INTRODUCTION
All organisms have an influence on their surrounding habitat and modify the environment to some degree. For example, some organisms modify physical conditions or alter the flow of resources and therefore influence the composition of surrounding assemblages (e.g. Jones et al., Reference Jones, Lawton and Shachak1997). Through the provision of a modified habitat these organisms can increase habitat complexity and, depending on environmental conditions, ameliorate stress and provide refuges (Crain & Bertness, Reference Crain and Bertness2006). Other organisms may modify the environment and effectively reduce the diversity of species associated with it (Hall et al., Reference Hall, Basford, Robertson, Raffaelli and Tuck1991).
Space is a limiting resource in many shallow water habitats (e.g. Seed & O'Connor, Reference Seed and O'Connor1981) and the presence of large macrophytes such as kelp considerably increases the heterogeneity, often transforming relatively two-dimensional habitats into complex three-dimensional environments (Bruno & Bertness, Reference Bruno, Bertness and Bertness2001). The surface of kelp and the spaces between the haptera of their holdfasts provide a substantial amount of space for colonization (Seed & O'Connor, Reference Seed and O'Connor1981). Biologically generated habitats such as this can substantially increase diversity since they create a patchwork of environmentally variable conditions (e.g. Thompson et al., Reference Thompson, Wilson, Tobin, Hill and Hawkins1996). The holdfast is of interest in this study because it exhibits the highest diversity of all the kelp structures (Thiel & Vasquez, Reference Thiel and Vasquez2000; Norderhaug et al., Reference Norderhaug, Christie and Rinde2002; Christie et al., Reference Christie, Jorgensen, Norderhaug and Waage-Nielson2003; Arroyo et al., Reference Arroyo, Maldonado, Perez-Portela and Benito2004). Moore (Reference Moore1973a), for example, lists 389 species found on holdfasts collected from the north-east coast of Britain. Hence, kelp holdfasts are an important biologically generated habitat and have a significant modifying influence on the environment (Christie et al., Reference Christie, Jorgensen, Norderhaug and Waage-Nielson2003).
To date most studies of kelp forests as a habitat have focused on the abiotic factors that affect the flora and fauna associated with them, such as geography (Sheppard et al., Reference Sheppard, Bellamy and Sheppard1977), depth (Arroyo et al., Reference Arroyo, Maldonado, Perez-Portela and Benito2004), seasonality (Christie et al., Reference Christie, Jorgensen, Norderhaug and Waage-Nielson2003), wave exposure (Lippert et al., Reference Lippert, Iken, Rachor and Wiencke2001; Arroyo et al., Reference Arroyo, Maldonado, Perez-Portela and Benito2004), water flow (Duggins et al., Reference Duggins, Eckman and Sewell1990) and pollution (Jones, Reference Jones1971). Most studies have examined a single kelp species and its associated fauna and flora (Jones, Reference Jones1971; Moore, Reference Moore1973b; Christie et al., Reference Christie, Jorgensen, Norderhaug and Waage-Nielson2003; Arroyo et al., Reference Arroyo, Maldonado, Perez-Portela and Benito2004), and comparisons of the flora and fauna associated with the holdfasts of two or more species of kelp are rare (however, see Berdar et al., Reference Berdar, Conato, Cavallaro and Giacombe1978; Schultze et al., Reference Schultze, Janke, Kruss and Weidemann1990; Thiel & Vasquez, Reference Thiel and Vasquez2000; Lippert et al., Reference Lippert, Iken, Rachor and Wiencke2001). The consequences of subtle shifts in kelp distribution for epibiont species richness have not previously been described.
The current study set out to examine the consequences of subtle shifts in the relative abundance of biologically generated habitats provided by different kelp species. This was achieved by comparing the holdfast assemblages of two Laminaria species: Laminaria digitata (Hudson) Lamouroux and Laminaria ochroleuca De La Pylaie. These species have different geographical distributions but are very similar in their morphology. Laminaria digitata is known to have diverse holdfast fauna and flora, and there is anecdotal evidence that L. ochroleuca also supports a diverse holdfast species assemblage but there are no quantitative data. Both species are found in low intertidal and shallow subtidal rocky habitats (Gibson et al., Reference Gibson, Hextall and Rogers2001; Smirthwaite, Reference Smirthwaite2006). Laminaria digitata is a cold water species distributed from Norway to the Atlantic coast of Portugal where its southern limit is set by high summer temperatures (Hoek, Reference Hoek and van1982; Figure 1). In contrast, Laminaria ochroleuca has a more southerly distribution and ranges from Morocco to north-west Europe, reaching its northern limit around the south-west coast of England (Norton, Reference Norton1985; Figure 1). Laminaria ochroleuca is of interest in the context of range shifts because it is progressively extending its range northward. From its recorded appearance in the far south-west of England (John, Reference John1969), it has progressed along the south-west coast (Norton, Reference Norton1985) to its current recorded distribution as far east as the Isle of Wight and northwards on the north Devon coast (Smirthwaite, Reference Smirthwaite2006). These two species of kelp therefore provide an ideal model system to examine the effects of relatively subtle species replacements on the species richness of associated organisms living within such biologically generated habitats.

