Fisheries, China. Upon arrival at the laboratory facilities of City University of Hong Kong, the fertilized eggs were kept in indoor tanks (300 × 40 × 20 cm) equipped with a water circulating system (flow rate: 13 l min−1) consisting of bio-rings, a protein skimmer, filter pumps and electric heaters. The fertilized eggs were gradually acclimatized to the following environmental conditions: temperature 28–30°C, salinity 30‰, pH 7.5, dissolved oxygen 6.0 mg l−1 and photoperiod 12 h light: 12 h dark cycle. The filtered seawater was monitored twice a week for quality assurance (salinity, pH and ammonia) and water renewal was carried out week-wise. Tachypleus tridentatus embryogenesis ended with hatching after 30 days and all 1st instars were relocated into a holding tank containing filtered charcoal and sand as substrate. All instars were not fed until first moult took place (Brown & Clapper, Reference Brown, Clapper, Hinegardner, Atz, Fay, Fingerman, Josephson and Meinkoth1981; Carmichael et al., Reference Carmichael, Gaines, Sheller, Tong, Clapp, Valiela, Tanacredi, Botton and Smith2009; Schreibman & Zarnoch, Reference Schreibman, Zarnoch, Tanacredi, Botton and Smith2009).
The feeding assay lasted 12 months and juvenile T. tridentatus were between 7th and 8th instars (Chen et al., Reference Chen, Lau, Cheung, Ke and Shin2010). Dried brine shrimp eggs and frozen brine shrimp (San Francisco Bay Brand, Inc., California, USA) were purchased from a local aquarium store, whereas fresh clam and shrimp were bought from a local wet market. Brine shrimp larvae were hatched from the cysts after incubating for 24 h under sufficient aeration with filtered seawater at temperature 28°C and salinity 30‰. Clam and shrimp meat were cut into pieces (~0.3 cm) before the feeding (Hong, Reference Hong and Hong2011). Excess food was removed 2 hours after feeding. The juveniles were fed to satiation once a day and 7 days weekly with different food sources at various growth stages: freshly hatched larvae of A. salina for 2nd–3rd instars (3 months), defrosted frozen adults of A. salina for 4th–5th instars (3 months), and a mixed feed of fresh R. philippinarum and M. ensis meat (1:1 wet weight) for 6th and larger instars (6 months), according to the optimized rearing methods for juvenile T. tridentatus reported by Chen et al. (Reference Chen, Lau, Cheung, Ke and Shin2010). Brine shrimp and clam meat provided optimum growth for 2nd–4th instars (Hu et al., Reference Hu, Wang, Cheung and Shin2013) and 6th instar (Kwan et al., Reference Kwan, Chan, Cheung and Shin2014) because growth and moulting were rapid.
Fatty acid sample preparation
Hard (spine and appendage) and soft (gill) tissues were removed from juvenile T. tridentatus after the feeding assay. Haemolymph extraction (1 ml) was carried out on 7th instar (carapace width ≥35 mm) juvenile horseshoe crabs through lumbar puncture using 25 gauge 0.5 × 16 mm needle and 1 ml Tuberculin syringe (Terumo Medical Products, Somerset, NJ, USA) and transferred into sterile 1.5 ml microcentrifuge tubes (Kwan et al., Reference Kwan, Chan, Cheung and Shin2014, Reference Kwan, Chan, Cheung and Shin2015b). The arthrodial membrane was cleaned with 70% ethanol (v/v) before and after haemolymph collection to avoid sepsis. All samples were dried at 80°C for 72 h, grounded into powder and stored in a −20°C freezer.
Fatty acid composition analysis
A total of 100 mg from each sample was used for the extraction of fatty acid methyl esters of phospholipids and neutral lipids, following the 2:1 (volume: volume) chloroform-methanol method modified from Budge et al. (Reference Budge, Iverson and Koopman2006). The crude extract was washed with 0.2× its volume using 0.04% CaCl2 solution and left to stand until separate layers were formed. The organic and aqueous layers were separated by centrifugation. The upper phase was removed, and the extract was rinsed twice with 0.15 fold of its volume of 0.02% CaCl2 solution in methanol. The lower layer was dried by a stream of nitrogen.
