INTRODUCTION
The pouting, Trisopterus luscus (Linnaeus, 1758), is a species with a wide distribution across Atlantic waters, occurring from Skagerrak and the British Isles to southern Morocco and the western Mediterranean, preferably in rocky and sandy areas on the continental shelf at depths of 30–100 m (Wheeler, Reference Wheeler1978; Whitehead et al., Reference Whitehead, Bauchot, Hureau, Nielsen and Tortonese1986). Pouting on the Galician shelf has a protracted spawning season, spanning from mid-winter to early spring with a spawning peak between February and April (Alonso-Fernández et al., Reference Alonso-Fernández, Domínguez-Petit, Bao, Rivas and Saborido-Rey2008), which is in agreement with studies conducted on ichthyoplankton in those same waters (Ferreiro & Labarta, Reference Ferreiro and Labarta1988). This teleost of the Gadoid family is of major commercial importance for the artisanal fleet of a number of European countries, but especially for France, Portugal and Spain.
Studies on eggs and larvae of this species are scarce (Ferreiro & Labarta, Reference Ferreiro and Labarta1988; Fox et al., Reference Fox, Dickey-Collas and Winpenny1997). Despite some existing literature concerning the developmental biology of this species, the description of its embryonic development remains incomplete (Ehrenbaum, Reference Ehrenbaum1905–1909; D'Ancona, Reference D'Ancona and Lo Bianco1933) and all the data regarding incubation times and embryonic development are exclusively from Erhenbaum (1905–1909). The current work presents the first successful attempt at achieving the natural spawning of pouting in captivity. It is the intent of this paper to contribute to filling the gap of knowledge on the subject by describing and illustrating, for the first time ever, the development of Trisopterus luscus eggs from spawning to hatching time.
MATERIALS AND METHODS
The reproductive specimens used for this study were obtained via either of the following methods: (i) hook-and-line techniques performed with conventional recreational gear; or (ii) standard commercial fishery traps set in the Ría de Vigo (Figure 1) in September 2008 and subsequently transported to the facilities of the Institute of Marine Research, in Vigo. The specimens were randomly distributed in two 250 l cylindrical aeration tanks. Both tanks received a constant inflow of sand–filtered seawater at ~13°C, with natural photoperiod. Before the experiment was started, the fish were allowed to acclimate to these conditions until the beginning of the spawning season (mid-winter–early spring). All specimens were fed to satiation once a day with squid and shrimp during the acclimation and experimental periods.

Fig. 1. Pouting (Trisopterus luscus) collection location.
In December, just before the beginning of the spawning season, the fish were randomly separated in pairs consisting of a female and a male each. The pairs were distributed in six tanks under the same conditions of water flow, photoperiod and feeding regime. Females spawned and males ejaculated in the tanks spontaneously. Tanks were checked and eggs collected and counted on a 24-hour basis. The ratio between dead eggs and fertilization rate was not assessed during the experimental procedure. Eggs were examined under a stereo microscope (Leica MZ6). Fertilized eggs were transferred to 10 l aquaria with permanent aeration and kept at a constant temperature of 13°C until hatching. A sample of eggs was removed from the tanks on a 12-hour basis for the description of their embryonic development stage, and a picture was taken with a video camera connected to the stereo microscope. The embryonic development stages were adapted from the Norway pout (T. esmarki) egg classification, elaborated by Friðgeirsson (Reference Friðgeirsson1978). All measurements were performed using the software QWin (© Leica Imaging Systems). At the end of the experiment individual spawning frequency was assessed dividing the duration of the spawning activity (total number of days from the first batch released to the last batch recorded) by the total number of batches spawned for each female.
Every female either died or was sacrificed within the first three months after the onset of the spawning season, which therefore could not be tracked fully. Ovaries were removed from all specimens and fixed immediately after in 3.6% buffered formalin. The central portions of the fixed ovaries were extracted, dehydrated, embedded in paraffin, sectioned at 3 µm and stained with haematoxylin–eosin for microscopic analysis.
