INTRODUCTION
In most of the species of the family Octopodidae adults are predominately benthic. Their hatchlings can either exhibit an adult-like bottom-dwelling mode of life or, as in many species, they spend a period of variable duration in the plankton during which they are free-swimming animals (Hochberg et al., Reference Hochberg, Nixon, Toll, Sweeney, Roper, Mangold, Clarke and Boletzky1992; Villanueva, Reference Villanueva1995; Boyle & Rodhouse, Reference Boyle and Rodhouse2005; Villanueva & Norman, Reference Villanueva and Norman2008). During this free-swimming period, as they grow and their body proportions change, they gradually switch to a benthic mode of life (Itami et al., Reference Itami, Izawa, Maeda and Nakai1963; Villanueva, Reference Villanueva1995). Cephalopod hatchlings that exhibit an ecologically distinct stage from the adults have been termed paralarvae (Young & Hartman, Reference Young and Hartman1988).
The family Octopodidae contains over 200 species and hatchlings of the subfamily Octopodinae constitute the majority of planktonic octopod fauna (Hochberg et al., Reference Hochberg, Nixon, Toll, Sweeney, Roper, Mangold, Clarke and Boletzky1992; Norman & Hochberg, Reference Norman and Hochberg2005). However, due to the lack of adequate sampling methods, the uncertainties of species identifications and difficulties with rearing experiments, many of these young cephalopods in their first growth stages after hatching are still unknown (Rodhouse et al., Reference Rodhouse, Symon and Hatfield1992; Sweeney et al., Reference Sweeney, Roper, Mangold, Clarke and Boletzky1992; Villanueva, Reference Villanueva1995; Boyle & Rodhouse, Reference Boyle and Rodhouse2005). In addition, locating eggs in the field when they are directly linked to spawning adult females can provide insight into biogeography and reproductive patterns (Boletzky, Reference Boletzky1998; Barón, Reference Barón2001; Ortiz et al., Reference Ortiz, Ré and Márquez2006), and avoid a potential misidentification of the species or an oversimplification of the diversity of taxa represented in a region (Villanueva & Norman, Reference Villanueva and Norman2008).
Taxonomic keys to identify cephalopods often use characters of adults, which are not yet developed in the early life stages (Kubodera & Okutani, Reference Kubodera and Okutani1981). Nevertheless, when octopus hatchlings emerge from the eggs, they possess chromatophores of particular sizes, numbers and densities in fixed arrangements in the skin. They create a pattern of chromatophore distribution that may differ in different regions of the body. These are referred to as ‘chromatophore fields’ in morphological terms, and can have taxonomic value enabling species identification (Young et al., Reference Young, Hartman and Hochberg1989; Hochberg et al., Reference Hochberg, Nixon, Toll, Sweeney, Roper, Mangold, Clarke and Boletzky1992; Messenger, Reference Messenger2001). In addition, morphological characteristics such as the relative length of different arms and the number, arrangement and relative size of suckers also can be used to separate species (Young et al., Reference Young, Hartman and Hochberg1989; Hochberg et al., Reference Hochberg, Nixon, Toll, Sweeney, Roper, Mangold, Clarke and Boletzky1992; Vecchione, Reference Vecchione, Voss, Vecchione, Toll and Sweeney1998). In the case of the eggs of the family Octopodidae, the length of the chorion stalk and the size and shape of the chorion chamber containing the embryo are species-specific. Characterization of egg masses including the number of eggs and egg strings and the attachment of the chorion stalk to a substratum is also important for species identification (Boletzky, Reference Boletzky1998).
There is very limited knowledge on the life history of R. fontaniana (d'Orbigny, Reference d'Orbigny1834). It is a small benthic octopus, with a maximum-recorded total length of 280 mm for males, 260 mm for females, and oocyte lengths of 3–5 mm (Pickford, Reference Pickford1955; Ré, Reference Ré and Boschi1998). According to Pickford (Reference Pickford1955), this species is endemic to the sub-Antarctic region of South America. It has been cited for the south-east Pacific Ocean, from Peru (6°S) to Tierra del Fuego (55°S), and for the south-west Atlantic Ocean from San Matías Gulf (41°S) to the south, with a bathymetric distribution ranging from littoral coastal areas up to 225 m depth (d'Orbigny, Reference d'Orbigny1834; Adam, Reference Adam1938; Pickford, Reference Pickford1955; Castellanos, Reference Castellanos1967; Castellanos & Menni, Reference Castellanos and Menni1969; Nesis, Reference Nesis1987; Ré, Reference Ré1989, Reference Ré and Boschi1998; Ibáñez et al., Reference Ibáñez, Sepúlveda, Guerrero and Chong2008).
