INTRODUCTION
Numerous endemic animals densely inhabit and create typical aggregations with specific distribution patterns around deep-sea hydrothermal vents (reviewed in Van Dover, Reference Van Dover2000). One of these animals, Shinkaia crosnieri, which is the sole member of the subfamily Shinkaiinae in the family Galatheidae (Baba & Williams, Reference Baba and Williams1998) aggregates to dense population around hydrothermal vent fields in the Okinawa Trough, Japan, and at methane seeps off south-east Taiwan (Chan et al., Reference Chan, Lee and Lee2000; Ohta & Kim, Reference Ohta and Kim2001; Liu et al., Reference Liu, Morita, Liao, Ku, Machiyama, Lin and Soh2008). In the crater of the Hatoma Knoll in the southern Okinawa Trough, thousands of S. crosnieri were observed to surround a hydrothermal vent in patches (Chan et al., Reference Chan, Lee and Lee2000; Tsuchida et al., Reference Tsuchida, Fujiwara and Fujikura2003). The aggregation of S. crosnieri studied here was distributed within a zone 0.2–2 m (4.0–6.2°C) from the active vent (301°C). More than 2 m from the vent, where the temperature was 3.0–3.7°C (ambient seawater 3.0°C), a dense bed of the deep-sea mussel Bathymodiolus platifrons and an aggregation of the vent shrimp Alvinocaris longirostris replaced the Shinkaia bed. This distribution pattern indicated that of all benthic animals at that location, S. crosnieri inhabited the sites nearest to active vents (Tsuchida et al., Reference Tsuchida, Fujiwara and Fujikura2003). Bathymodiolus platifrons is known to host endosymbionts (methanotrophic bacteria) in the epithelial cells of its gill tissue (Fujiwara et al., Reference Fujiwara, Takai, Uematsu, Tsuchida, Hunt and Hashimoto2000). The nutrient acquisition pattern of A. longirostris is not well known, but it was often observed to prey actively on other animals (unpublished). Distribution pattern of S. crosnieri which was close to the active vent, suggests the nutrition of the crab should be closely related to some symbiotic association rather than some predation.
Shinkaia crosnieri has strong, stout, sparse setae on the dorsal surface of the carapace, chelipeds, and walking legs. However, it has long, soft, dense plumose setae on the ventral surface of the body, such as the ventral surface of chelipeds and walking legs, the sternum, and lateral surface of the body (pterygostomian flap) (Baba & Williams, Reference Baba and Williams1998). This plumose pilosity on the ventral body distinguishes the Shinkaiinae from other Galatheidae like Munidopsinae and Galatheinae. Previously, we found a mass of filamentous microorganisms attached to the plumose setae on the ventral surfaces of the crabs and inferred that they were related to their feeding ecology (Miyake et al., Reference Miyake, Kitada, Tsuchida, Okuyama and Nakamura2007). These filamentous microorganisms were also common on the iron sulphide-coated scales of scaly-foot snails, the dorsal setae of Alvinella pompejana, the branchial chamber of Rimicaris exoculata, Kiwa hirsute and described as episymbiotic associations (Polz & Cavanaugh, Reference Polz and Cavanaugh1995; Cary et al., Reference Cary, Cottrell, Stein, Camacho and Desbruyères1997; Goffredi et al., Reference Goffredi, Warèn, Orphan, Van Dover and Vrijenhoek2004, Reference Goffredi, Jones, Erhlich, Springer and Vrijenhoek2008). To investigate the potential role of the epibiotic association between the filamentous microbes and the crab, we used various methods, such as microscopic observations of the epibionts with field-emission scanning electron microscopy (FE-SEM) and transmission electron microscopy (TEM), observation of crab feeding behaviour, cloning and fluorescence in situ hybridization (FISH) analysis to identify the epibionts, and fatty acid and isotope analysis to determine whether the epibionts are the nutrient source of the crab.
MATERIALS AND METHODS
Sample collection and video capture
Specimens of Shinkaia crosnieri were collected from the Hatoma Knoll in the Okinawa Trough using a suction sampler loaded on the submersible ‘Shinkai 2000’ from 17 May to 1 June 2000 (for microscopy, SE-SEM and TEM analyses), and the remotely operated vehicle (ROV) ‘Hyper-Dolphin’ from 20 April to 28 April 2005 (for the other analyses). Feeding behaviour of the galatheid crabs were recoded by the super harp TV camera loaded on the ‘Shinkai 2000’ and the high-definition TV camera loaded on ‘Hyper-Dolphin’. The sampling site was located in the southern crater of the Hatoma Knoll (24°51.3′N 123°50.6′E) at a depth of 1480 m, which is covered by a dense bed of S. crosnieri and exteriorly with Bathymodiolus platifrons and Alvinocaris longirostris aggregations, close to an active hydrothermal vent site with temperature higher than 300°C (Figure 1A). Collected specimens were preserved in a deep freezer (–80°C) until examination.
