INTRODUCTION
Population patterns of benthic invertebrates are determined by a variety of biological and physical factors acting on both larval and benthic stages (Thorson, Reference Thorson1966). The first determinant of population patterns is often the abundance of larvae ready to settle (Gaines & Roughgarden, Reference Gaines and Roughgarden1985; Underwood & Fairweather, Reference Underwood and Fairweather1989; Minchington & Scheibling, Reference Minchington and Scheibling1991), which is influenced by such factors as larval dispersal and mortality (Eckman, Reference Eckman1983; Roughgarden et al., Reference Roughgarden, Gaines and Possingham1988; Rumrill, Reference Rumrill1990; Gaines & Bertness, Reference Gaines and Bertness1992; Morgan et al., Reference Morgan, Zimmer-Faust, Heck and Coen1996; Levin, Reference Levin2006). Population patterns are also affected by larval behaviour and responses to settlement cues (Butman, Reference Butman1987; Pawlik, Reference Pawlik1992; Harvey et al., Reference Harvey, Bourget and Ingram1995; Kingsford et al., Reference Kingsford, Leis, Shanks, Lindeman, Morgan and Pineda2002; Li et al., Reference Li, Lin, Guang, Cai, Cai, Chang and Xing2006). One can infer larval responses to substrata by studying the abundance and distribution of early settlers before patterns are masked by the mortality that follows settlement (Keough & Jones, Reference Keough and Jones1982; Connell, Reference Connell1985). Post-settlement mortality of benthic invertebrates is often high, particularly from predation (see reviews by Gosselin & Qian, Reference Gosselin and Qian1997; Hunt & Scheibling, Reference Hunt and Scheibling1997). Gosselin & Qian (Reference Gosselin and Qian1997) indicate that juvenile mortality was >90% in 20 of the 30 benthic invertebrate studies they examined (and ~98.6% for the studies on bivalves).
The effect of predation on bivalves, as on most benthic invertebrates, can vary with habitat, site, time and the kinds of predators (Barbeau & Scheibling, Reference Barbeau and Scheibling1994; Barbeau et al., Reference Barbeau, Scheibling, Hatcher, Taylor and Hennigar1994). Prey selection by predators often plays a role in determining the size–structure of prey populations (Moran, Reference Moran1985; Kvitek et al., Reference Kvitek, Oliver, DeGange and Anderson1992; Arsenault & Himmelman, Reference Arsenault and Himmelman1996). Physical disturbances and inadequate food resources can also cause mortality leading to changes in population structure (see reviews by Olafsson et al., Reference Olafsson, Peterson and Ambrose1994; Constable, Reference Constable1999; Lenihan & Micheli, Reference Lenihan, Micheli, Bertness, Gaines and Hay2001). Most studies focus on single factors causing mortality, yet a comprehensive understanding of population dynamics requires the examination of various factors together (Gaines & Roughgarden, Reference Gaines and Roughgarden1985; Underwood & Fairweather, Reference Underwood and Fairweather1989; Bertness et al., Reference Bertness, Gaines, Stephens and Yund1992; Eggleston & Durham, Reference Eggleston and Durham1995).
The present study considers the amber penshell Pinna carnea Gmelin 1791, a sessile filter-feeding bivalve that is distributed from southern Florida to Brazil (Turner & Rosewater, Reference Turner and Rosewater1958; Narváez et al., Reference Narváez, Lodeiros, Freites, Nunez, Pico and Prieto2000). It may reach 30 cm in length (Yonge, Reference Yonge1953) and occurs in medium to coarse sand or mixed substrata (sand–rocks–corals) in Columbia (Urban, Reference Urban2001), in fine calcareous sandy mud in eelgrass (Zostera sp.) beds in the Bahamas (Yonge, Reference Yonge1953), and mainly in the sandy substrata in turtle grass (Thalassia testudium) beds in the Dominican Republic (Aucoin, Reference Aucoin2008). No information on its embryology is available, but Rosewater (Reference Rosewater1961) reports that the embryonic valves of juvenile Pinna sp. are quickly worn away after they start growing from the posterior edge (personal observations on P. carnea).
The anterior portion of the penshell is buried and attached by byssal filaments to sub-surface materials (sand, pebbles and seagrass roots), whereas the posterior portion (beyond the posterior adductor muscle) protrudes vertically above the bottom surface. Although this position allows penshells to filter water from above silty bottoms, it could also increase exposure to predators (Yonge, Reference Yonge1953). Penshells often show breaks or scars on the posterior shell margin that likely result from predatory attacks (Turner & Rosewater, Reference Turner and Rosewater1958; Dietl & Alexander, Reference Dietl and Alexander2005). Octopuses are likely an important predator of adult penshells (Anderson et al., Reference Anderson, Wood and Mather2008; personal observations). Anderson et al. (Reference Anderson, Wood and Mather2008) examined shell remains near Octopus vulgaris dens in Bonaire, Netherlands Antilles, and stated that one octopus appeared to feed exclusively on P. carnea. Few studies report on the biology of P. carnea. Yonge (Reference Yonge1953) describes its morphology, Aucoin & Himmelman (Reference Aucoin and Himmelman2010) report the frequency of symbiotic shrimp (Pontonia sp.) and cardinalfish (Astrapogon stellatus) in its mantle cavity, and others discuss aspects of its reproduction and potential for aquaculture (Castellanos et al., Reference Castellanos, Urban and Borrero1997; Garcia-Valencia et al., Reference Garcia-Valencia, Urban and Borrero1997; Narváez et al., Reference Narváez, Lodeiros, Freites, Nunez, Pico and Prieto2000; Urban, Reference Urban2001; Núñez et al., Reference Núñez, Lodieros, Acosta and Castillo2006).
The present study first documents population patterns of P. carnea at a number of locations in the south-western Dominican Republic, and then describes experiments performed to examine: (1) substratum and habitat choice for settlement; (2) early post-settlement mortality; (3) subsequent mortality in relation to size in two contrasting habitats, seagrass beds and sandflats; and (4) the growth rate of penshells of different sizes in the two habitats. We also examine the importance of food availability and sedimentation because P. carnea and other members of the family Pinnidae have a unique waste canal that facilitates living in silty habitats (see Winckworth, Reference Winckworth1929; Yonge, Reference Yonge1953; Turner & Rosewater, Reference Turner and Rosewater1958; Rosewater, Reference Rosewater1961).