On the north and central Patagonian Atlantic coasts, R. fontaniana is found in submerged abrasion platforms in subtidal areas and infrequently under rocks or in scattered holes of intertidal areas (Ré, Reference Ré and Boschi1998). These habitats are also commonly occupied by commercial octopodid species such as the large-sized Enteroctopus megalocyathus (Gould, 1852) and the small-sized Octopus tehuelchus (d'Orbigny, Reference d'Orbigny1834). Although R. fontaniana is not the target species of these fisheries, it is occasionally caught and frequently misidentified by fishermen as O. tehuelchus (Ré, Reference Ré and Boschi1998) or as a juvenile stage of E. megalocyathus (N. Ortiz & M.E. Ré, personal observations).
In a recent work, a new diagnosis of the genus Robsonella Adam, Reference Adam1938 and a redescription of R. fontaniana based on immature and mature males and females, allowed differentiating both Robsonella from Octopus Cuvier, 1797 and R. fontaniana from other sympatric species (Ibáñez et al., Reference Ibáñez, Sepúlveda, Guerrero and Chong2008). In addition, the eggs, clutches and hatchlings of R. fontaniana were obtained from aquaria and briefly described for aquaculture purposes (González et al., Reference González, Arraigada, López and Pérez2008). However, no descriptions have been published that enable correct identification of samples taken from the wild.
The objectives of the present study were to describe the eggs, egg strings and hatchlings of R. fontaniana, to compare with those of other Patagonian coastal octopodids and to provide valuable ecological information for this poorly known species.
MATERIALS AND METHODS
Three clutches of eggs were obtained by scientific SCUBA divers from coastal waters of the San José Gulf (42°15′S 64°14′W), Argentina. One was found in April 2005, another in June 2005, and the last in May 2007. These clutches were encountered in artificial dens that were monitored monthly. Another clutch was found inside a natural subtidal refuge in the Malaspina Inlet (45°10′S 66°33′W) in April 2007. Except for those of April 2007, all clutches were found with the brooding females, which died shortly after capture. Stages of embryonic development at the time of egg collection were established according to Naef (Reference Naef1928). Time of embryonic development was obtained from laboratory rearing experiments when possible.
Egg clutches found in April 2005, June 2005 and April 2007 were removed from the dens and were kept indoors at the Centro Nacional Patagónico Laboratory, Puerto Madryn, Argentina. The clutches were reared in 5 l aerated glass aquaria with natural seawater previously filtered with 500 µm-filters, kept in darkness and cleaned with a fine brush twice a week. In order to minimize artificial hatching stimuli, cleaning of eggs was done only until most of the embryos underwent a second inversion in the chorion chamber. Seawater was changed every four days. To avoid thermal shock, incubations were conducted with seawater previously acclimated to experimental conditions for a period of 12 hours at approximately the same temperatures as registered at the specific site of collections by a temperature data logger (On Set-Tidbit). For these clutches, incubation water temperatures ranged from 10 to 12.1°C.
The May 2007 clutch was collected along with the artificial den and transported to the laboratory. We used this clutch to obtain the total number of eggs per clutch. The egg strings were removed and reared in the same conditions as the others, but randomly assigned to two incubation temperature regimes: one group from 13.7 to 14.5°C and the other group from 11.0 to 11.5°C. In order to register the duration of the hatching period, newly hatched animals were daily separated from this clutch.
The eggs and freshly dead paralarvae were measured with an ocular micrometer under a dissecting microscope. The egg length (EL), egg width (EW) and chorion stalk length (StL) were measured. The average length of egg strings and the average number of eggs per string were determined using only complete strings. Paralarvae were characterized by their total length (TL), dorsal mantle length (DML), ventral mantle length (VML), mantle width (MW), head width (HW), arm length (AL), eye diameter (ED), funnel length (FL), number of suckers per arm and chromatophore fields. In addition, several indices of relative size were calculated as the ratio of VML, MW, HW, TL, AL, ED or FL, and DML of the same paralarva. Two-tailed paired-sample t-tests (Zar, Reference Zar1996) were performed to compare: (1) the number of dorsal chromatophores with the number of ventral chromatophores; and (2) the number of dorsal mantle chromatophores against the number of ventral mantle chromatophores of hatchlings. Results of rearing experiments and morphological measurements presented include only hatchlings without external yolk at hatching (i.e. non-premature hatchlings). For hatchling descriptions, the terminology and measurements recommended by Young et al. (Reference Young, Hartman and Hochberg1989) and Hochberg et al. (Reference Hochberg, Nixon, Toll, Sweeney, Roper, Mangold, Clarke and Boletzky1992) were used.
A few newly hatched animals were anaesthetized at 3°C, fixed for 3 hours in 2.5% glutaraldehyde in phosphate buffer, and subsequently transferred to phosphate buffer for observations with scanning electronic microscopy (SEM). Samples were treated using the critical point dehydration method followed by gold covering.
For adult females the total weight (TW), DML, and TL were measured to the nearest g and mm respectively, and the ovaries were dissected in order to count the remaining oocytes.
Samples of the eggs and hatchlings were deposited at the Centro Nacional Patagónico-Colección de Invertebrados Marinos de la Patagonia (Voucher: CNP-CIP201), Puerto Madryn, Argentina.