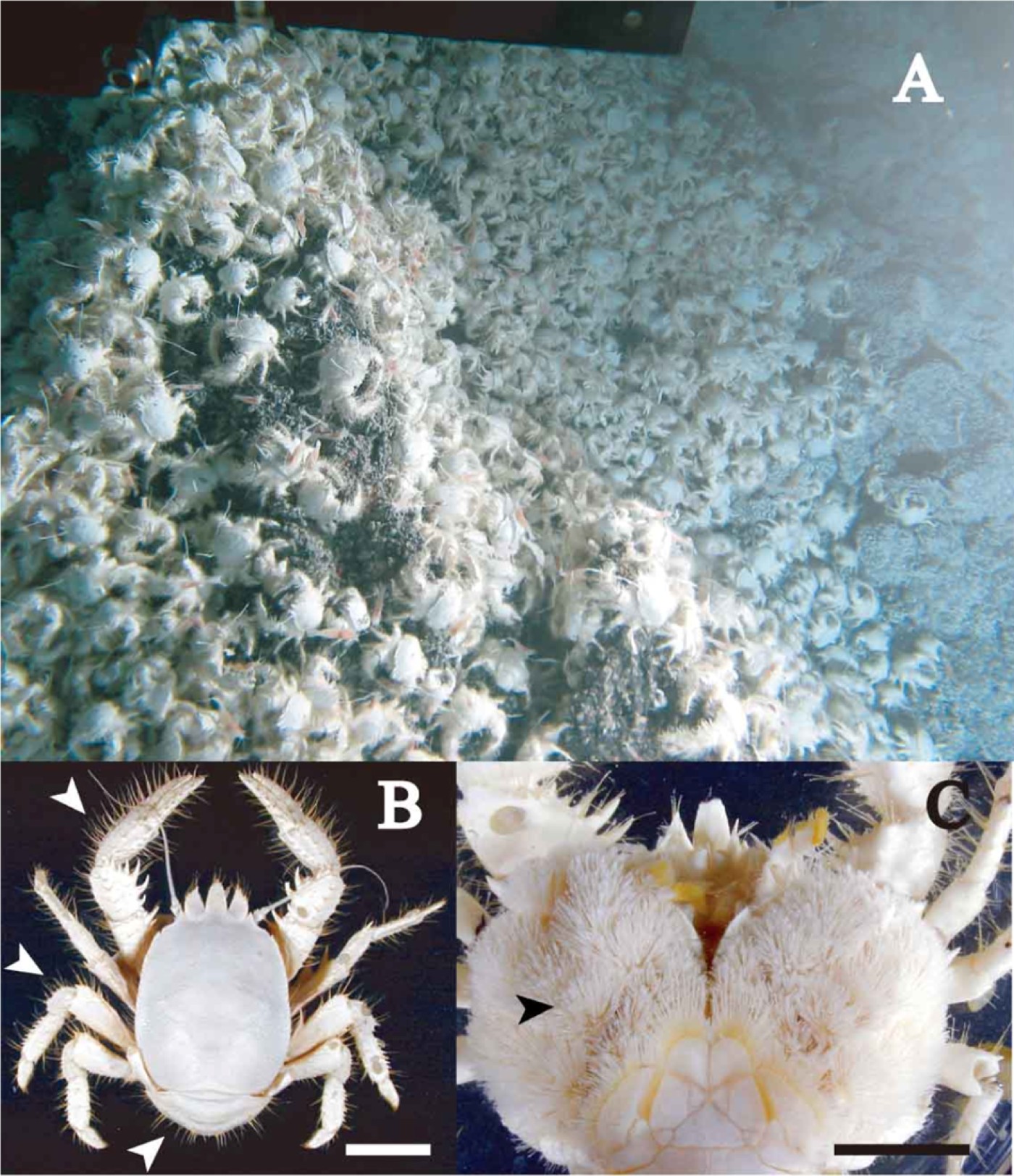
Fig. 1. In situ habitat of Shinkaia crosnieri at a depth of 1580 m on the Hatoma Knoll (photograph taken by the ROV ‘Hyper-Dolphin’) (A), and dorsal (B) and ventral (C) views of a specimen. Scale bars indicate 20 mm. Arrows: setae on the dorsal surface without epibionts in (B), and plumose setae on the ventral surface with epibionts in (C).