Fig. 1. Position of the survey site and the north-east Atlantic distribution (bold line) of (A) Laminaria digitata and (B) Laminaria ochroleuca.
MATERIALS AND METHODS
The survey site (Figure 1), Tinside on the south-west coast of England (50°21.75′N, 4°08.60′W), situated in Plymouth Sound behind a breakwater, is moderately exposed to wave action and consists of a bedrock substrate. Sampling was undertaken using SCUBA between September and November 2004. Fifteen specimens of Laminaria ochroleuca and fifteen Laminaria digitata were collected during this period. All specimens were sampled from an area parallel to the shore, below mean low water spring (MLWS) at a depth of 1–3 m, where stands of the two Laminaria species overlapped in their distribution, in order to minimize any potentially confounding effects associated with the depth/immersion gradient. The stipe and fronds were cut from each specimen about 5 cm above the holdfast which was then immediately covered in a fine muslin bag. The holdfast was very carefully dislodged from the substrate with a lever and immediately secured in the muslin bag to prevent the loss of mobile fauna.
The age of each holdfast was determined using a method set out by Kain (Reference Kain1963). A thin cross-section of the stipe was taken just above the holdfast. This was examined under a stereo dissection microscope to count the appropriate growth rings and the age of all the kelp specimens used for analysis was standardized to two years. Holdfast volume was determined by wrapping each holdfast in plastic food wrapping film and dipping it into a bucket of water, to mould the film to the outer shape of the holdfast. The total volume of the holdfast was then determined by displacement. The tissue volume was calculated by multiplying the wet weight of the cleaned holdfast tissue after epibiont collection by 1.3 (specific gravity of the tissue; after Jones, Reference Jones1971). Tissue volume was then subtracted from the total volume of the holdfast to give the volume of potentially habitable space amongst the haptera of the holdfast. Haptera were removed to expose the inner structure and any associated flora and fauna. Identification was to species level wherever possible using Hiscock (Reference Hiscock1986) and Hayward & Ryland (Reference Hayward and Ryland2002).
Data analysis
For each species of kelp linear regression was used to establish relationships between holdfast volume and the number of associated epibiont species. To determine if the number of epibiont species differed significantly between the two species of Laminaria, analysis of covariance (ANCOVA) was used to compare the relationship between kelp species and number of epibiont of species, with habitat volume as the covariate. All data were tested for normality using a Kolmogorov–Smirnov test and for homogeneity of variances using Levene's test. For all epibionts, particularly colonial organisms, data were reduced to presence/absence of species on each holdfast (N = 15). A Bray–Curtis similarity matrix was generated from the presence/absence data using PRIMER (Version 5.2.0) to give the similarity in assemblage composition between kelp species. To visualize the similarity of each holdfast a multidimensional scaling (MDS) ordination was plotted giving the position of each holdfast in two-dimensional space based on its epibiont composition. Analysis of similarity (ANOSIM) was then carried out to test for differences between the epibiont assemblages of the two kelp species. Similarity percentages (SIMPER) analysis was also used to identify characteristic epibiont species for L. digitata and L. ochroleuca and indicate their contribution to the level of similarity (within a species of kelp) and dissimilarity (between the two species of kelp).