The extracted lipids were subsequently analysed using an Agilent 6890 series GC-FID with an autosampler equipped with a DB-225 capillary column (30 m, 0.25 mm internal diameter, 0.25 pm film thickness) (J&W Scientific, Folsom, CA, USA). Authentic methylated fatty acid standards were purchased from Sigma and Supelco (Bellefonte, PA, USA), and methyl nonadecanoate (C19:0) was used as an internal standard (Chan et al., Reference Chan, Gao, Yip, Wong, Shin and Cheung2003). Triplicate measurements were performed for each pooled sample and data were expressed as total lipids. The summary of the assay method is depicted in Supplementary Figure 1.
Data analysis
All values represented the mean (N = 3) ± standard deviation. Normalized principal component analysis (PCA; Pielou, Reference Pielou1984) was employed to compare fatty acid composition differences among juvenile haemolymph and different part of tissues as well as the feed provided during the rearing period. For the six fatty acid components (21:0, 23:0, 24:0, 22:1ω9, 20:2ω6 and 20:3ω6) that could be detected in horseshoe crab samples but not in the feeds, a zero value was assigned for the PCA analysis. All statistical analyses were performed with the SPSS software for version 16.0 (SPSS, Inc., Chicago, IL, USA), while PCA was conducted with software PRIMER 6 statistical package (PRIMER-E, Plymouth Marine Laboratory, UK).
RESULTS
Saturated fatty acids
The primary saturated fatty acid (SFA) detected in the four food sources provided during the feeding experiment was palmitic acid (16:0; 11–29%) (Table 1). The shrimp meat had the highest number of SFA (n FA = 6), followed by clam meat (n FA = 5). There was an absence of SFAs 17:0 and 20:0 in brine shrimp and its larvae, and 20:0 in clam meat. A greater proportion of total SFAs was measured in shrimp (59%) and clam (48%) meat if compared with brine shrimp (19%) and its larvae (18%). After the feeding experiment, there were nine SFAs detected in different tissues of juveniles (Table 2). Relatively richer amounts of stearic acid (18:0; 13–20%) and palmitic acid (9–15%) were observed in various juvenile tissues. Percentage of total SFAs in juvenile tissues was in the range of 39–59%, in which book gill had the lowest while haemolymph had the highest proportion.
Table 1. Fatty acid composition of various food sources provided to juvenile horseshoe crabs: brine shrimp (Artemia salina) and larvae, short-neck clam meat (Ruditapes philippinarum) and greasyback shrimp (Metapenaeus ensis).

All values were expressed as the mean percentage of total fatty acids (triplicate measurements) ± standard deviation.– = not detected.
Table 2. Fatty acid composition of juvenile haemolymph and different tissues after feeding experiment.

All values represent the mean percentage of total fatty acids the mean (triplicate measurements) ± standard deviation.– = not detected.
Monounsaturated fatty acids
Regarding the food sources provided, the greatest number of monounsaturated FA (MUFA) was observed in clam meat (n FA = 7), followed by brine shrimp and its larvae (n FA = 6, 6), and shrimp meat (n FA = 4) (Table 1). More abundant amounts of MUFAs oleic acid (18:1ω9; 6–25%), palmitoleic acid (16:1ω7; 4–11%) and cis-vaccenic acid (18:1ω7; 4–10%) was measured among the feed. MUFA 15:1 was absent in brine shrimp, larvae and shrimp meat but present in a small quantity in clam meat (0.39%). Brine shrimp and its larvae had the relatively higher proportion of total MUFAs (44–48%) compared with that in the clam (21%) and shrimp meat (18%). For juvenile tissues, oleic acid was the most dominant MUFA measured (7–34%) (Table 2). MUFAs 15:1 and 18:1ω7 were only detected in the haemolymph of juveniles but not the other tissues. Juvenile hard tissues had relatively higher percentages of total MUFAs, including swimming appendage (41%), chelicerae (38%) and opisthosomal spine (34%).