Electron microscopic observations
Ventral plumose setae dissected from three Shinkaia crosnieri individuals were fixed with 2.5% glutaraldehyde in 0.22 µm-filtered seawater for 24 hours at 4°C and preserved in filtered seawater with 10 mM sodium azide at 4°C. Samples were then washed in filtered seawater and postfixed with 2% osmium tetroxide in filtered seawater for 2 hours at 4°C. For FE-SEM observations, conductive staining was performed by incubation with 1% aqueous tannic acid (pH 6.8) for 1 hour at 4°C after setae had been rinsed with distilled water, and then the samples were washed with distilled water and treated with 1% aqueous osmium tetroxide for 1 hour at 4°C. The setae were dehydrated in a graded ethanol series and critical point-dried (JCPD-5; JEOL Ltd., Tokyo, Japan). The samples were coated with an osmium plasma coater (POC-3; Meiwa Shoji Co., Osaka, Japan) and observed under an FE-SEM (JSM-6700F; JEOL Ltd., Tokyo, Japan) at an acceleration voltage of 5 kV. For TEM observations, the postfixed setae were rinsed with distilled water and stained en bloc with 1% aqueous uranyl acetate for 2 hours at 4°C. After rinsing with distilled water, the samples were dehydrated in a graded ethanol series and embedded in Epon 812 resin (TAAB, Aldermaston, UK). Ultrathin sectioning was performed using an ultramicrotome (Reichert Ultracut S; Leica, Wetzlar, Germany). Ultrathin sections of the setae were stained with uranyl acetate and lead citrate and observed under the TEM (JEM-1210; JEOL Ltd., Tokyo, Japan) at an acceleration voltage of 80 kV.
DNA analysis
Total DNA was extracted from the dissected setae on the ventral body of three frozen (–80°C) crabs (numbers 4, 12 and 26) using the DNeasy Blood & Tissue Kit (Qiagen, Valencia, CA, USA). Using the extracted DNA as templates, 16S rRNA gene sequences were amplified through the polymerase chain reaction (PCR) using Ex Taq polymerase (TaKaRa, Tokyo, Japan) with the bacterial 16S rRNA gene-universal oligonucleotide primers Bac27F and 1492R (Lane, Reference Lane, Stackebrandt and Goodfellow1991). Thermal cycling was performed using the GeneAmp PCR System 2700 (Applied Biosystems, Foster City, CA, USA), with a preliminary denaturation at 96°C for 1 minute, followed by 35 cycles of denaturation at 96°C for 20 seconds, annealing at 55°C for 45 seconds, and elongation at 72°C for 2 minutes, and then final elongation at 72°C for 4 minutes. The amplified 16S rRNA gene-sequence products were cloned using the TA Cloning Kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. Ninety-six recombinant colonies were directly used as a template for the PCR using the Insert Check-Ready-Blue kit (Toyobo Co. Ltd., Osaka, Japan). The PCR products containing appropriately sized inserts (around 1500 base pair (bp)) were identified by 1.2% (w/v) agarose gel electrophoresis. The appropriately sized inserts were partially sequenced with the primer 519R (5′-GTATTACCGCGGCTGCTG-3′). Subsequent clones (548 bp) were used for preliminary phylogenetic analysis to distinguish unique and duplicate clones. Twenty-two representatives of the diversity were used as a template for PCR amplification using Bac27F and 1492R and sequenced directly with the ABI PRISM 3100 DNA Analyzer and the BigDye Terminator Cycle Sequencing Ready Reaction Kit, version 3.1 (PE Biosystems, Foster City, CA, USA). The sequences obtained in this study were deposited in the database of the DNA data bank of Japan (DDBJ) under accession numbers AB 440161–AB 440176 and compared with those available in databases using the Basic Local Alignment Search Tool (BLAST) network service to determine approximate phylogenetic affiliations. Sequences were manually aligned, and phylogenetic analyses were restricted to nucleotide positions that were unambiguously alignable in all sequences. Phylogenetic trees of partial 16S rRNA sequences for Epsilon- (1224 bp) and Gammaproteobacteria (1266 bp) were constructed using the maximum likelihood (ML) method with the program PhyML ver. 2.4.4 (Guindon et al., Reference Guindon, Lethiec, Duroux and Gascuel2005) with the GTR nucleic substitution matrix, eight rate categories, a BIONJ tree as a starting point. Bootstrap values were calculated with the same parameters for 500 replicates in ML using the same program and 1000 replicates in the neighbour-joining (NJ) method using CLUSTAL_X (Thompson et al., Reference Thompson, Gibson, Plewniak, Jeanmougin and Higgins1997).
FISH analysis
Ventral plumose setae were dissected from three Shinkaia crosnieri individuals and fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS, pH 7.4) for 2 hours at 4°C, then mounted on slides with gelatin coating and dehydrated in an ethanol series. Hybridization was conducted at 46°C in a solution containing 20 mM Tris–HCl (pH 7.4), 0.9 M NaCl, 0.1% sodium dodecyl sulphate, 30% formamide and 50 ng µl−1 of two oligonucleotide probes. The rRNA-targeted oligonucleotide probe GAM42a, which was known to detect the 23S rRNA of most Gammaproteobacteria, and a partial group of Betaproteobacteria (Manz et al., Reference Manz, Amann, Ludwig, Wagner and Schleifer1992; Amann & Fuchs, Reference Amann and Fuchs2008), labelled at the 5′ end with Alexa546 was used here. The other rRNA-targeted oligonucleotide probe Rim656 was designed to detect the 16S rRNA of the epibiont of Rimicaris exoculata (Polz & Cavanaugh, Reference Polz and Cavanaugh1995) and labelled at the 5′ end with Alexa488 and shortened in the present study (5′-CTTCCCCTCCCAGACTC-3′). After hybridization, each slide was washed at 48°C for 15 minutes in a solution without any probes and formamide adjusted to the same concentration with NaCl (Lathe, Reference Lathe1985) and then stained with 0.4 mg ml−1 of 4′,6-diamidino-2-phenylindole (DAPI). The slides were examined using a confocal laser-scanning microscope FV5000 (Olympus, Tokyo, Japan). A negative control probe, in which a two-base mismatch was introduced in the middle of Rim656 (5′-CTTCCCCTAACAGACTC-3′), was used to determine whether non-specific labelling occurred.