MATERIALS AND METHODS
Population patterns and habitat description
Our first observations of Pinna carnea populations were conducted at 6 locations near Pedernales and Barahona (Figure 1) during surveys (4–8 transects per location) from February to June 2002 in the context of benthic foodweb studies by Tewfik et al. (Reference Tewfik, Rasmussen and McCann2005, Reference Tewfik, Rasmussen and McCann2007). In 2005, we again examined locations near Pedernales (Figure 1). We examined 6 transects at Trudillé and 4 at Cabo Rojo during May, and followed with 5 transects at each location during late-August and early-September. These locations were exposed to Hurricane Dennis on 5–7 July 2005. All survey locations supported sheltered seagrass habitats, although Trudillé had markedly more calcareous green algae (family Halimedacea). In each survey, two SCUBA divers carefully searched through 3 × 60 m transect corridors across the seagrass bed (4–8 m depth), recording the hinge length (anterior to posterior dorsal tip) of each penshell, the numbers of other large (>3 cm) benthic invertebrates and several fish that could be penshell predators. We also conducted extensive searches for P. carnea in adjacent sandflats at each location, but not in a systematic way as no penshells were encountered.

Fig. 1. Locations where the penshell Pinna carnea was studied in the Dominican Republic. Black dots ![]() are locations where surveys were made in 2002 and open dots
are locations where surveys were made in 2002 and open dots ![]() are Cabo Rojo and Trudillé, where surveys and experiments were conducted in 2005. The doted line represents the boundaries of Jaragua National Park.
are Cabo Rojo and Trudillé, where surveys and experiments were conducted in 2005. The doted line represents the boundaries of Jaragua National Park.
To provide information on factors that could affect penshell populations, we first examined the density of potential predators, the yellow stingray Urolophus jamaicensis, echinoderms Oreaster reticulates and Meoma ventricosa, and all crabs (Brachyurans) at both sites (11 transects at Trudillé and 9 at Cabo Rojo). We also evaluated some above- and below-ground features of the seagrass beds at both locations. We determined the dry mass of seagrasses and macrophytes (dried at 80°C for 24 hours) by sampling 15 quadrats at Trudillé and 16 at Cabo Rojo. Quadrats were 10 × 10 cm2 and placed randomly at least 15 m from each other. The below-ground portion of seagrass habitats was quantified from 196 cm3 sediment cores (5 cm diameter and 10 cm depth) taken in the middle of each of the above quadrats. Contents from each sediment core were washed on a 3.5-mm sieve to remove small loose sediment (mainly sand). Then the materials that did not pass through the sieve were separated into two components: (1) consolidated sediment with roots and algal rhizoids (removed manually); and (2) large loose sediment (the remaining materials), and weighed after drying at 80°C for 24 hours.
Substratum preference for larval settlement
Since penshells were only found in seagrass beds, we evaluated the suitability of various potential settlement substrata from the seagrass habitat using caged spat collectors (Figure 2A). The substrata tested were: (1) only sand; (2) seagrass blades only (the bottom portions of the blades implanted into sand); (3) roots (mostly buried in sand); (4) seagrass blades with roots (the root portion in sand); and (5) 2-cm-wide strips of plastic filament scouring pads (similar to Scotch-BriteTM pads) with a total surface area of 0.15 m2. The latter treatment also allowed us to compare the settlement in this experiment with that in subsequent experiments where scouring pads were the only settlement substratum deployed.

Fig. 2. Types of collectors used to investigate larval settlement of the penshell Pinna carnea, (A) is the caged collector used to evaluate substratum preference for settlement, and (B) is the collector (which was either caged or uncaged) used to evaluate variations in numbers of larvae settling, and early post-settlement survival, in different habitats.
We deployed 25 collectors, 5 replicates for each of the above treatments (substrata), at both Trudillé and Cabo Rojo. Each collector (Figure 2A) consisted of a large PVC cap (9 cm depth and 13 cm diameter), which held the substratum, and was protected in a cylindrical cage (12-mm2 mesh, 30 cm height and 13 cm diameter). The collector was attached to floats and suspended at ~1 m above the seagrass bed. In this and the following experiments, the experimental units were placed randomly at least 5 m from one another within a hectare (10,000 m2). We counted penshells and other bivalves in the collectors after ~75 days at Trudillé (21–23 March to 1–4 June 2005) and after ~130 days at Cabo Rojo (11–12 April to 16–20 August 2005). As the egg cockle Laevicardium laevigatum turned out to be an abundant bivalve in these trials, we also evaluated its settlement preference. In this and several other experiments, the delayed retrieval of data at Cabo Rojo was due to unforeseen circumstances (a fieldwork injury).
Settlement intensity and early post-settlement mortality
As numbers of settlers and their subsequent survival can be major determinants of population patterns, we evaluated larval settlement and early post-settlement mortality from predators by deploying spat collectors at both Trudillé and Cabo Rojo. The collectors were either caged or uncaged and were deployed on the seafloor and at ~1 m above the bottom, both in seagrass beds and adjacent sandflats. There were 10 caged and 10 uncaged replicates for each position in each habitat at both locations (80 experimental units per location). Each collector (Figure 2B) consisted of two 10 × 15 cm2 plastic filament scouring pads wrapped around a sealed plastic bottle and held in place by a crinoline (onion-sac material) sleeve and tie-wraps. In the caged collectors (6-mm2 mesh, 30 cm height and 19 cm diameter) the scouring pad substratum was separated from the cage walls by ~6 cm. We counted the penshells and all other bivalves in each collector after ~75 days at Trudillé (14–15 March to 1–4 June 2005) and after ~140 days at Cabo Rojo (5–6 April to 16–20 August 2005).
To provide further information on the settlement of P. carnea larvae in other habitats, we also suspended 5 caged collectors ~1 m above the bottom in a rocky–coral–sponge habitat (~6 m depth) and in a seagrass bed off a headland, where the brown drift alga Lobophora variegata was abundant (~12 m depth). These collectors were deployed at Cabo Rojo and retrieved ~140 days later (8–13 April to 22–25 August 2005).
Penshell mortality in seagrass beds and sandflats
Penshells for survival trials were collected from offshore moorings located ~3 km offshore south of Barahona (Figure 1). They were transported to Cabo Rojo in buckets and then maintained in underwater cages for at least 2 weeks to allow them to acclimatize to conditions at Cabo Rojo.
We evaluated the impact of predators on these penshells by quantifying the survival of 3 size-groups (10–30, 50–70 and 90–110 mm in hinge length) in a seagrass bed and an adjacent sandflat at Cabo Rojo. Each replicate consisted of a 50 × 50 cm2 plot into which we transplanted 4 penshells (evenly dispersed) of a given size-group. We deployed 5 replicates with predator exclusion cages (12-mm2 mesh, 50 × 50 × 50 cm3) and 5 replicates without cages for each of the 3 size-groups in both habitats (60 plots). Marked stakes were planted in the middle of each replicate and a map was drawn to record the positions of the plots. The above experimental design was run for three different experimental periods in 2005. In the first trial, survival was measured after a period of 1 day (12 to 13 May), in the second after 10 days (14 to 23 May) and in the third after 100 days (24 May to 31 August). Hurricane Dennis passed through the region during the 100-day trial (5–8 July).