RESULTS
Spawning under controlled conditions
This has been the first successful attempt ever at achieving the natural spawning of pouting in captivity. Coincident with previous studies, the onset of the spawning period for the captive fish took place in early winter, and no hormonal induction was required. Captive female fish spontaneously and repeatedly spawned during the breeding season, even in absence of males.
Despite some individual variation in the spawning frequency, ranging from 1.8 to 4.6 days, no significant (P > 0.05) size-dependent trend could be determined (Table 1). An average amount of one released batch per female every 2.7 days was hence established. Once the females either died or were sacrificed, the histological examinations of their gonads revealed that some of the females remained spawning-capable. This finding thus suggests that the spawning season could extend for longer than three months at an individual level.

Fig. 2. Fitted curves of average batch size–length (log-transformed) and batch size–weight relationships. Grey triangles correspond to batch size–length (log-transformed) relationship and black dots batch size–weight relationship.
Table 1. Statistical results for linear models of (i) spawning frequency–length and weight relationships and (ii) batch size–length and weight relationships for pouting, Trisopterus luscus (Linnaeus, 1758).

Batch fecundity, i.e. the number of eggs released per batch, ranged approximately between 1900 and 80,000 hydrated oocytes (fish length ranged between 20 and 28 cm) throughout the experiment. Relative batch fecundity yielded a mean of 51 ± 15 (mean ± SD) hydrated oocytes per gram of female throughout the experimental procedure. Batch fecundity–size (length and weight in cm and g respectively) relationships were evaluated and the correspondent equations are presented below:
The average batch size, i.e. the number of eggs released per batch, was significantly dependent (P < 0.05) on length (cm, Equation 1) and weight (g, Equation 2). Parameter estimates for both relationships are listed in Table 1 and fitted models are shown in Figure 2. In Table 2 complete individual spawning data are provided.
Table 2. Individual spawning data of the pouting, Trisopterus luscus (Linnaeus, 1758), in captive conditions at 13°C. Batch size expressed as number of eggs released per batch, spawning frequency as average elapsed time in days between batches and egg diameter in microns.

Embryonic development of pouting, Trisopterus luscus, at 13°C
Recently released pouting eggs are pelagic, with a smooth, clear and spherical chorion, and a homogeneous yolk. The perivitelline space is narrow and oil globules are absent. Live eggs fertilized were 0.95–1.10 mm in diameter (N = 160, mean 1.03 mm, SD 0.03 mm), showing scant variation in their development stage. No manipulation was required for egg fertilization: the released eggs were naturally fertilized in the tanks. The development of pouting eggs was divided into six stages, based on the artificially-reared material. A detailed description of each development stage is provided below and Table 3 provides information on timing at each stage and organogenesis.
Table 3. Embryonic development of the pouting, Trisopterus luscus (Linnaeus, 1758), at 13°C.

*, mean duration taken by Friðgeirsson (Reference Friðgeirsson1978).
Stage 1. Fertilization—preparation for cleavage (precell—early stage)
The egg is quite transparent. Cell division has not yet begun. Cytoplasm is concentrated at the animal pole, appearing as a raised cap of undivided cellular material, easily differentiated from the yolk mass (blastodisc: preparation for cleavage) (Figure 3A). The membrane is thin and a small content of carotin gives the egg a faint yellow hue. This, together with the small size of the egg, makes it almost invisible in the water to the naked eye.
Stage 2. Cleavage of the blastodisc
The cleavage of the blastodisc begins with the division of the single cell into two daughter cells (blastomeres), becoming multicellular by repeated cleavages:
2 cells—Cleavage 1. Cytoplasm splits into two equal-sized cells (Figure 3B).
4 cells—Cleavage 2. Cell cap consists of four cells of equal size (Figure 3C).