Bulk carbon, nitrogen and sulphur isotope analyses
The abdominal muscle and filamentous epibionts of two specimens were dissected, and the dissected tissues were lyophilized. A small portion of each lyophilized tissue sample was powdered and then acid-fumed for 6 hours. The remaining untreated lyophilized tissue was stored at –80°C for fatty acid extraction. Preparation for sulphur isotopic measurement followed the procedures described in previous studies (Mizota et al., Reference Mizota, Shimoyama and Yamanaka1999; Yamanaka et al., Reference Yamanaka, Mizota, Maki, Fujikura and Chiba2000). The dissected soft tissues of each specimen were centrifugally washed repeatedly with 0.1 M LiCl solution to eliminate seawater sulphates and then freeze-dried. The carbon and nitrogen isotopic compositions of the muscle tissues of crabs were analysed using the DELTAplus Advantage mass spectrometer connected to an elemental analyser (EA1112) through the ConFlo III interface (Thermo Electron Corp., Bremen, Germany). For sulphur analysis, some parts of the freeze-dried samples were pulverized and then combusted in a Parr bomb #1108, a stainless steel vessel filled with oxygen gas under high pressure (30 kg cm−2) and a few millilitres of distilled water. After combustion, organic sulphur in the dried samples was completely converted into sulphurous acid gas and sulphate, and the sulphur was trapped as sulphate ion in distilled water in the vessel. The resulting sulphate dissolved in the distilled water was recovered as BaSO4 precipitate by adding 0.5 M BaCl solution. The recovered BaSO4 was converted using the procedure of Yanagisawa & Sakai (Reference Yanagisawa and Sakai1983) into SO2 gas as follows: an aliquot (~10 mg) of the dry BaSO4 was mixed with V2O5-SiO2 (1:1 by weight, total 200 mg) oxidant. The mixture was gradually heated to 950°C in a quartz tube under a vacuum. Liberated SO2 was cryogenically purified and then introduced into a gas-source mass spectrometer (VG SIRA 10, VG Isogas Ltd, UK).
All values are shown as δ13C, δ15N and δ34S notations, per mil variation relative to V-PDB air dinitrogen and V-CDT, respectively. The analytical precision for δ13C, δ15N and δ34S measurements was greater than ±0.2‰ in the present analysis.
Analysis of fatty acid methyl ester profiles
The method described by Komagata & Suzuki (Reference Komagata and Suzuki1987) was used for the extraction of cellular fatty acids. Approximately 20 mg of muscle tissues of crabs was incubated in 1 ml of anhydrous methanolic hydrochloric acid at 100°C for 3 hours. After the addition of 1 ml of deionized, distilled water (DDW) to the cooled aliquots, the fatty acid methyl esters (FAMEs) were extracted three times with 3 ml of n-hexane. The n-hexane fractions were washed with an equal volume of DDW and dehydrated with anhydrous Na2SO4. The concentrated FAMEs were stored at –20°C for subsequent carbon isotopic analyses.
The identities of the FAMEs were determined by comparison of the retention times and spectra with Supelco 37 Component FAME Mix for the external standards and C19 saturated fatty acid (19:0) for the internal standard (Supelco Inc., Bellefonte, PA, USA) in gas chromatography–mass spectrometry (GC-MS) using a Shimadzu GC-MS system (Shimadzu, Kyoto, Japan). The oven temperature was set at 140°C for 3 minutes and then increased to 250°C at the rate of 4°C min−1 with He at a constant flow of 1.1 ml min−1 through the DB-5MS column (30 m × 0.25 m × 0.25 mm; J&W Scientific, Folsom, CA, USA). The standard nomenclature for fatty acids was used: fatty acids are designated X:Y, where X is the number of carbon atoms, and Y is the number of double bonds.