To evaluate survival rates of still larger penshells, we compared 50–100 mm and 150–170 mm individuals transplanted to the sandflat at Cabo Rojo. We ran 5 consecutive 3-day trials (23 August to 8 September 2005). Each trial was set up with three 50–100 mm penshells and three 150–170 mm individuals without cages.
Sedimentation in seagrass beds and sandflats
The amount and types of material falling to the seafloor from the water column and from resuspended sediment can be either beneficial (e.g. provide food) or detrimental (e.g. block gills) to bivalves and other suspension feeders. In 2005, we quantified particulate organic matter (POM) and silt mass using sediment traps that were deployed in seagrass beds and adjacent sandflats at both Trudillé and Cabo Rojo (8 traps/habitat/location, placed at least 20 m from one another in the same general area as the other experiments). The traps were made of PVC pipes (30 cm length and 2.5 cm diameter) that were driven into the sediment (~2 cm of each pipe extended above the seafloor). These traps collect both particles sinking from the water column and particles in suspension by means of eddies formed at the trap mouth (Butman, Reference Butman1989). The traps can also collect settling bivalves and thus permitted us to examine settlement at each location. To preserve collected materials, the bottom half of each trap was filled with a tinted 10% formalin–seawater solution and topped off with filtered seawater. The higher density of the bottom formalin layer reduces resuspension of settled materials and the added colour provides an indication of loss of material from the traps (Yund et al., Reference Yund, Gaines and Bertness1991). The traps were retrieved after ~75 days at Trudillé (16 March to 29–30 May) and after 145 days at Cabo Rojo (8 April to 30 August).
The contents from each trap were first sieved using a 0.45-µm filter and a manual vacuum pump to remove any remaining formalin. We then separated the invertebrates using a stereomicroscope. Finally, we determined the silt content and organic content of the remaining materials. For silt content, we removed large pebbles and detritus with a 2.8-mm sieve and then calculated the percentage weight loss resulting from thorough washing of the sediments on a 63-µm sieve. Prior to the washing process, a sodium polyphosphate solution was stirred into the sediments to aid in the dispersion of particles (Bale & Kenny, Reference Bale, Kenny, Eleftheriou and McIntyre2005). Organic content was determined from the weight loss after ashing all of the materials that did not pass through the 63-µm sieve (including the materials collected on the 2.8-mm sieve). The muffle furnace was set at 350°C to avoid decomposition of shell carbonates (Welikey et al., Reference Welikey, Suess, Ungerer, Muller and Fisher1983) and the prior removal of silt should have removed bias due to structural water that could be retained within clay interstices (Craft et al., Reference Craft, Seneca and Broome1991).
To evaluate potential pico- to micro-plankton food sources in the water, we analysed 3.8-l water samples collected just above the seafloor in seagrass beds and adjacent sandflats. At Trudillé, we took 3 water samples from the seagrass (26 May 2005) and 2 samples from the sandflat (27 May 2005). At Cabo Rojo, we took 7 water samples from the seagrass and 7 samples from the sandflat (2 samples on 22 April, 2 samples on 22 May and 3 samples on 31 August 2005, from each habitat). Each water sample was first pre-filtered through a 263-µm sieve and then filtered through a 0.45-µm combusted and pre-weighed Whatman GF/F glass filter. The material collected on the filter was weighed after being dried at 80°C for 24 hours.
Growth
To obtain an approximate growth curve for Pinna carnea, we measured increases in shell hinge length of 62 individuals (ranging from 10.5 to 220 mm in hinge length) transplanted to seagrass beds between March to June 2002 and May to August 2005. For the small penshells, growth increments were recorded after a few days to a week, whereas for larger individuals measurements were taken after a few weeks to a month. Each individual was identified with a marked stake and the smallest penshells were maintained in cages to protect them from predators.
During 23 August to 19 September 2005, we also compared the increase in hinge length of penshells transplanted to the seagrass bed and adjacent sandflat at our study area in Cabo Rojo. We ran three 9-day trials with an array of similar-sized penshells (11 to 60 mm). In each trial, 5 penshells per habitat were individually maintained in cages (6-mm2 mesh, 30 cm height and 19 cm diameter) to exclude predators.
Statistical analyses
To compare population density across the south-western Dominican Republic, we applied an ANOVA to survey data from the 6 locations sampled in 2002, and t-tests to survey data from Trudillé and Cabo Rojo in 2005. The above and following analyses of variances were treated as fixed models unless otherwise indicated.
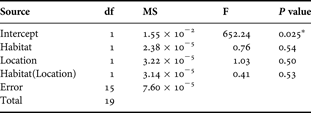
To examine penshell habitat, we compared the structure of seagrass beds at Trudillé and Cabo Rojo by applying a MANOVA to data on seagrass and algal dry mass (from quadrats) and a second MANOVA to data on dry mass of loose sediment and consolidated sediment with roots and rhizoids (from sediment cores). The data in these analyses, except for that on seagrass biomass, did not meet the assumption of equal variances. However, the validity of the tests and the probabilities associated with the F-ratio distribution were probably not greatly affected because sample size was relatively large and the numbers of samples per location or treatment were almost the same (Weerahandi, Reference Weerahandi1995; Underwood, Reference Underwood1997). As P values are the same for MANOVAs and ANOVAs (when using the SPSS statistical package version 11.04), we set the α-level at 0.025 (0.05 divided by the number of univariate tests conducted) to control for Type I error (Green & Salkind, Reference Green and Salkind2003).
To compare substratum preference for larval settlement, we applied an ANOVA to the data on P. carnea recruitment. We also applied an ANOVA to data on recruitment of the egg cockle Laevicardium laevigatum, an infaunal species that was a conspicuous recruit. The data on L. laevigatum did not meet the assumption of equal variances because there were many zero values. Nevertheless, we continued with post hoc pair-wise comparisons using the conservative Holm's sequential Bonferroni approach (to control for Type I error).
In our experiment examining settlement and early mortality from predation, the data did not support the use of factorial analysis because there were no penshells in many collectors and all of the caged collectors on the seafloor were lost to Hurricane Dennis. Thus, to examine post-settlement mortality we applied unequal variance t-tests to recruitment data on penshells, and the other bivalves, in caged and uncaged collectors suspended at ~1 m above the bottom, as well as for the data from uncaged collectors on the bottom and at ~1 m above the bottom. We used an ANOVA to compare the numbers of recruits in caged collectors in the four different habitats (seagrass with L. variegata, rocky–coral–sponge habitat, seagrass and sandflat) and followed with a least significant difference (LSD) test.
To compare penshell survival for different size-groups placed in a seagrass bed and sandflat, we applied a factorial ANOVA to data after the 1-day and 10-day experiments and followed with LSD tests. To increase the power of each analysis we did not include the cage factor because penshell survival in cages was very high. The assumption of equal variances was not met, but as the number of treatments was relatively large and there were equal numbers of samples per treatment we again used the LSD tests.