RESULTS
A total of 130 species of epibionts were found on the Laminaria holdfasts, of these 57 were associated solely with Laminaria digitata and 19 were solely with Laminaria ochroleuca. The most commonly occurring groups unique to L. digitata were the family Tubificidae and the anemone Urticina felina (Linnaeus). The most common species unique to L. ochroleuca were the ascidians Molgula spp, the entoproct Pedicellina nutans Dalyell and the molluscs Heteranomia squamula (Linnaeus) and Modiolarca tumida (Hanley). Appendix 1 gives a complete list of the epibiont species found on both kelps. The fauna common to both species were predominantly annelids, molluscs and bryozoans. The annelids were in turn dominated by polychaetes; 27 out of the 28 species found, and the molluscs by bivalves. The most common epibiont on L. digitata was the bryozoan, Callopora lineata (Linnaeus), which was present on 14 out the 15 holdfasts and the mollusc, Modiolus barbatus (Linnaeus), which was present on 13 holdfasts. For L. ochroleuca, the most common species was the ascidian, Dendrodoa grossularia (Van Beneden), which was present on 14 holdfasts. The majority of epifauna found on both species of Laminaria were filter feeding sessile species.
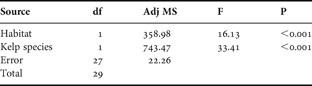
The number of epibiont species increased with holdfast habitat volume in both species of Laminaria (Figure 2). Linear regression found the slopes to be significantly different from zero, indicating a positive relationship between habitat volume and number of epibiont species for both L. ochroleuca (R2 = 0.3855, F1,13 = 8.16, P < 0.05) and L. digitata (R2 = 0.4815, F1,13 = 12.07, P < 0.05). Regression slopes were homogeneous (F1,26 = 2.92, P = 0.099) allowing an ANCOVA (Table 1) to be carried out which showed the effect of habitat volume as a covariate was highly significant for both species (F1,27 = 16.13, P < 0.001). The difference in epibiont species number was highly significant (F1,27 = 33.41, P < 0.001) with L. ochroleuca having far fewer epibiont species, a mean of 0.62 species per cm3, when compared to L. digitata which had a mean of 1.13 species per cm3.

Fig. 2. The relationship between holdfast habitat volume and number of epibiont species per holdfast for Laminaria digitata and Laminaria ochroleuca (N = 15). Regression lines found to be homogeneous.
Table 1. Analysis of covariance data for number of epibiont species vs kelp species with habitat volume as the covariate.
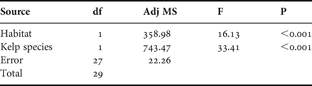
df, degrees of freedom; Adj MS, adjusted mean square.
An MDS plot of the holdfast assemblages for the two Laminaria species (Figure 3) had a stress value of 0.22 indicating the data are only partially represented by a two-dimensional plot (Clarke & Warwick, Reference Clarke and Warwick1994) but were better represented by a three-dimensional plot (stress = 0.16). Hence although Figure 3 helps illustrate the separation in assemblage composition between holdfasts of L. ochroleuca and L. digitata it does not fully encapsulate the data therein. Subsequent ANOSIM indicated a significant difference in assemblage composition (R = 0.336, P = 0.001). SIMPER analysis showed that the L. digitata holdfast assemblage had an average similarity of 38.96%. The epibiont species Celleporella hyalina (Linnaeus), Callopora lineata, Modiolus barbatus, Fabricia stellaris (Blainville), Anomia ephippium Linnaeus, Palmaria palmata (Linnaeus) Kuntze and Nematodes contributed 50.08% of this similarity. Laminaria ochroleuca holdfast assemblages had an average similarity of 36.41%. The epibiont species Dendrodoa grossularia, Celleporella hyalina and Celleporina hassallii (Johnston) contributed 46.37% of this. These three species had the highest individual contributions towards similarity for any of the species found inhabiting the holdfasts. Celleporella hyalina contributed towards the similarity of both species of Laminaria, which is indicated by its presence on 29 out the 30 holdfasts sampled. The average dissimilarity between the two Laminaria species was 68.71%. A wide variety of fauna and flora contributed towards this dissimilarity with no individual species contributing more than 2.42%. So while assemblages differed between L. digitata and L. ochroleuca this effect was not generated by marked differences in the occurrence of one or two particular epibiont species but rather the occurrence of numerous different epibionts on each species of kelp.

Fig. 3. Two-dimensional multidimensional scaling plot of the 30 holdfasts; 15 Laminaria digitata (filled squares) and 15 Laminaria ochroleuca (open circles), based on a presence/absence Bray–Curtis similarity matrix of epibiont species collected from each holdfast (stress = 0.22).