8 cells—Cleavage 3. Eight cells in 2 parallel rows form a single-layer rectangle (Figure 3D).
16 cells—Cleavage 4. Fourth and last division of cells on a single layer. Cells form a rough square, four cells wide and four cells long (Figure 3E).
>16 cells. After the 5th or 6th division, the blastodisc attains a berry-like appearance that is oftentimes referred to as the ‘mulberry stage’ (Moser & Ahlstrom, Reference Moser, Ahlstrom and Lasker1985) (Figure 3F). During all of the aforementioned stages, cells multiply and become smaller. However, the size of the blastodisc remains constant. The result of this process is a regular morula (Figure 3G, H).

Fig. 3. Development stages of eggs of pouting, Trisopterus luscus, reared from known adults at 13°C. (A) Stage 1; (B) Stage 2 (2 cells); (C) Stage 2 (4 cells); (D) Stage 2 (8 cells); (E) Stage 2 (16 cells); (F) Stage 2 (>16 cells, ‘mulberry’); (G) Stage 2 (morula); (H) Stage 2 (morula). Photographs by A. A–F.
The border of the morula thickens and forms the germ ring of the blastula (Figure 4A, B).

Fig. 4. Development stages of eggs of pouting. (A) Stage 2 (blastula); (B) Stage 2 (blastula); (C) Stage 3 (beginning of gastrulation); (D) Stage 3 (late); (E) Stage 4; (F) Stage 4; (G) Stage 4; (H) Stage 4 (late). Photographs by A. A–F.
Stage 3. Formation of basic embryonic tissue layers—gastrulation
The formation of the basic embryonic tissue layers marks the beginning of gastrulation (Figure 4C). The beginning of this stage is best defined by the visual aspect of the blastoderm, which resembles tissue rather than a collection of individual cells. The margin of the blastodisc becomes slightly thickened and is termed the germ ring. On one region of the germ ring the thickening extends inwards to form the embryonic shield, which defines the future axis of the embryo.
Gastrulation proceeds by further proliferation and downward movement of the cells in the region of the germ ring by a process known as epiboly, i.e. the expansion of the cellular blastoderm over the yolk. At the end of this stage, the germ ring has enclosed one-third of the yolk mass and the embryo is beginning to form along the median region of the embryonic shield. The head region of the embryo is becoming apparent (Figure 4D).
Stage 4. Formation of pre-organs—organogenesis 1
Formation of the first rudimentary organs marks the end of gastrulation and the beginning of organogenesis. During this stage, the head and part of the embryonic body are formed.
This stage is characterized by the development of the notochord, which can be seen from a dorsal viewpoint, and the differentiation of the optic vesicles from the brain (the optic bulbs; Figure 4E). Subsequently, the germ ring has closed, has almost completed overgrowth of the yolk, but the blastopore remains open.
The ear primordia have formed and can be seen on both sides of the hindbrain (Figure 4F). The embryo has expanded over approximately one-half of the yolk sac circumference. Subsequently, the blastopore becomes closed. At the time of the closure of the blastotopore, the first pigmentation of the embryonic body begins (Figure 4G). First somites have been formed at the middle of the embryonic body. The tail is plump and the tip appears to be lifting from the yolk surface, but the tail margin is still attached (Figure 4H).
Stage 5. Full development of main organs — organogenesis 2
During the second part of the organogenesis, the organs and systems of organs become functional one after another. The head and tail regions of the embryo are discernible. The tail is developing. This stage begins when the tail starts separating from the yolk mass (Figure 5A, B). Initially, the tail is broadly rounded and then begins to narrow as it rapidly elongates. On both sides of the embryonic body, the rudimentary pectoral fin lobes are taking shape and myomeres are clearly visible (Figure 5C). Fin lobes are developing ventrally and dorsally on the tail and on the dorsal side of the embryonic body (Figure 5C, D).