To compare the predation on even larger penshells, we performed a binary regression analysis of the data on the survival of 50–100 mm and 150–170 mm penshells in the sandflat (3-day trials). To evaluate growth of penshells in different habitats, we applied an ANCOVA to growth data on small penshells maintained in the seagrass bed and adjacent sandflat (9-day trials; covariate: length at t0).
Finally, we performed t-tests to compare materials collected in sediment traps placed in both habitats at Trudillé. We also performed t-tests to compare plankton-sized materials collected from water samples taken in seagrass and sandflat habitats at each of the two locations, Trudillé and Cabo Rojo, and then followed with a nested ANOVA to compare the two locations (nested factor: location, analysed as a random factor in SPSS 11.04).
RESULTS
Population patterns
Low numbers of Pinna carnea (0.016 penshells·m−2, SD = 0.010) were found in the seagrass beds surveyed in 2002 and densities were similar across the locations surveyed (F5,25 = 1.06 P = 0.41). Surveys were carefully conducted (each taking >1 hour) so it was unlikely that any penshells protruding 20 mm above the bottom were missed. The population size–structure was always skewed towards large individuals (Figure 3). Comparison of the numbers of penshells encountered in transects surveyed before and after Hurricane Dennis in 2005 showed no change in either penshell density or mean size at either Trudillé (density t 0.05(2)9 = −0.39 P = 0.70; size t 0.05(2)93.9 = −0.92 P = 0.36) or Cabo Rojo (density t 0.05(2)7 = −1.10 P = 0.31; size t 0.05(2)13 = 0.67 P = 0.52). The mean density of penshells at Cabo Rojo in 2005 (0.012·m−2, SD = 0.013; the 9 transects pooled) was similar to the values observed at the 6 locations studied in 2002, but the density at Trudillé (0.076·m−2, SD = 0.026; the 11 transects pooled) was >6-fold greater than at Cabo Rojo (t 0.05(2)13.6 = −6.9 P < 0.001). At both Trudillé and Cabo Rojo, the size-range was similar with larger individuals predominating (Figure 3); however, the mean size was smaller at Trudillé (134 mm, SD = 39) than at Cabo Rojo (171 mm, SD = 55) (t 0.05(2)152 = 3.3 P = 0.001).

Fig. 3. Population size–frequency distributions for the penshell Pinna carnea from transect surveys near Barahona (4 transects) and Pedernales (4–8 transects at 5 locations) in 2002, and at Cabo Rojo (9 transects) and Trudillé (11 transects) in 2005.
Our counts of potential predators during the 2005 penshell surveys revealed that the stingray Urolophus jamaicensis, the sea star Oreaster reticulates, and crabs (Brachyurans) occurred in similar numbers at Trudillé and Cabo Rojo (U. jamaicensis t 0.05(2)18 = −1.31 P = 0.21; O. reticulates t 0.05(2)11.5 = 3.9 P = 0.71; crabs t 0.05(2)8.0 = 1.63 P = 0.14). However, the large red-heart urchin Meoma ventricosa, which slowly ploughs through sub-surface sediments to feed (potentially disturbing small penshells), was present at Cabo Rojo (0.084 urchins·m−2, SD = 0.14) and absent at Trudillé.
Comparison of seagrass habitats
Seagrass dry mass was similar at Trudillé and Cabo Rojo (MANOVA F1,29 = 4.1 P = 0.05, α-level = 0.025), but algal dry mass was ~50 times greater at Trudillé (MANOVA F1,29 = 42.1 P < 0.001). There was more loose sand in the seagrass beds (i.e. unconsolidated sediment) at Cabo Rojo than at Trudillé. The comparisons of the below-ground portion of the habitat at the two locations indicated that the dry mass of large loose sediment (>3.5 mm) was >3 times greater at Trudillé than at Cabo Rojo (F1,29 = 31.0 P < 0.001) and the dry mass of consolidated matter (with roots and rhizoids) was >5 times greater at Trudillé than at Cabo Rojo (F1,29 = 55.1 P < 0.001) (Table 1; Figure 4).

Fig. 4. Comparison of seagrass beds at Trudillé ![]() and Cabo Rojo
and Cabo Rojo ![]() in relation to the dry mass of (1) seagrass, (2) algae, (3) consolidated sediment with roots and rhizoids, and (4) large loose sediment (>3.5 mm diameter). Vertical lines represent standard deviations and * indicate a significant difference between the two locations (MANOVA).
in relation to the dry mass of (1) seagrass, (2) algae, (3) consolidated sediment with roots and rhizoids, and (4) large loose sediment (>3.5 mm diameter). Vertical lines represent standard deviations and * indicate a significant difference between the two locations (MANOVA).
Table 1. Results of MANOVAs applied to data on above- and belowground samples of seagrass beds at Trudillé and Cabo Rojo. Dependent variables: Trudillé and Cabo Rojo.

*, significant difference (Levene's test for seagrass, P = 0.43).
a, α-level at 0.025 (0.05/number of univariate tests conducted).
b, corrected total, adjusted r 2
Substratum choice for larval settlement
Our collectors at Trudillé contained only 2 large penshells, and 147 other bivalves, after the ~75-day deployment. At Cabo Rojo there were 50 penshells (2.1/collector, SD = 1.6) and 169 other bivalves after the ~130-day deployment. The posterior ends of the penshell recruits were buried in the same way as the individuals encountered in the field surveys. In the scouring pad treatment the recruits were attached to the pads by byssal filaments. There were similar numbers of penshells settled on the five substrata tested at Cabo Rojo (F4,20 = 0.48 P = 0.75; Figure 5).

Fig. 5. Mean number of penshell Pinna carnea and cockle Laevicardium laevigatum spat on collectors with five different substrata after a deployment period of ~75 days at Trudillé and ~130 days at Cabo Rojo. At Trudillé there were only 2 penshell recruits and the number of cockle recruits differed among treatments (‘sand’ and ‘blades’ were similar and greater than in the other treatments). At Cabo Rojo there was no difference among treatments in penshell recruits (P = 0.75) or in cockle recruits (P = 0.06). Vertical lines represent standard deviations (ANOVA and post hoc Bonferroni tests).
The egg cockle Laevicardium laevigatum was the most abundant bivalve in the collectors at Cabo Rojo and was also common on collectors at Trudillé. Laevicardium laevigatum was also the second largest bivalve recruit after P. carnea. At Trudillé numbers of L. laevigatum varied on different substrata (F4,19 = 4.77 P = 0.01). Post hoc comparisons (Bonferroni) indicated that cockle recruits were similar in the treatments with no roots (‘sand’ and ‘blades’), but no cockles settled in the treatments with roots and with scouring pads. At Cabo Rojo, the number of cockles tended to vary on different substrata (F4,20 = 2.71 P = 0.06) and the pattern was similar to that at Trudillé, there being more cockle recruits in the treatments with no roots (‘sand’ and ‘blades’) than in the other treatments (Figure 5).