Polyunsaturated fatty acids
Polyunsaturated fatty acids (PUFAs) 18:3ω3 and 20:5ω3 were commonly observed in all the diets (Table 1). The major PUFAs detected in clam and shrimp meat were eicosapentaenoic acid (20:5ω3; 9–13%) and docosahexaenoic acid (22:6ω3; 8–13%), while brine shrimp and its larvae had the highest proportions of eicosapentaenoic acid (10–11%) and linoleic acid (18:2ω6; 9–18%). Percentage of total PUFAs among various tissues of juveniles was similar (22–23%), except book gill had relatively richer total PUFAs (36%) (Table 2). EPA was the most dominant PUFA among juvenile tissues. While nine PUFAs were detected in juvenile gill, spine and appendage, PUFAs 18:3ω6, 18:3ω3 and 22:6ω3 were absent from the haemolymph of juveniles.
Principal component analysis
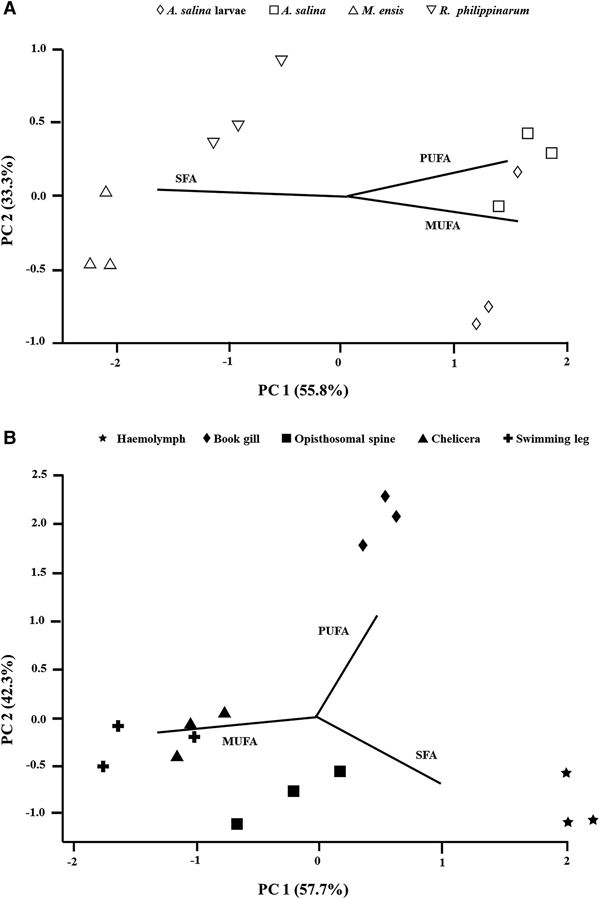
Principal component analysis (PCA) of feed provided (Figure 1A) revealed that there was a distinctly broad separation between two clusters: shrimp and clam meat on the left, which was characterized by its higher total proportion of SFAs (48–59%); and brine shrimp and its larvae on the right that featured considerable amounts of MUFAs (44–48%) and PUFAs (33–38%). For juvenile tissues (Figure 1B), three distinct clusters were observed, including haemolymph at the bottom right corner characterized by its greatest amount of SFAs (59%) and lowest percentage of total MUFAs (19%); book gill on the top of the plot featured by its highest proportion of total PUFA (36%); and hard tissues (appendages and spine) at the bottom left corner with relatively richer amount of MUFAs (36–41%). By considering the fatty acid profile of both food sources and juvenile tissues, the PCA (Figure 2) plot ordinations illustrated a clear separation between two clusters: juvenile haemolymph and food sources of clam and shrimp meat on the top; and a variety of juvenile tissues and the diets provided during early experiment including brine shrimp adults and larvae at the bottom right corner.

Fig. 1. Principal component plots illustrating the fatty acid composition of (a) feed and (b) soft (haemolymph, gill) and hard tissues (appendage, spine) of juvenile T. tridentatus after the feeding experiment. Each of the points represents triplicate measurements.

Fig. 2. Principal component plot showing the relationship of fatty acid composition of juvenile haemolymph and different tissues to the provided food sources. Each of the points represents triplicate measurements.