RESULTS
Microscopic observation of filamentous bacteria attached to setae on the ventral surface of crabs
A typical Shinkaia crosnieri specimen had strong, stout, sparse setae without epibionts on its dorsal surface (Figure 1B), while it had dense plumose setae with numerous milky-white epibionts on the ventral surface of the walking legs, chelipeds, thoracic sternum and lateral carapace (Figure 1C). FE-SEM images showed numerous segmented filaments attached to the whole surface of setae (Figure 2A). Larger filaments colonized directly on the setae of crabs, and smaller filaments were attached to the surface of larger filaments (Figure 2B). These bundles of filaments were bacteria-like cells of various lengths of from 1 to more than 500 µm and cell diameter of 0.5 to 3 µm (Figure 2B). Sections of typical larger filaments were observed in TEM image (Figure 2C), which showed that the filaments had a segmented structure composed of cells covered by a sheath-like membrane. The diameter of the cells varied from 1.6 to 2.1 µm, and the length varied from 0.6 to 1.6 µm, as shown in Figure 2C. Filamentous bacteria completely covered the surface of setae and attached directly to the setae cuticle (Figure 2D). This demonstrates that the filamentous bacteria grew on the surface of setae and were not hanging from or hooked to setae.

Fig. 2. Images of filamentous bacteria attached to the ventral setae of the galatheid crab Shinkaia crosnieri using a field-emission scanning electron microscope (A & B), and transmission electron microscope (C 7 D). (A) Each seta (arrows) of crab covered by bacteria-like filaments; (B) magnified image of (A) indicates various sizes of filaments were observed on setae of crab; (C) cross-section of the bacteria-like filaments (fb); and (D) the chitinous layer of the setae (st) attached by the bacteria-like filaments. Scale bars: A, 100 µm; B, 5 µm; C, D, 1 µm.
Feeding behaviour
Video images from the two expeditions of the submersible ‘Shinkai 2000’ and the ROV ‘Hyper-Dolphin’ (total 14 dives), and observations in aquaria of Shinkaia crosnieri did not provide evidence for any predatory behaviour or active motion toward other animals. The only possible feeding behaviour observed in the video images captured by the submersible and ROV was ‘combing’. Shinkaia crosnieri was often seen combing out its ventral setae using the third maxilliped with a dense patch of strong setae on the tip. After the combing behaviour, the crab brought the third maxilliped to its mandible. This combing was also thought to be a form of grooming to furnish the filamentous bacteria with reduced chemicals from hydrothermal vents. Another appendage, the fifth pereopod, is slender and flexible with a small patch of strong setae on the tip and was also used for combing and scraping bacteria-like filaments toward the mouth.
Phylogenetic analysis of microbial flora on the ventral setae of crabs
The 16S rRNA gene was sequenced for 22 representatives among 81 positive clones from bacteria-like filaments on the ventral setae of Shinkaia crosnieri. These sequences were clustered within three groups: Epsilonproteobacteria including 60 clones (74% of the total 81 clones); Gammaproteobacteria including 16 clones (20%); and Bacteroidetes including 5 clones (6%). The Epsilonproteobacteria were thus the dominant group among bacterial clones collected from the ventral setae of the crabs. Numbers of clones for each specimen (Epsilon/Gamma/Bacteroidetes) were 20/6/2 in specimen No. 4, 21/4/2 in specimen No. 12 and 19/6/1 in specimen No. 26, respectively, indicating low variation among specimens.
Within the clade of Epsilonproteobacteria, the clones were closely related to the epibiotic bacteria found in invertebrates from deep-sea hydrothermal vent fields. As shown in Figure 3A, 11 phylotypes representing 60 clones were related to the epibionts of the polychaete worm Alvinella pompejana, scaly-foot snail, stalked barnacle Vulcanolepas osheai, alvinocaridid shrimp Rimicaris exoculata, and kiwaid crab, Kiwa hirsuta, and numerous uncultured clones from hydrothermal vent environments like chimneys and sediments in the Okinawa Trough, mid-Atlantic, eastern Pacific, and Kermadec Arc (Polz & Cavanaugh Reference Polz and Cavanaugh1995; López-García et al., Reference López-García, Gaill and Moreira2002, Reference López-García, Duperron, Philippot, Foriel, Susini and Moreira2003; Dhillon et al., Reference Dhillon, Teske, Dillon, Stahl and Sogin2003; Goffredi et al., Reference Goffredi, Warèn, Orphan, Van Dover and Vrijenhoek2004, Reference Goffredi, Jones, Erhlich, Springer and Vrijenhoek2008; Inagaki et al., Reference Inagaki, Takai, Nealson and Horikoshi2004; Kormas et al., Reference Kormas, Tivey, Von Damm and Teske2006; Tokuda et al., Reference Tokuda, Yamada, Nakano, Arita and Yamasaki2008).

Fig. 3. Phylogenetic trees of the Epsilonproteobacteria group (A) and Gammaproteobacteria group (B) related to the epibiont clones on the setae of Shinkaia crosnieri based on partial 16S rRNA gene sequences (1224 and 1266 bp) with an outgroup of Alphaproteobacteria. This tree is constructed using maximum likelihood (ML) analysis with the bootstrap values greater than 50% from the ML (first value) and neighbour-joining (second value) methods obtained by 500 and 1000 replicate samplings, respectively. Numbers in parentheses indicate the number of clones represented by each phylotype. Arrows indicate phylotypes detected using a probe, Rim656, in fluorescence in situ hybridization (FISH) analysis.