Settlement and early post-settlement mortality
In the experiment examining recruitment on scouring pads (caged and uncaged) in two different habitats (seagrass bed and sandflat) and at two positions (seafloor and ~1 m above the bottom), 69 bivalves, but no penshells, were collected after the ~75-day deployment at Trudillé. In the parallel experiment at Cabo Rojo, but with a ~140-day deployment period, 26 penshells and 115 other bivalves were collected. Values were greater for caged than for uncaged suspended collectors for both the number of penshells (t 0.05(2)21.04 = 2.20 P = 0.04) and the number of other bivalves (t 0.05(2)24.34 = 5.09 P < 0.001). The difference, which likely reflects post-settlement mortality due to predation, was 84% for penshells and 63% for the other bivalves. The number of penshells in uncaged collectors did not vary between the bottom and ~1 m above (t 0.05(2)25.17 = 1.14 P = 0.27) but the number of other bivalves was less on the bottom (t 0.05(2)22.79 = 2.87 P = 0.009).
The caged collectors in the two additional habitats, the rocky–coral–sponge habitat and the seagrass bed with the brown drift alga Lobophora variegata, showed that penshell larvae are present outside coastal sandflats and seagrass beds. More penshell recruits were found on suspended collectors just above the seagrass bed with L. variegata than in the other habitats (F3,24 = 0.40 P = 0.02), which had similar numbers of recruits (Figure 6).

Fig. 6. Mean number of penshell Pinna carnea spat observed on the same type of caged collector (with scouring pads) deployed for ~140 days at ~1 m above four different habitats at Cabo Rojo, (1) a seagrass bed where the brown drift alga Lobophora variegata was abundant, (2) a rocky–coral–sponge habitat, (3) a sandflat, and (4) a seagrass bed. Vertical lines represent standard deviations. Bars not sharing the same letter are significantly different (ANOVA and post hoc LSD test).
Penshell mortality in seagrass beds and sandflats
In our experiments on penshell survival, we considered there had been predation when we found penshell remains and when individuals went missing. Missing penshells were likely either completely consumed or carried away by predators. On occasion we observed broken penshells next to octopus dens located outside of our experimental area. Individuals could not have been transported away by water motion, since underwater conditions were very calm during all trials, except in the 100-day trials that were exposed to Hurricane Dennis (5–7 July). The high survival of penshells in caged control plots indicated that mortality from sources other than predators was negligible after 1 day (97% survival in the seagrass bed and 100% in the sandflat; 3 small penshells perished in the seagrass).
The 1-day predation trials evaluating survival of different size-groups in the two different habitats indicated that any effect related to penshell size also depended on the type of habitat since there was an interaction between factors (Table 2). Post hoc comparisons (LSD) indicated that the mortality of medium-sized and large penshells was greater in the sandflat and that predation decreased with penshell size in the seagrass habitat (Figure 7).

Fig. 7. Mean survival after 1 day and after 10 days of different size-groups of penshell Pinna carnea transplanted to a seagrass bed ![]() and an adjacent sandflat
and an adjacent sandflat ![]() at Cabo Rojo. Vertical lines represent standard deviations. Bars not sharing the same letter are significantly different (ANOVA and post hoc LSD tests).
at Cabo Rojo. Vertical lines represent standard deviations. Bars not sharing the same letter are significantly different (ANOVA and post hoc LSD tests).
Table 2. Results of factorial ANOVAs applied to data on survival of different size-groups of Pinna carnea in seagrass and sandflat habitats after 1-day and 10-day predation trials. Dependent variable: survival.

*, significant difference.
a, corrected total, adjusted r 2
Survival was also high in the caged control plots after 10 days (89% in the seagrass bed and 100% in the sandflat; 6 small penshells and 1 medium-sized individual perished in the seagrass). Predation mortality once more varied with both penshell size and habitat, and again there was an interaction between these factors (Table 2). In the uncaged plots, there was almost no survival of small penshells in either habitat (only 1 small individual remained in the sandflat) but there was an increase in survival with size in the seagrass bed. Post hoc comparisons (LSD) indicated a similar predation pattern as in the preceding experiment, although with a greater mortality (Figure 7). During the 10-day trials, 16 individuals in seagrass (8 in caged plots and 8 in uncaged plots) died of unknown causes and were replaced during daily inspections of the plots. These ‘dead-standing’ individuals were upright and were not broken or unearthed. The equal number of dead-standing penshells in both caged and uncaged plots in the seagrass suggested that potential cage artefacts were not involved. Further, no dead-standing individuals were ever found in the caged sandflat plots.
At the end of the 100-day predation trials, during which our study site at Cabo Rojo was subjected to Hurricane Dennis, all plots (caged and uncaged) in the sandflat were gone, as well as all plots with small individuals in the seagrass. However, 7 of the 10 plots with medium-sized penshells and all of the 10 plots with large penshells had remained in the seagrass bed. The only living penshells were from the largest size-group (90–110 mm). The survival of these large individuals did not vary between the caged (30%, SD = 37) and uncaged (30%, SD = 33) plots (t 0.05(2)8 = −15.8 P = 0.88). In the caged plots all of the mortalities were dead-standing individuals. In the uncaged plots about one-third of the mortalities were dead-standing individuals and two-thirds were missing. For medium-sized penshells (50–70 mm), we found only 2 of the caged plots, but all of 5 uncaged plots. In the caged plots, 62% of the individuals were dead-standing and 38% were missing, whereas in the uncaged plots 25% were dead-standing and 75% missing.
The binary regression applied to the data on the survival of 50–100 mm and 150–170 mm penshells (five 3-day trials) in the sandflat (where our 10-day predation trials indicated survival was much less than in the seagrass bed) indicated a difference in survival between these two size-groups (Wald = 11.53, df = 1, P = 0.001) and a constant rate of loss in the different 3-day periods (Wald = 0.42, df = 1, P = 0.52). There was 6% survival (SD = 26) for 50–100 mm penshells, compared to 93% (SD = 26) for 150–170 mm individuals.
Sedimentation in seagrass beds and sandflats
Most materials collected in the sediment traps deployed at Trudillé were likely retained, as the tinted formalin that had been placed in the traps was clearly visible. At Trudillé, we found 2 penshells (~3 mm) in one trap in the seagrass bed, which represented <2% of the bivalves collected by all the traps. Although the mean number of all bivalves collected in traps was greater in the seagrass bed (9.6 bivalves/trap, SD = 5.3) than the sandflat (5.6 bivalves/trap, SD = 4.4), the difference was not significant (t 0.05(2)14 = −1.63 P = 0.13). The percentage of silt in the traps was ~3-fold greater in the seagrass bed than in the sandflat (t 0.05(2)14 = −7.25 P < 0.001). The tint was not visible in any of the traps at Cabo Rojo (after Hurricane Dennis) and the materials that filled most of the traps were decaying. The traps at Cabo Rojo provided a comparison of bottom stability between the sandflat and seagrass bed at this location. Traps in the sandflat had been unearthed on average by 38% of their initial burial depth. In contrast, in the seagrass bed, two of the traps were undisturbed and one was buried under <1 cm of sediment. We suspect the remaining 5 traps, which were not found, were also slightly buried.