DISCUSSION
The total amount of unsaturated FAs detected in various tissues of juvenile T. tridentatus was consistent with those naturally existing in adult L. polyphemus (Van der Horst et al., Reference Van der Horst, Oudejans, Plug and Van der Sluis1973), in which the inter-specific differences for tissues of gill, opisthosomal spine (carapace) and swimming leg (muscle) were smaller than 4%. Identical to those from the American counterpart (Van der Horst et al., Reference Van der Horst, Oudejans, Plug and Van der Sluis1973), a high amount of polyunsaturated FAs, particularly eicosapentaenoic acid (EPA; 20:5ω3), was widely detected in various tissues of juvenile T. tridentatus, including gill and appendage. Although FA profiles of juvenile T. tridentatus were not documented, the presence of predominant FAs such as stearic, arachidonic and EPA in juvenile T. tridentatus were comparable with L. polyphemus (MacPherson et al., Reference MacPherson, Pavlovich and Jacobs1998).
Since long-chain FAs, notably omega-3 and omega-6 FAs, cannot be effectively produced in some marine predators, these indicator FAs are believed to be specific to certain groups of organisms or even individual species (Iverson et al., Reference Iverson, Frost and Lang2002). For example, EPA and docosahexaenoic acid (DHA; 22:6ω3) were the most common primary PUFA components detected in marine molluscs (Passi et al., Reference Passi, Cataudella, Di Marco, De Simone and Rastrelli2002) and shrimp (Iverson et al., Reference Iverson, Frost and Lang2002; Passi et al., Reference Passi, Cataudella, Di Marco, De Simone and Rastrelli2002), in which these two components made up 66–77% of total PUFAs. Meanwhile, horseshoe crab eggs and different tissues were characterized by the two predominant PUFAs EPA and AA that contributed to 41–54% of total PUFAs (Van der Horst et al., Reference Van der Horst, Oudejans, Plug and Van der Sluis1973; MacPherson et al., Reference MacPherson, Pavlovich and Jacobs1998). In the present study, the most abundant PUFA components observed in R. philippinarum and M. ensis (Table 1) as well as different tissue samples of juvenile horseshoe crabs (Table 2) were consistent with those previous studies (Van der Horst et al., Reference Van der Horst, Oudejans, Plug and Van der Sluis1973; MacPherson et al., Reference MacPherson, Pavlovich and Jacobs1998). While the role of EPA and AA in different tissue cells of horseshoe crabs is unknown, MacPherson et al. (Reference MacPherson, Pavlovich and Jacobs1998) suggested that these two PUFA components are essential in horseshoe crab amoebocytes to activate rapid degranulation during the immune responses, so that clotting factors and antimicrobial substances can be released from amoebocytes against invading pathogens or external injury. A recent study by Kwan et al. (Reference Kwan, Chan, Cheung and Shin2017) also showed that the microalgae-supplement diets with a higher amount of EPA improved amoebocyte viability and the ratio of the functionally active morphological form of amoebocytes in juvenile T. tridentatus. For other marine invertebrates, EPA was demonstrated to control membrane fluidity in the Atlantic deep-sea scallop Placopecten magellanicus (Hall et al., Reference Hall, Parrish and Thompson2002). Meanwhile, reports about growth enhancement under dietary treatments with EPA and DHA were also widely documented in marine invertebrate larvae (e.g. Levine & Sulkin, Reference Levine and Sulkin1984; Suprayudi et al., Reference Suprayudi, Takeuchi and Hamasaki2004; Alkanani et al., Reference Alkanani, Parrish, Thompson and McKenzie2007).