DISCUSSION
At broad spatial scales environmental factors, including depth, wave action and tidal elevation influence holdfast morphology and epibiont species richness, therefore affecting assemblage composition (Christie et al., Reference Christie, Jorgensen, Norderhaug and Waage-Nielson2003). In addition, Arroyo et al. (Reference Arroyo, Maldonado, Perez-Portela and Benito2004) suggest that factors operating at a much smaller scale of resolution may have an even stronger influence on the distribution of meiofauna within a particular Laminaria bed. The epibionts found in this study were both macrofauna and meiofauna, therefore, as well as broad scale factors, subtle and small scale effects may have been important in generating the differences between Laminaria digitata and Laminaria ochroleuca observed here.
The holdfast habitat volumes of both species of Laminaria examined had a positive relationship with epibiont species richness which was in agreement with previous studies (Jones, Reference Jones1971; Sheppard et al., Reference Sheppard, Bellamy and Sheppard1977; Thiel & Vasquez, Reference Thiel and Vasquez2000). Most of the epiflora found on the holdfasts were from the class Rhodophyceae, which accounted for 12 out of the 14 species of algae found, together with Himanthalia elongata (Linnaeus) Gray (Phaeophyceae) and Ulva lactuca (Linnaeus) (Chlorophyceae), however, these species only occurred on one L. digitata sample. Nine of the species of algae were unique to L. digitata and the most abundant of these were Ptilota gunneri Silva, Maggs & Irvine (four of the holdfasts) and Delesseria sanguinea (Hudson) Lamouroux (three of the holdfasts). Palmaria palmata was the single most abundant species and was common to both species of holdfast. Hill (Reference Hill2006) made the same observation in her description of L. digitata and other studies have also found a diverse flora associated with Laminaria hyperborea (Gunnerus) Foslie in Scotland (Whittick, Reference Whittick1983) and Helgoland (Schultze et al., Reference Schultze, Janke, Kruss and Weidemann1990) which tend to be dominated by Rhodophyceae.
In a study of marine algal epifaunas, Seed & O'Connor (Reference Seed and O'Connor1981) reported that the majority of kelp epifauna consist of filter feeding, sessile species with bryozoans, hydroids, sponges and ascidians well represented and this was very much in agreement with the results of the present work. Filter feeding molluscs were well represented on both species of Laminaria in this study. Grazing molluscs were rare on L. digitata and completely absent on L. ochroleuca. Herbivores are generally rare amongst kelp epibionts and only a few species are known to directly graze on kelp (Nybakken, Reference Nybakken2001). Three species of bryozoan were present on nearly all of the holdfasts sampled. One of which, Celleporella hyalina, was the most common species in this study and contributed the greatest towards any similarity of these two kelp species. A similar pattern of bryozoan abundance was obtained by Lippert et al. (Reference Lippert, Iken, Rachor and Wiencke2001) who examined the macroalgal epibionts in Kongsford (Spitsbergen). Polychaetes dominated the annelid epibiont species found in the present study and were also amongst the most abundant species present in the holdfast communities from Norway (Christie et al., Reference Christie, Fredriksen and Rinde1998) and the Cantabrian Sea (Arroyo et al., Reference Arroyo, Maldonado, Perez-Portela and Benito2004).
Laminaria ochroleuca had significantly lower epibiont species richness than L. digitata. Shepperd et al. (Reference Sheppard, Bellamy and Sheppard1977) and Arroyo et al. (Reference Arroyo, Maldonado, Perez-Portela and Benito2004) also found faunal richness to be lower on L. ochroleuca compared to other species of macroalgae but did not examine L. digitata. ANOSIM showed epibiont community composition to be significantly different between the two species of Laminaria. However, SIMPER analysis suggested a wide variety of fauna and flora contributed towards this dissimilarity explaining some of the variance within the kelp holdfast assemblages. This pattern may in part be a consequence of reducing the data to presence/absence scores. Analysis of abundance and biomass data places more emphasis on both rare and abundant species. However, it is not appropriate to use untransformed data in this manner when they consist of both counts and percentage cover information, as was the case in the present study. The outcomes may also be modified by low taxonomic resolution in some of the groups such as ‘nematodes’ and ‘red encrusting algae’. None the less our data provide a robust indication of the patterns of the assemblages on these two species of kelp and indicate biologically, as well as statistically, important differences and are indicative of small scale differences between biological habitats as described by Arroyo et al. (Reference Arroyo, Maldonado, Perez-Portela and Benito2004).