Our various measurements of food availability did not indicate a difference between seagrass beds and adjacent sandflats. The materials collected in the sediment traps at Trudillé provided an index of potential food materials for penshells in the two habitats. Although the dry mass of organic matter per trap varied from 0.38 g (SD = 0.23) for the sandflat to 0.18 g (SD = 0.14) for the seagrass bed, the difference was not significant (t 0.05(2)11.7 = 2.1 P = 0.06).
In our analysis of the 3.8-l water samples taken just above seagrass beds and sandflats, there were no differences in the dry mass of plankton-sized materials (i.e. particulate matter measuring 0.45–263 µm) between the two habitats at Trudillé (t 0.05(2)1.19 = 0.21 P = 0.86) and Cabo Rojo (t 0.05(2)12 = 0.21 P = 0.31). Although we collected water samples at different times for each location (and amounts of material in the water would be expected to vary at different times), no differences in the dry mass of plankton-sized materials with either habitat or location were observed when we proceeded with a nested ANOVA (Table 3). The mean values were 8.54·10−3 g l−1 for seagrass beds (SD = 4.11·10−4) and 9.70·10−3 g l−1 for sandflats (SD = 3.27·10−3).
Table 3. Results of a nested ANOVA applied to data on the mass of plankton-sized materials (i.e. particulate matter measuring 0.45–263 µm) in 3.8-l water samples from seagrass and sandflat habitats at locations Trudillé (sampled 26–27 May 2005) and Cabo Rojo (sampled 22 April, 22 May and 31 August 2005). Dependent variable: dry mass of plankton-sized materials.
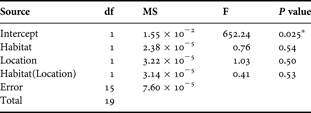
*, significant difference (Levene's test, P = 0.13).
Growth
The penshell growth data were best described by an exponential growth curve (r 2 = 0.63; Figure 8). Small penshells grew rapidly (up to 2.2 mm·d−1) but the growth rate dropped markedly at ~150 mm in hinge length (the beginning of the asymptote in growth). Small penshells that had been maintained for three 9-day trials in the seagrass bed and adjacent sandflat at Cabo Rojo showed no difference in growth rate with habitat or period (Table 4). The mean growth rate was 1.2 mm·d−1 (SD = 0.66), and varied with initial length (F1,12 = 7.83 P = 0.02), being greater for smaller individuals.

Fig. 8. Relation of daily increase in hinge length to initial hinge length as recorded for the penshell Pinna carnea during various periods between March to June 2002 and May to August 2005 in south-western Dominican Republic. An exponential curve best fits the data points.
Table 4. Results of an ANCOVA applied to data on the growth of the penshell Pinna carnea in seagrass and sandflat habitats at Cabo Rojo (3 trials conducted between 23 August and 19 September 2005). Dependent variable: growth rate.

*, significant difference (Levene's test, P = 0.43).
a, covariate.
b, corrected total, adjusted r 2
DISCUSSION
Population patterns
Numbers of Pinna carnea encountered in seagrass beds in the Dominican Republic were low and large individuals predominated. Low densities and large mean sizes have also been reported for 7 other penshell species in seagrass beds in Malaysia (Idris et al., Reference Idris, Arshad, Bujang, Daud and Ghaffar2008a, Reference Idris, Arshad, Bujang, Ghaffar and Daudb). A predominance of large individuals is further reported for Pinna nobilis in seagrass beds in the Mediterranean (García-March et al., Reference García-March, García-Carrascosa and Peña2002, Reference García-March, García-Carrascosa, Peña Cantero and Wang2006) and for Pinna bicolor in habitats absent of seagrass in Australia (Butler & Brewster, Reference Butler and Brewster1979). The latter penshell occurred at much higher densities than P. carnea.
Settlement
Penshells are not restricted to seagrass beds because of substratum preferences at the time of larval settlement. Our trials with five substrata showed similar numbers of recruits for each treatment. This suggests that competent larvae do not actively select a specific substratum, but likely settle wherever they can attach byssal filaments (including on the offshore moorings where we collected penshells for our experiments on penshell survival). In contrast, these same trials indicated a substratum preference for larvae of the egg cockle Laevicardium laevigatum, as recruits settled more on collectors that contained sand but no roots (i.e. the treatments ‘only sand’ and ‘seagrass blades’). Laevicardium laevigatum were completely buried in the sand or wedged into spaces in the scouring pad treatment. There were markedly reduced numbers of L. laevigatum in the treatments with roots and in the scouring pad treatment (where sand was absent). This pattern suggests L. laevigatum larvae avoid roots. They may have difficulty burrowing in sand held together with roots. Although most studies focus on cues that trigger settlement (i.e. positive cues) (see review by Rodriguez et al., Reference Rodriguez, Ojeda and Inestrosa1993), Woodin (Reference Woodin1991) hypothesizes that inhibitors or negative cues should be as important as positive ones. Our data suggest that larvae of L. laevigatum avoid a specific substratum, thus providing an example supporting Woodin's hypothesis.
Our comparisons of numbers of penshells settling on a common substratum (scouring pad collectors in cages) suspended ~1 m above contrasting habitats indicated no difference in settlement intensity between seagrass and sandflat habitats, or in comparison with a rocky–coral–sponge habitat (all at ~6 m in depth). Although we recorded markedly greater settlement above a seagrass bed in which the brown drift alga Lobophora variegata was abundant, the seagrass bed with L. variegata also differed from the other habitats as it was deeper, more exposed (being off a headland) and subjected to stronger tidal currents. Thus, the greater settlement in this habitat may have been related to depth and current rather than to the presence of L. variegata (which occasionally occurs attached to P. carnea shells). The heavy settlement of penshells that we observed on offshore moorings was also in a deeper open area with stronger currents.
Mortality from predation
Predation is likely a major factor determining the abundance and distribution of penshells. Our 140-day predation trials with caged and uncaged scouring pad collectors indicated that predation of recently settled penshells on collectors suspended ~1 m above the bottom was high (84%). Unfortunately, we did not obtain a measure of predation intensity on the bottom as all of the caged collectors on the bottom were destroyed by Hurricane Dennis. The fact that there was only 1 penshell recruit (in seagrass) in the 19 remaining uncaged collectors on the bottom could indicate high predation intensity or very low settlement. Our predation trials with older individuals transplanted to plots indicated that losses differed between seagrass and sandflat habitats. For penshell transplants measuring 10 to 110 mm the loss was >4-fold greater in the sandflat than the seagrass bed in our 1-day trials, and 27-fold greater in our 10-day trials. The field trials indicated that predation decreases in the seagrass bed as penshells increase in size. In both the 1-day and 10-day trials, most penshells of all sizes in the uncaged plots in the sandflat were found as shell remains or went missing in the first 24 hours. The loss of new recruits likely acts as a recruitment bottleneck for the population as described for numerous other benthic invertebrates (Underwood & Denley, Reference Underwood, Denley, Strong, Simberloff, Abele and Thistle1984; Gaines & Roughgarden, Reference Gaines and Roughgarden1985; Smith & Herrnkind, Reference Smith and Herrnkind1991).