The Gammaproteobacteria were the second most dominant group of clones. As shown in Figure 3B, five phylotypes representing 16 clones were closely related to epibionts on vent-associated invertebrates such as the scaly-foot snail, stalked barnacle Vulcanolepas osheai, and kiwaid crab, Kiwa hirsuta, along with some uncultured clones from hydrothermal vent environments on the Mid-Atlantic Ridge (Goffredi et al., Reference Goffredi, Warèn, Orphan, Van Dover and Vrijenhoek2004, Reference Goffredi, Jones, Erhlich, Springer and Vrijenhoek2008; Brazelton et al., Reference Brazelton, Schrenk, Kelley and Baross2006; Suzuki et al., Reference Suzuki, Suzuki, Tsuchida, Takai, Horikoshi, Southward, Newman and Yamaguchi2009), and also, Leucothrix mucor, which is a representative of filamentous bacteria that often form colonies on the eggs and pleopods of benthic crustaceans (Johnson et al., Reference Johnson, Sieburth, Sastry, Arnold and Doty1971), supported by high bootstrap values and high sequence similarities (>91.0%).
FISH analysis of filamentous bacteria
FISH analysis was conducted to identify the distribution of the dominant bacteria in the microbial flora on setae. Numerous filamentous bacteria entangled thickly in the crab setae were all stained with DAPI (Figure 4A). Using the probe Rim656 for members of the Epsilonproteobacteria clustering with the episymbiont of Rimicaris exoculata, we identified a dominant group of bacteria which covered the setae of the crab specimens. However, other members of the Epsilonproteobacteria present in the clone library, and branching with uncultured clones from the gill filaments of Alvinocaris longirostris, were not stained with this probe and could not be further studied here. Gammaproteobacteria (20% of the clones in the clone library) were stained with the probe GAM42a, which however is not fully specific and may also include Betaproteobacteria (Figure 4B). The FISH analysis confirmed that Gammaproteobacteria were not dominant, but regularly appeared around the setae. Five clones of Bacteroidetes did not react with either probe in this study.

Fig. 4. Fluorescence in situ hybridization (FISH) photographs of epibionts on the ventral setae of Shinkaia crosnieri. (A) DNA staining of filamentous bacteria attached to the ventral setae of crab by DAPI; (B) Epsilonproteobacteria labelled by Rim656 with Alexa488 (green) and Gammaproteobacteria labelled by GAM42 with Alexa546 (red) occupied in the majority of filamentous bacteria. Scale bars indicate 30 µm.
Comparison of fatty acid profiles and carbon, nitrogen, and sulphur isotopic compositions between filamentous bacteria and galatheid crabs
The FAME profiles of Shinkaia crosnieri muscle indicated that total fatty acids were composed of 22.3% polyunsaturated fatty acids (PUFAs), 62.3% monounsaturated fatty acids (MUFAs), and 15.4% saturated fatty acids (SFAs), with high levels of monounsaturated 16:1 and 18:1 and saturated 16:0 and constant levels of polyunsaturated 18:3, 18:2, 20:5, and others (Figure 5). The total fatty acids of filamentous bacteria were composed of 37.8% PUFAs, 45.7% MUFAs, and 16.4% SFAs, with high levels of monounsaturated 16:1 and 18:1 and polyunsaturated 16:2 and 18:2 and constant levels of the other fatty acids 14:0, 14:1, 16:0, and 18:3.

Fig. 5. Fatty acid profiles of the crab muscle dissected from a walking leg (upper) and filamentous bacteria on the ventral setae (lower) from three specimens of Shinkaia crosnieri. Vertical bars indicate standard deviations.
The bulk carbon isotopic (δ13C) composition of Shinkaia crosnieri muscle (–21.4 and –22.2‰) was almost identical to that of filamentous bacteria (–21.1 and –21.4‰). The bulk nitrogen isotopic (δ15N) composition of S. crosnieri muscle (+2.0 and +2.2‰) was slightly higher than that of filamentous bacteria (–2.1 and –2.3‰). The sulphur isotopic composition of S. crosnieri muscle was δ34S = +8.3 (‰), as measured in a composite sample of two specimens. The δ34S values of the filamentous bacteria could not be measured because the sample amount was too small for the recovery of sufficient sulphur for isotope analysis.