A possible explanation for the difference in the epibiont species richness between these two Laminaria species is the increased production of antifouling chemicals by L. ochroleuca compared to L. digitata. Many large brown algae, including kelps, produce antifoulants and these exudates are known to hinder growth and settlement (Al-Ogily & Knight-Jones, Reference Al-Ogily and Knight-Jones1977) and may be significant in determining epibiont abundance and species richness. Both L. digitata (Al-Ogily & Knight-Jones, Reference Al-Ogily and Knight-Jones1977) and L. ochroleuca (Sheppard, Reference Sheppard1976) are known to exude such antifoulants. Hellio et al. (Reference Hellio, Bremer, Pons, Le Gal and Bourgougnon2000) extracted the exudates of a variety of marine algae and examined their effects on the development of microorganisms. They found that the extracts from L. ochroleuca had high levels of antimicrobial activity, particularly against marine fungi (Hellio et al., Reference Hellio, Bremer, Pons, Le Gal and Bourgougnon2000) and exudates were also found to inhibit microalgal growth and the attachment and germination of a variety of macroalgal spores (Hellio et al., Reference Hellio, Berge, Beaupoil, Le Gal and Bourgougnon2002). In contrast, exudates of L. digitata only had slight antimicroalgal activity and were inactive against macroalgal spores (Hellio et al., Reference Hellio, Berge, Beaupoil, Le Gal and Bourgougnon2002). Marine fungi and bacteria are significant contributors to biofilm formation which provides a substrate for the subsequent attachment of other epibiont organisms (Hellio et al., Reference Hellio, Bremer, Pons, Le Gal and Bourgougnon2000). Therefore, the inhibition of this process by antifouling chemicals could potentially limit settlement of larger organisms and result in an impoverished epibiont assemblage on L. ochroleuca.
One of the primary factors that regulate the physiology and biogeography of marine algae is temperature (Adey & Steneck, Reference Adey and Steneck2001). The ‘climate envelope’ approach, which forecasts the response of a species' geographical distribution to a single climatic variable, e.g. temperature, has been the focus of many studies (Berry et al., Reference Berry, Dawson, Harrison and Pearson2002; Pearson & Dawson, Reference Pearson and Dawson2003; Huntley et al., Reference Huntley, Green, Collingham, Hill, Willis, Bartlein, Cramer, Hagemeijer and Thomas2004). Organisms at the limit of their geographical ranges are likely to be some of the first to respond to temperature changes (Lewis, Reference Lewis1996; Herbert et al., Reference Herbert, Hawkins, Sheader and Southward2003) and a general poleward movement of species ranges is predicted in response to warming (Parmesan, Reference Parmesan1996; Sagarin et al., Reference Sagarin, Barry, Gilman and Baxter1999). For example, warm water species have been seen to extend their northern range and abundance in the English Channel in response to climate changes (Herbert et al., Reference Herbert, Hawkins, Sheader and Southward2003) and any future temperature rises in this location will favour warm water species such as L. ochroleuca over the cold water Laminaria species. Hence, the northward extension of L. ochroleuca's range and the consequences in terms of reduced epibiont species richness could influence an important biologically generated habitat and potentially have consequences at a broad scale of resolution. However, species will not necessarily react as predicted by climate envelope based on their current range alone (Simkanin et al., Reference Simkanin, Power, Myers, McGrath, Southward, Mieszkowska, Leaper and O'Riordan2005). While species distributions are anticipated to change, a variety of factors including biological interactions, which are considered here, are likely to modify the outcomes that would be predicted using climate envelope in isolation. For instance, in the present data local populations of epibiont species are likely to be strongly influenced by shifts in the relative abundance of major habitat modifying species such as kelp. Depending on the extent of alternative habitat that is available, changes in distribution of these two habitat-forming species of kelp will likely influence the abundance and possibly the presence or absence of epibiont species. Therefore, while climate envelope predictions are invaluable for initial assessments at a broad scale of resolution, in order to provide information at a scale relevant to management of habitats, predictions of species level responses to climate change should also take a more extensive account of biological interactions (Moore et al., Reference Moore, Hawkins and Thompson2007).
ACKNOWLEDGEMENTS
Thanks to A. Foggo and M.P. Johnson for comments on an earlier draft and also two anonymous referees' who greatly helped to improve this manuscript. This study could not have been conducted without the support of the University of Plymouth Diving and Sailing Centre, particularly D. Clarke, M. Whelan, S. Syson and S. Jacques. Thanks also to P. Smithers and M. Browne for technical support and a special thanks to E. Dalton.
Appendix 1. Species list and number of holdfasts occupied by each of the species recorded (L.d, Laminaria digitata; L.o, Laminaria ochroleuca).