Fig. 5. Development stages of eggs of pouting. (A) Stage 5; (B) Stage 5; (C) Stage 5; (D) Stage 5 (late); (E) Stage 6; (F) Stage 6; (G) Stage 6; (H) Stage 6 (before hatching). Photographs by A. A–F.
Stage 6. Preparation for hatching
The beginning of the preparation for hatching marks the end of the organogenesis (Friðgeirsson, Reference Friðgeirsson1978). This is the final stage before hatching and it is defined by an embryo length exceeding three-quarters of the egg's circumference. It lasts until the tail reaches the head (Figure 5E, F). The tail is well separated from the yolk mass and the rest of the embryo remains tightly associated with the yolk. The tail continues to separate from the yolk as the embryo grows.
Late stage: the tail completes a full circle around the upper half of the yolk. The tail of the embryo almost touches the head (Figure 5G, H). Pupils can be discerned in the eyes, optic cups remain unpigmented (Figure 5G). Embryo pigment intensifies, including melanophores over the snout, and additional melanophores develop laterally along the trunk and tail.
Stage 7. Hatching
The embryo hatches as a yolk-sac larva with closed mouth and gut. The head has not yet lifted from the yolk sac (Figure 6A). The pouting eggs hatch during the latter half of the fifth day (4.54 ± 0.17 days, approximately).

Fig. 6. Newly-emerged yolk-sac larva of pouting, emerged after 109 hours of rearing at 13°C. (A) Ventral view of larva; (B) 3.18 mm body length. Photographs by A. A–F.
The yolk-sac is large and facing upwards, and the embryo is floating on its surface. The newly hatched larva is 3.13 mm ± 0.15 SD long. Eyes still almost unpigmented. The whole body shows black star-shaped chromatophores (Figure 6B). The farthest third of the postanal portion of the body is free from pigment.
Numerous minute yellow chromatophores were distributed over the whole of the body, yolk-sac and primordial fin (Figure 6B). These chromatophores provided the larva with a dim yellowish gleam.
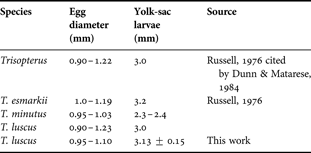
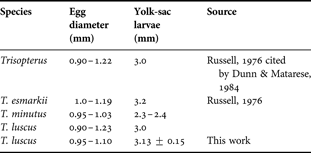
Previously published characteristics of eggs and yolk-sac larvae of the pouting are presented in Table 4 to facilitate comparisons with those derived in the present study (Table 4). Also comparative data for eggs and yolk sac larvae for T. luscus and other species from genus Trisopterus are shown in Table 5.
Table 4. Characteristics of eggs and yolk-sac larvae of the pouting, Trisopterus luscus (Linnaeus, 1758).

Table 5. Comparative data for eggs and yolk-sac larvae for T. luscus and other species from genus Trisopterus.
DISCUSSION
The present paper aims to contribute to the study of the spawning of one of the fish species with the highest commercial importance for the artisanal fisheries of several European countries, primarily France, Portugal and Spain. Morphological identification and correct ageing of pelagic eggs in ichthyoplankton surveys is crucial in the egg production method (Moser & Ahlstrom, Reference Moser, Ahlstrom and Lasker1985), and despite pouting being a determinate spawner (Alonso-Fernández et al., Reference Alonso-Fernández, Domínguez-Petit, Bao, Rivas and Saborido-Rey2008), the biological information provided in this paper could help to estimate realized annual fecundity (Murua & Saborido-Rey, Reference Murua and Saborido-Rey2003).