Interestingly, in our 1-day and 10-day predation trials in the sandflat, the penshells that survived the longest in uncaged plots were the smallest individuals (10–30 mm). Possibly the small surviving penshells on the sandflat were less visible to some large predators found in this habitat. These small penshells were translucent in contrast to the larger penshells that were much darker and likely more visible on the sand. Alternatively, predators of small penshells may be less abundant in sandflats than in seagrass beds because they themselves are vulnerable to higher-order predators. We occasionally observed small lizardfish and crustaceans inside penshell cages in the seagrass bed, but never inside cages in the sandflat. In our 10-day predation trials, ~12% of small penshells in cages in the seagrass bed were found as shell remains or went missing, whereas all small penshells in cages in the sandflat were found alive. This suggested that small predators (<12-mm2 cage mesh) that prey on small penshells are less abundant in sandflats than seagrass beds.
It is likely that the impact of predators varies between seagrass beds and sandflats because of the differing numbers, types and activities of the predators found in each habitat. For example, the adult seastar Oreaster reticulates, an opportunistic omnivore known to consume bivalves (Scheibling, Reference Scheibling1982), was rarely encountered in seagrass beds but frequently occurred in some adjacent sandflats (0.32 seastars·m−2, SD = 0.06; S. Aucoin, unpublished data).
Faunal abundance and diversity are greater in seagrass beds than in unvegetated areas (Heck & Wetstone, Reference Heck and Wetstone1977; Orth, Reference Orth, Coull and Belle1977; Peterson, Reference Peterson and Livingstone1979; Heck & Orth, Reference Heck, Orth and Kennedy1980; Stoner, Reference Stoner1980). Many benthic predators stay inside seagrass beds during daylight and move to sandflats during the night (Summerson & Peterson, Reference Summerson and Peterson1984; Wielderholm, Reference Wielderholm1987). We observed several goatfish and wrasse species foraging in seagrass beds during the day and in sandflats at night. Although such predators may prey on penshells in both habitats, penshells appear to be slightly less vulnerable in seagrass beds than in sandflats. The seagrass likely provides camouflage against visually oriented predators. Seagrass cover provides protection from foraging fish for a variety of small invertebrates (see reviews in Orth et al., Reference Orth, Heck and Van Montfrans1984; Heck & Crowder, Reference Heck, Crowder, Bell, McCoy and Mushinsky1991). In reviewing the literature on early post-settlement mortality of benthic invertebrates, Hunt & Schiebling (1997) emphasize that predation intensity is generally reduced in vegetation and other structurally complex habitats. Their review mainly covers sedentary and infaunal species. The reviews by Orth et al. (Reference Orth, Heck and Van Montfrans1984) and Worthington et al. (Reference Worthington, Ferrell, McNeill and Bell1992) report that there is no clear relationship between predation intensity and the density of seagrass cover for mobile epibenthic species. The latter was also the case for P. carnea, which is sedentary and partly buried. Although penshell survival was clearly enhanced in seagrass beds, a linear regression analysis applied to the 2002 survey data (quadrats taken along 3–4 transects conducted at five locations) indicated no correlation between penshell abundance and estimates of seagrass dry biomass as a proxy of seagrass density (F1,20 = 0.01 P = 0.86; values ranged from 52 to 206 g/m2; A. Tewfik, unpublished data). This is possibly because predation intensity only drops once a threshold seagrass density is attained, as hypothesized by Nelson (Reference Nelson1979).
Habitat stability
Why penshells are found in seagrass beds and not in sandflats may also be related to the greater long-term stability of the seafloor in seagrass habitats, given that seagrass roots and associated algal rhizoids consolidate the bottom sediment. Our observations at Cabo Rojo before and after Hurricane Dennis showed that seagrass beds were less affected by disturbance than sandflats. For example, all sediment traps in the sandflat, but not in the seagrass beds, had been largely unearthed by the hurricane. Moreover, all penshells that had been transplanted in caged and uncaged plots to the sandflat went missing, whereas surviving penshells remained in both caged and uncaged plots in the seagrass bed. Hurricanes and tropical storms frequently affect offshore sites in the Dominican Republic, particularly along the southern coast of the island (Geraldes, Reference Geraldes and Cortés2003). If penshells were to escape predators in the sandflat they would likely later be removed by perturbations caused by hurricanes and storms.
An increase in sediment stability in seagrass beds has been shown to increase the density of a number of benthic species (Orth, Reference Orth, Coull and Belle1977). The differences between penshell populations at Trudillé and Cabo Rojo, specifically the higher density and greater proportion of small individuals at Trudillé, may also be related to the greater stability of the seafloor, due to the increased consolidation of the sediments. Several parameters indicated greater bottom stability at Trudillé than at Cabo Rojo: (1) the total mass of consolidated sediment (with roots and algal rhizoids) was >5-fold greater; (2) the mass of large loose sediment (>3.5-mm) was 3-fold greater; and (3) the above-ground algal biomass was ~50-fold greater. Calcareous algae (Halimeda spp.) accounted for 96% of algal biomass, but only slightly contributed to above-ground cover. Decaying calcareous algae likely formed most of the coarse carbonate sands at Trudillé (see Scullion Littler et al., Reference Scullion Littler, Littler, Bucher and Norris1989). The more consolidated sediment and coarser grain size at Trudillé appeared to better anchor penshells (see Young, Reference Young1983). This was evident when we collected the penshells, as it was more difficult to unearth penshells at Trudillé than at Cabo Rojo.
Coarse sands and seagrass roots have been shown to provide a barrier against digging crabs and whelks (Reise, Reference Reise, Keegan, Ceidigh and Broaden1976; Peterson, Reference Peterson1982; see references in Orth et al., Reference Orth, Heck and Van Montfrans1984). The more consolidated sediment at Trudillé may also provide protection from some predators. For example, the red-heart urchin Meoma ventricosa was found in seagrass beds at Cabo Rojo, but not in seagrass beds examined at Trudillé. Trials in which red-heart urchins were maintained in caged plots with penshells on the less consolidated sediments at Cabo Rojo showed that the urchins could readily unearth small penshells (Aucoin, Reference Aucoin2008). It is possible this large urchin was absent in our study area at Trudillé because the roots, algal rhizoids, and consolidated sediment at this site would prevent it from plowing through the sediments to feed.