Most field studies assumed that FAs transfer to predators with minimal modification, despite the fact that some benthic invertebrates possess active elongase and desaturase enzymes, and may have greater ability to alter dietary FAs (Hall et al., Reference Hall, Lee and Meziane2006; Bell & Tocher, Reference Bell, Tocher, Arts, Kainz and Brett2009). Therefore, controlled feeding studies in a laboratory setting are necessary to further understand how FAs are transferred from prey, catabolized or excreted by the consumer before applying FAs as dietary tracers in field studies (Kelly et al., Reference Kelly, Krumhansl and Scheibling2012). Many feeding experiments of benthic invertebrates include artificial feed enriched with high levels of specific FAs to isolate the growth and reproduction responses of FAs of interest (Calado et al., Reference Calado, Rosa, Morais, Nunes and Narciso2005), but these aquaculture-focused results are not useful for seeking tracers for use in field studies. On the other hand, documenting changes in FA signatures over time during a controlled feeding experiment, as demonstrated in the present study, can be used for validating FAs as dietary indicators. Liyana-Pathirana et al. (Reference Liyana-Pathirana, Shahidi and Whittick2002) noted that the FA indicator for kelp decreased and an indicator for artificial diet increased when the diet of green sea urchin Strongylocentrotus droebachiensis switched from kelp- to grain-based feed. Previous studies also reported the identification of indicator FAs of primary producers (e.g. diatoms, dinoflagellates, green algae) in their invertebrate predators (Shin et al., Reference Shin, Yip, Xu, Wong and Cheung2008; Kelly et al., Reference Kelly, Scheibling and Iverson2009). Some sea urchin species were reported to have greater capability to convert dietary FA. For example, Psammechinus miliaris can convert α-linolenic acid (18:3ω3) in the diet to EPA at a prolonged conversion rate (Bell et al., Reference Bell, Dick and Kelly2001). Strongylocentrotus droebachiensis that were fed artificial diets rich in oleic acid (18:1ω9) contained high levels of both oleic acid and its elongation product 20:1ω9 (Castell et al., Reference Castell, Kennedy, Robinson, Parsons, Blair and Gonzalez-Duran2004). The present study demonstrated that juvenile horseshoe crabs may have limited ability to modify dietary FA, and must obtain it from their diets.
The extent of FA profile from diets that incorporate into tissues of marine invertebrates can be species- and tissue-specific, owing to the different modes of lipid storage and metabolic turnover (Skonberg et al., Reference Skonberg, Rasco and Dong1994). For marine invertebrates, previous studies reported that FA composition in digestive glands of European common cuttlefish Sepia officinalis (Fluckiger et al., Reference Fluckiger, Jackson, Nichols, Virtue, Daw and Wotherspoon2008) and Atlantic brief squid Lolliguncula brevis (Stowasser et al., Reference Stowasser, Pierce, Moffat, Collins and Forsythe2006) indicate their recent diets. This is because cephalopods do not accumulate dietary lipids in their digestive glands for long-term storage. Nevertheless, FA profile in various fish species, including European sea bass (Skalli et al., Reference Skalli, Robin, Le Bayon, Le Delliou and Person-Le Ruyet2006), Atlantic salmon (Benedito-Palos & Bendiksen, Reference Benedito-Palos and Bendiksen2003), sea bream (Benedito-Palos et al., Reference Benedito-Palos, Navarro, Kaushik and Pérez-Sánchez2010), turbot (Regost et al., Reference Regost, Arzel, Robin, Rosenlund and Kaushik2003) and pike perch (Schulz et al., Reference Schulz, Knaus, Wirth and Rennert2005) reflected their diet composition regardless of the tissue. In this study, the potential of utilizing juvenile haemolymph in providing recent dietary information was evident, since the haemolymph FA profile apparently responded to the change in food sources from brine shrimp to shrimp and clam meat, as illustrated in the PCA plot (Figure 2). On the other hand, juvenile horseshoe crab tissues were more resistant to nutrient alteration (Figure 2). Such differences were believed related to mobilized FAs found in juvenile horseshoe crab haemolymph, but not in tissues which tended to accumulate FAs and store lipids.
CONCLUSION
In conclusion, the present feeding experiment demonstrated that the FA composition of juvenile horseshoe crab haemolymph reflected recent food sources, as the haemolymph FA composition was profoundly influenced by the feed alteration. Juvenile soft (gill) and hard tissues (appendage, carapace), nevertheless, were more resistant to dietary changes and useful in indicating previous diets. Further investigation is required to validate the usefulness of FA signatures as a dietary tracer for horseshoe crabs in their natural habitat so that knowledge of their foraging biology and ecology can be enhanced.
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at https://doi.org/10.1017/S0025315418000279
ACKNOWLEDGEMENTS
We would like to thank Prof. Peter B. Armstrong from the University of California, Davis and Prof. S.G. Hong from Xiamen University for their helpful advice.
FINANCIAL SUPPORT
This work was funded by Ocean Park Conservation Foundation of Hong Kong, National Natural Science Foundation of China (41706183), and Start-up Research Grant from Qinzhou University (2017KYQD106).