The presence of pelagic eggs of pouting on the Galician shelf (north-western Spain) was associated with the colder months of the year (12–14°C), while salinity was relatively constant, around 350/00 (Ferreiro, Reference Ferreiro1985). Therefore, the highest egg densities for Trisopterus luscus were found during icthyoplankton cruises in the Ría of Vigo in winter and spring (Ferreiro & Labarta, Reference Ferreiro and Labarta1988), in agreement with the spawning season data described in some studies on the Iberian shelf (Labarta et al., Reference Labarta, Ferreiro, Fenandez and Martinez1982; Merayo, Reference Merayo1996; Alonso-Fernández et al., Reference Alonso-Fernández, Domínguez-Petit, Bao, Rivas and Saborido-Rey2008). Pouting's peak reproductive activity seems to vary with latitude, from December to April on the Asturian coast (Merayo Reference Merayo1996), from January to March on the coast of Brittany (Gherbi-Barre, Reference Gherbi-Barre1983), from February to June in the English Channel, and in March in the North Sea (Desmarchelier, Reference Desmarchelier1985). Larvae began to appear in significant numbers in the eastern Irish Sea from early April onwards (Nichols et al., Reference Nichols, Haynes, Fox, Milligan, Brander and Chapman1993; Fox et al., Reference Fox, Dickey-Collas and Winpenny1997), a little bit later than on the Galician shelf due to spawning season delay. This study was the first attempt at achieving the reproduction in captivity of this species. Poutings spontaneously spawned successful batches of viable eggs in controlled conditions; artificial hormonal induction was not necessary. The average spawning frequency estimated at an individual level during the experiment was of 3 days. This result was inferior to the average spawning frequency assessed at a population level on the Galician shelf (north-western Spain) (Alonso-Fernández et al., Reference Alonso-Fernández, Domínguez-Petit, Bao, Rivas and Saborido-Rey2008), which was approximately of 6–7 days. The disparities found between captivity and field studies might be due to the different approaches used: individual level versus population. Additional likely sources of variation could be the effects of capture, confinement and repeated sampling, which generate stress in captive fish. Stress is known to cause a variety of physiological responses in fish, affecting fecundity, egg size and egg survival, and spawning behaviour (Morgan et al., Reference Morgan, Wilson and Crim1999). The duration of the spawning season seems to be consistent with field studies (Labarta et al., Reference Labarta, Ferreiro, Fenandez and Martinez1982; Merayo, Reference Merayo1996; Alonso-Fernández et al., Reference Alonso-Fernández, Domínguez-Petit, Bao, Rivas and Saborido-Rey2008). Captive pouting corroborates the observations in the wild concerning a protracted spawning season, longer than 3 months even at an individual level. Nonetheless, results concerning this respect should be carefully interpreted, since the spawning season was not fully tracked for any of the individuals studied in the experiment. As expected, batch fecundity proved to be significantly dependent on length and weight, and the average relative batch fecundity, i.e. the number of released eggs per batch and gram of female, was quite similar to the one estimated in field studies (Alonso-Fernández et al., Reference Alonso-Fernández, Domínguez-Petit, Bao, Rivas and Saborido-Rey2008), namely 51 and 46 eggs/g respectively.
The development of pouting eggs was divided into six stages, based on the artificially reared material and adapted from Friðgeirsson (Reference Friðgeirsson1978). The embryonic development sequence described in this paper is consistent with previous studies on Trisopterus luscus (Ehrenbaum, Reference Ehrenbaum1905–1909) and the egg classification for Trisopterus esmarki (Nilsson) (Friðgeirsson, Reference Friðgeirsson1978). The temperature selected for the experimental period, 13°C, was on par with the typical conditions of surface water in late winter on the Galician shelf (Domínguez-Petit, Reference Domínguez-Petit, Korta, Saborido-Rey, Murua, Sainza and Piñeiro2008). Additionally, the prevalence of pouting eggs is higher in Galician waters at temperature ranges between 12° and 14°C for that period (Ferreiro, Reference Ferreiro1985). Under artificially controlled conditions at a constant 13°C, hatching time was estimated in approximately 4.54 ± 0.17 days, while Ehrenbaum (Reference Ehrenbaum1905–1909) determined this period to be 10–12 days in the south-western North Sea. However, no reference temperature was provided by Ehrenbaum (Reference Ehrenbaum1905–1909) in his studies. Friðgeirsson (Reference Friðgeirsson1978) found that T. esmarki hatches after 5 days and 20 hours of incubation at 7.2°C. The disparities between this study and Ehrenbaum's observations could be due to differences concerning incubation temperature, since fertilized eggs came from northern waters containing reared material. It has been clearly demonstrated for several species that egg development is strongly temperature-dependent, and the decrease of the developmental time for higher temperatures is known for a number of fish species (Blaxter, Reference Blaxter, Hoar and Randall1969; Miranda et al., Reference Miranda, Cal and Iglesias1990; Cunha et al., Reference Cunha, Vendrell and Gonçalves2008). Thus, further research is needed to properly ascertain pouting's developmental rates for the likely temperature range at different latitudes.