Sedimentation
Particulate organic matter from resuspended sediment can be a valuable food source for some suspension feeders (Grant et al., Reference Grant, Enright and Griswold1990; Navarro et al., Reference Navarro, Iglesias and Ortega1992), but elevated levels of resuspended sediment can be deleterious and limit where animals can live, as reported for the penshell Atrina zelandica (Norkko et al., Reference Norkko, Hewitt, Thrush and Funnell2001; Ellis et al., Reference Ellis, Cummings, Hewitt, Thrush and Norkko2002). Increasing concentrations of fine sediment such as silt or clay can have detrimental effects on numerous filter-feeding invertebrates, including bivalves (Peddicord, Reference Peddicord1977; Robinson et al., Reference Robinson, Wehling and Morse1984; Rogers, Reference Rogers1990; Shumway et al., Reference Shumway, Frank, Ewart and Ward2003). Our sediment traps showed that silt levels were ~3-fold greater in the seagrass habitat than in the adjacent sandflat. It is possible the dead-standing penshells, which were upright without showing signs of predatory attacks, were individuals that had succumbed to the negative effects of silt. These dead individuals were only found in seagrass beds. In the seagrass, we replaced 13% of the penshells in the 10-day predation trials (during daily inspection of the plots) and ~27% of the penshells in the growth experiments were found as dead-standing individuals. Food conditions (POM and potential plankton) were similar in seagrass beds and adjacent sandflats, and thus could not have accounted for the dead-standing individuals in seagrass. During transect surveys, we often found silt in the gaping valves of old dead-standing penshells. We also found dead-standing penshells carrying silt among the individuals transplanted to the seagrass bed for our 100-day predation trials. However, we did not inspect for silt build-up in the gills of individuals during the 10-day predation trials and the growth experiments.
Growth and refuge in size
Growth rates of young Pinna carnea are among the highest known for molluscs (Castellanos et al., Reference Castellanos, Urban and Borrero1997; Garcia-Valencia et al., Reference Garcia-Valencia, Urban and Borrero1997; Narváez et al., Reference Narváez, Lodeiros, Freites, Nunez, Pico and Prieto2000). Other species of penshells also exhibit rapid early growth (Cendejas et al., Reference Cendejas, Carvallo and Juarez1985; Butler, Reference Butler1987; Wu & Shin, Reference Wu and Shin1998). Our measurements of penshells transplanted to a seagrass bed and adjacent sandflat indicated no difference in growth rates between these habitats. The approximate growth curve we calculated for P. carnea showed a rapid decrease in growth rate as individuals attained ~150 mm in shell hinge length. A reduction in growth at a similar size (155 mm) was also reported for P. carnea in suspended culture in Venezuela (Narváez et al., Reference Narváez, Lodeiros, Freites, Nunez, Pico and Prieto2000).
We observed that large penshells attain a refuge in size, as reported for other bivalves (Seed & Brown, Reference Seed and Brown1978; Commito, Reference Commito1982; Arsenault & Himmelman, Reference Arsenault and Himmelman1996). As small benthic organisms are generally vulnerable to a larger suite of predators than large individuals, survival often increases with increasing size (Paine, Reference Paine1976; Peterson & Wroblewski, Reference Peterson and Wroblewski1984; Seed, Reference Seed and Dame1993; Gosselin & Qian, Reference Gosselin and Qian1997). This is the case for P. carnea, as our field trials showed that the probability of survival increased with size. We hypothesize that the extremely rapid growth of small penshells represents an evolutionary adaptation for attaining a size that is less vulnerable to predators as soon as possible (a response to the intense predation on small individuals). In the sandflat where mortality was highest, the survival rate of transplanted penshells increased from 6% for 50–100 mm individuals to 93% for 150–170 mm individuals. We also observed that only larger penshells survived the passing of Hurricane Dennis. Thus, the extremely rapid growth rate of small penshells could at the same time reflect a strategy for limiting vulnerability to environmental perturbations.
The predominance of large individuals in P. carnea populations in the Dominican Republic likely represents the merging of successive recruitment events caused by the decrease in growth rate at ~150 mm. Butler (Reference Butler1987) suggests a similar scenario for Pinna bicolor in Australia. The size–structure for P. carnea provides little information about the age–structure of the population. We suggest using measurements of shell thickness as well as hinge length in future studies, as thickness may be a better indicator of age. Measurements of shell thickness (at the centre of the posterior adductor muscle scar) indicated that growth in thickness increased coincident with the decrease in growth in length at ~150 mm (Aucoin & Himmelman, Reference Aucoin and Himmelman2010). Considering our growth curve and observations on the largest recruits at Trudillé (111 mm in hinge length after 79 days) and Cabo Rojo (162 mm after 135 days), we estimate it would take at least 4–5 years to attain 270 mm, the maximum size observed during our fieldwork. Other species of penshells in the Mediterranean and tropical regions have been estimated to live between 8 and 17 years (Butler, Reference Butler1987; Wu & Shin, Reference Wu and Shin1998; Richardson et al., Reference Richardson, Kennedy, Duarte, Kennedy and Proud1999; Rodriguez-Jaramillo et al., Reference Rodriguez-Jaramillo, Maeda-Martinez, Valdez, Reynoso-Granados, Monsalvo-Spencer, Prado-Ancona, Cardoza-Velasco, Robles-Mungaray and Sicard2001; Garcia-March et al., 2002).
Further research
To provide further understanding of the population dynamics of the penshell Pinna carnea, future studies should focus on quantifying recruitment over a longer period with frequent sampling to identify recruitment pulses. Studies are needed to quantify variations in recruitment in different habitats and locations as well as to document variations in different seasons. Our penshell survival experiments showed that mortality is extremely high in both sandflats and seagrass beds and that smaller penshells are most vulnerable. However, these trials were only conducted at Cabo Rojo, as we had a limited supply of small penshells (from a few offshore moorings). It is critical that similar trials be run at other locations to ensure that this pattern is not specific to Cabo Rojo. To elucidate the causes of mortality, the most productive approach would likely be videofilming of penshells transplanted to different habitats and locations. This would allow identification of the predators and provide information on their activity rhythms.
ACKNOWLEDGEMENTS
We are grateful to A. Tewfik for permitting us to take part in the field surveys in 2002 and to Grupo Jaragua, Cementos Andinos Inc., Proyecto Carey, the Acuario Nacional and the Secretaría de Estado del Medio Ambiente y Recursos Naturales (República Dominicana) for invaluable logistical support of the fieldwork. We also thank Y. Arias, Y. León, J. ‘Chapo’ Ledesma, J. ‘Ciguillo’ Ledesma, C. Gonzalez, ‘El Flaco’, E. Trupp, Å. Kestrup, and C. Dumont for their indispensable assistance. L. Johnson, K. Harper, C. Dumont and two anonymous referees provided advice that was helpful in improving the manuscript. This study was funded by an NSERC Discovery grant to J.H.H. and Québec-Océan provided financial support to S.A.