DISCUSSION
FAME profiles of the galatheid crabs were not completely identical to those of the bacteria on their ventral setae. However, MUFAs 16:1 and 18:1 of this crab muscle were much higher than those of Munidopsis sp. without epibionts collected from non-vent area (4100 m depth) in the north-east Pacific (Drazen et al., Reference Drazen, Phleger, Guest and Nichols2008) and almost the same as the vent adult shrimp Rimicaris exoculata with episymbionts collected from vent site in the Mid-Atlantic Ridge (Pond et al., Reference Pond, Gebruk, Southward, Southward, Fallick, Bell and Sargent2000). These MUFAs are known to be characteristic of sulphur-oxidizing bacteria in H2S-rich marine habitats (Conway & Capuzzo, Reference Conway and Capuzzo1991; Conway et al., Reference Conway, Howes, McDowell Capuzzo, Turner and Cavanaugh1992; Pranal et al., Reference Pranal, Fiala-Médioni and Guezennec1996, Reference Pranal, Fiala-Médioni and Guezennec1997; Suzuki et al., Reference Suzuki, Sasaki, Suzuki, Nogi, Miwa, Takai, Nealson and Horikoshi2005b; Zhang et al., Reference Zhang, Huang, Cantu, Pancost, Brigmon, Lyons and Sassen2005) suggesting that Shinkaia crosnieri feeds on the bacteria related to sulphur-oxidizing.
Identical values of the carbon isotopic ratios and slightly higher values of the nitrogen isotopic ratio in the crab muscle than in bacteria also support the hypothesis that the crab relies on bacterial production (Conway & Capuzzo, Reference Conway and Capuzzo1991; Conway et al., Reference Conway, Howes, McDowell Capuzzo, Turner and Cavanaugh1992; Minagawa & Wada, Reference Minagawa and Wada1984; Pond et al., Reference Pond, Bell, Dixon, Fallick, Segonzac and Sargent1998, Reference Pond, Gebruk, Southward, Southward, Fallick, Bell and Sargent2000; Suzuki et al., Reference Suzuki, Sasaki, Suzuki, Nealson and Horikoshi2005a, Reference Suzuki, Sasaki, Suzuki, Nogi, Miwa, Takai, Nealson and Horikoshib). Additionally, the significantly lower δ34S value in the muscle than in common marine animals (δ34S values range from +15– + 20‰; Conway et al., Reference Conway, Kennicutt, Van Dover, Lajtha and Michener1994) was nearly identical to that of hydrothermal sulphide, for which δ34S values range from +8– + 12‰ (Yamanaka et al., Reference Yamanaka, Mizota, Ishibashi, Nakayama, Morimoto, Okamoto, Kosaka, Maki, Tsunogai, Fujikura, Tsuchida and Fujiwara2002). This also suggests that the crab relies on hydrogen sulphide as nutrition source via sulphur-oxidizing bacteria. The absence of predation behaviour, the regular combing motions, and the distribution of fatty acids and isotope signatures indicate that the crabs feed on vent bacteria, most likely their own epibionts.
Huge biomasses of filamentous bacteria were attached only to the ventral setae of Shinkaia crosnieri. This type of setation was not observed on any other surface of this crab or in other vent-associated crustaceans except the sole species of Kiwaidae in the Galatheoidea, Kiwa hirsuta (Macpherson et al., Reference Macpherson, Jones and Segonzac2005). Among other Galatheidae, the genus Munidopsis is a typical member of hydrothermal vent communities. It was reported that three species of Munidopsis associated with vents in the Okinawa Trough lacked a dense patch of plumose setae and filamentous bacteria on their ventral surfaces (Cubelio et al., Reference Cubelio, Tsuchida and Watanabe2007). Kiwa hirsuta which was found from the Pacific–Antarctic Ridge has a pair of chelipeds densely covered by long plumose setae with clusters of filamentous bacteria (Goffredi et al., Reference Goffredi, Jones, Erhlich, Springer and Vrijenhoek2008). The parapagurid hermit crab Paragiopagurus ventilatus has a patch of long plumose setae with filamentous bacteria on its ventral surface (Lemaitre, Reference Lemaitre2004). These long plumose setae might be suitable for growth of filamentous bacteria rather than surfaces of chitin and thick setae.
Some phylotypes of the epibionts on the crab are closely related to uncultured clones that are common in hydrothermal vent and seep environments (López-García et al., Reference López-García, Gaill and Moreira2002, Reference López-García, Duperron, Philippot, Foriel, Susini and Moreira2003; Dhillon et al., Reference Dhillon, Teske, Dillon, Stahl and Sogin2003; Inagaki et al., Reference Inagaki, Takai, Nealson and Horikoshi2004; Kormas et al., Reference Kormas, Tivey, Von Damm and Teske2006). These might be common to the setae of crabs and the vent environment, although major phylotypes of Gamma- and Epsilonproteobacteria detected in FISH might be related to a specific association because of their phylogenetic similarities to the episymbionts on the alvinocaridid shrimp Rimicaris exoculata, kiwaid crab, Kiwa hirsuta, and the scaly-foot snail (Polz & Cavanaugh, Reference Polz and Cavanaugh1995; Goffredi et al., Reference Goffredi, Warèn, Orphan, Van Dover and Vrijenhoek2004, Reference Goffredi, Jones, Erhlich, Springer and Vrijenhoek2008). In the present study, we did not have quantitative data on microflora in the field, and the epibiotic bacteria detected in Shinkaia crosnieri might be common in that environment, similar to the episymbionts on R. exoculata (Polz & Cavanaugh, Reference Polz and Cavanaugh1995). However, filamentous bacteria are obviously more abundant on the setae of crabs than on chimney walls and mounds. The advantages to bacteria of attaching to the setae of crabs might be gaining a substratum to grow on and transport to habitats providing access to reducing chemicals, inorganic and organic compounds, etc.