Eggs float due to their high water content, nearing 90% as for other species from the same genus (Friðgeirsson, Reference Friðgeirsson1978). Due to an egg mean diameter of 0.95–1.10 mm as per the results of the present study, the identification of pouting eggs could be problematic given their similarities to other species. Pouting eggs cannot be readily separated from those of similar size with no oil globules. In Galician waters, the main sources of identification problems are Trisopterus minutus (Ferreiro, Reference Ferreiro1985) and Pollachius pollachius. In northern latitudes, however, Merlangius merlangus, Platicthys fesus and Limanda limanda can also be misleading (Fox et al., Reference Fox, Dickey-Collas and Winpenny1997). Regarding egg and larvae size, the present results are similar to those found in previous studies. Ferreiro (Reference Ferreiro1985) found eggs with 0.89–1.10 mm of diameter, which is consistent with our own findings, while Erhenbaum (1905–1909) and Russell (Reference Russell1976) established the diameter-range of fertilized eggs to be between 0.97–1.23 and 0.90–1.22 mm respectively, i.e. they found larger eggs. Nichols et al. (Reference Nichols, Haynes, Fox, Milligan, Brander and Chapman1993), Fox et al. (Reference Fox, Dickey-Collas and Winpenny1997) and Ré (Reference Ré1999) determined the egg diameter-range to be between 0.90 and 1.23 mm, in accordance with the latter references. Hatched larvae, measured within the first 24 hours after hatching, were 3.13 mm ± 0.15 SD long. Ferreiro (Reference Ferreiro1985) and Erhenbaum (1905–1909) described hatched larvae as slightly below 3.0 mm long. Larvae of T. luscus are quite similar to those of Micromessistius poutassou but they can be easily distinguished by the number of miomeres, namely 48–49 and 56–60 respectively (Ré, Reference Ré1999).
The development of the pelagic eggs and hatched larvae of Trisopterus luscus (Linnaeus, 1758) has been described in this study for the first time ever. The experiment has been based on reared eggs from adults captured on the Galician shelf of north-western Spain. This has also been the first natural spawning experience in captivity for this species and it opens a new way for experimental design. Spontaneous spawning activity was observed and viable and fertilized eggs obtained through the experimental procedure. The authors expect this study on embryonic development to be useful in future ichthyoplankton surveys for the correct identification of Trisopteurs luscus eggs and hatched larvae, being nevertheless highly desirable that it be complemented by future research with the description of the late larval and early juvenile stages of this species.
ACKNOWLEDGEMENTS
We would like to thank L. Alonso-Martínez and C. Sánchez-Espinel for providing the specimens for this work. We are grateful to A. Chamorro and R. Chamorro for their helpful fish husbandry advice and also to D. Fernández-Taboada for help with the English and editing. We appreciate anonymous referees' comments on the manuscript. This research was funded by the regional government of Galicia (Xunta de Galicia) under the coverage of Project DETEPRE (08MMA010402PR).