Rimicaris exoculata is very abundant on the chimney walls in vent sites at the Mid-Atlantic Ridge. These shrimp carry epibionts on the branchial chamber inside the carapace, dominated by single bacterial phylotype belonging to the Epsilonproteobacteria (Polz & Cavanaugh, Reference Polz and Cavanaugh1995). Also, Alvinella pompejana carry filamentous epibionts, which colonize the dorsal setae of the worms and are dominated by a few phylotypes of Epsilonproteobacteria (Haddad et al., Reference Haddad, Camacho, Durand and Cary1995; Cary et al., Reference Cary, Cottrell, Stein, Camacho and Desbruyères1997; Campbell et al., Reference Campbell, Jeanthon, Kostka, Luther and Cary2001). The ecological features of A. pompejana are a habitat inside tubes attached to chimney walls and tolerance to a wide range of high temperatures from 20 to 85°C (Gaill & Hunt, Reference Gaill and Hunt1991; Cary et al., Reference Cary, Cottrell, Stein, Camacho and Desbruyères1997). The preferred habitats of episymbiotic bacteria, such as the branchial chamber of R. exoculata and inside tubes of A. pompejana, might be a selective factor for other bacteria in that environment, so that a single or a few types of epibiotic bacteria belonging to Epsilonproteobacteria could exist in that habitat. However, the scaly-foot snail, an endemic species associated with hydrothermal vents on the Central Indian Ridge (Warèn et al., Reference Warèn, Bengtson, Goffredi and Van Dover2003), has the sole phylotype of endosymbiont belonging to the Gammaproteobacteria in its enlarged oesophageal gland and carries numerous filamentous episymbionts on its iron sulphide-coated scales. Its episymbionts are dominated by Epsilonproteobacteria with a minority of Deltaproteobacteria, Gammaproteobacteria and Cytophaga–Flavobacterium–Bacteroides groups (Goffredi et al., Reference Goffredi, Warèn, Orphan, Van Dover and Vrijenhoek2004). The stalked barnacle Vulcanolepas osheai is abundant in the hydrothermal vent fields on Brothers Seamount in the Kermadec Arc. The numerous filamentous bacteria attached to the cirri of this stalked barnacle are predominantly Epsilonproteobacteria, with a minority of Gammaproteobacteria that are thought to be episymbiotic (Suzuki et al., Reference Suzuki, Suzuki, Tsuchida, Takai, Horikoshi, Southward, Newman and Yamaguchi2009). Kiwaid crab, Kiwa hirsuta was reported from the hydrothermal vent field in the Pacific–Antarctic Ridge. It has dense setae on the chelipeds, walking legs, and the ventral surface of carapace with clusters of filamentous bacteria dominated by Epsilon- (56%) and Gammaproteobacteria (25%), Bacteroidetes (10%) and others (9%) (Goffredi et al., Reference Goffredi, Jones, Erhlich, Springer and Vrijenhoek2008). These episymbiotic bacteria are directly exposed to vent plumes with a high density of reducing chemicals. Epibiotic flora on the setae of Shinkaia crosnieri, are more diverse than those on R. exoculata and A. pompejana, because the setae on the ventral surface might be exposed to a vent environment similar to that of the scaly-foot snail, V. osheai, and K. hirsuta.
In the present study, we investigated six data sets, i.e. SEM and TEM observations, video images of feeding behaviour, phylotype trees detected by clone analysis, FISH signals, stable isotopic compositions, and fatty acid profiles, to clarify the epibiotic association between filamentous bacteria and the galatheid crab. However, many questions concerning the functional and ecological roles of epibionts and Shinkaia crosnieri remain. Quantitative analysis of the bacteria on the setae of crabs and in the environment and of the effects of crab activities on bacterial growth, especially after moulting when exuviae are shed with epibionts, should be carried out in future studies to determine the detailed epibiotic association between these bacteria and vent-associated galatheid crabs.
ACKNOWLEDGEMENTS
We thank the captain and crew of RV ‘Natsushima’ and the operation teams of the submersible ‘Shinkai 2000’ and the ROV ‘Hyper-Dolphin’ for their skilful collection of specimens. We are also grateful to Professor H. Tsutsumi, Kumamoto Prefectural University, for providing the facilities for carbon and nitrogen isotope measurements and Professor H. Chiba and Professor Emeritus M. Kusakabe for providing the facilities for sulphur isotope measurements.