RESULTS
Clutches and eggs
The clutches of eggs found in San José Gulf were attached underneath the cement anchor of a trap mooring, between the anchor and the sea sand bottom at depths from 4 to 26 m. Bottom water temperatures ranged from 11.5 to 15.0°C. The clutch from Malaspina Inlet was found underneath a rock at 12 m; temperature for this site is not available (Table 1).
Table 1. Robsonella fontaniana: data of sampling sites, morphological measurements of brooding females, and stages of embryonic development of the egg masses at time of egg collection.

ML, mantle length; n/a, not available; TL, total length; TW, total weight. Embryonic stages follow the scale of Naef (Reference Naef1928).
The small eggs showed an elongated-ovoid chorion shape, with a StL nearly 60% larger than the principal axis of the egg chamber. The chorion stalk was enlarged at its free end, and in almost all cases had a small swelling proximal to the egg chamber (Figure 1A & B). The stalks were entwined with one another in the central axis, which was surrounded with material secreted by the oviducal glands, and formed strings of a variable number of eggs (Figure 1C; Table 2). All brooding females were spent. In one complete clutch, the number of eggs was 2133 plus 20 mature oocytes which were observed free in the ovary lumen. The mean weights and lengths of the three captured brooding females were TW 81.9 g, DML 61.6 mm and TL 283 mm respectively (Table 1).

Fig. 1. Robsonella fontaniana: part of the clutch of eggs at the time of collection. (A) Eggs from the clutch of May 2007; from right to left embryos in stages of development VII, VIII and X, according to Naef (Reference Naef1928); (B) chorion stalk; (C) egg strings of the clutch of April 2005. Note the differences in the stages of embryonic development among eggs within a single egg string.
Table 2. Robsonella fontaniana: numerical and morphometric characteristics of egg clutches and eggs. For comparison, characteristics of other octopus species recorded from the Atlantic coast of Patagonia were included.

(a)Ortiz et al. (Reference Ortiz, Ré and Márquez2006) and Ortiz (Reference Ortiz2009); (b)Ré (Reference Ré1989, 1998); (c)Perez & Haimovici (Reference Perez and Haimovici1991). n/a, not available; *, mode; **, estimated fecundity; ***, nearly mature ovarian eggs.
Artificial egg incubation and hatching
In all cases, at the beginning of the rearing experiment and during the total time of incubation, individual eggs exhibited marked differences in the stages of embryonic development within egg clutches or even within a single egg string (Figure 1A & C; Table 1). In addition, high mortality was noticed during the period of embryonic development, and a large number of eggs hatched prematurely.
Eggs from the June 2005 clutch did not hatch, and eggs in the April 2007 clutch hatched with external yolk still attached to the hatchlings and thus in both cases these were not used for descriptions. The April 2005 clutch began hatching after 91 days of incubation at a mean temperature of 11.5 ± 0.5°C. The May 2007 clutch, incubated at 14.0 ± 0.1°C, began hatching 39 days after the start of the rearing experiment and hatching continued for 25 more days. The part of the same clutch incubated at 11.0 ± 0.1°C hatched after incubating for 68 days. Since these hatchlings were used for SEM images and due to high mortality, hatching period is not available for this group of animals.
The newly hatched paralarvae
Animals began swimming after hatching, with their mantle forward, in movements consisting of a series of short propulsions towards the surface. After several hours, this behaviour was followed by a period during which paralarvae were sinking down the water column and remained on the bottom of aquaria for a short period, before resuming propulsion movements.
MORPHOLOGICAL FEATURES OF PARALARVAE
The mantle is rounded, often appearing conical or ovoid. The arms are subequal in length but short relative to the mantle. They have long tapered tips, which are devoid of suckers, and occupy nearly 40% of the total length of the arms. The arms consistently possess five suckers. The two suckers closest to the mouth are arranged linearly, whereas the following three are in a zigzag row. The fourth sucker distally to the mouth, is noticeably elevated from the surface of the arms relative to the sucker closest to the mouth (Figures 2A & 3A). Suckers consist of an infundibulum encircled by a rim (Figure 2B). The infundibulum has numerous pegs endowed with pores and arranged in circles, with the outermost, last formed pegs having a smaller size (Figure 2B & C).

Fig. 2. Robsonella fontaniana: scanning electron micrographs of hatchlings. (A) Oral view showing the tapered tips of the arms and the number and position of the arm suckers; (B) oral view of an arm sucker: r, rim; i, infundibulum; (C) higher magnification of the infundibulum showing the projection of cuticular processes of the infundibulum or pegs (p) and pores over them; (D) Kölliker's organs with different degrees of expansion on the lateral and aboral sides of the arm: fascicle starting to emerge (head arrow), beginning to radiate (white arrow) and fully expanded (black arrow); (E) emerged fascicles of the Kölliker's organs distributed over the skin of the dorsal mantle; (F) oral view showing denticles of the upper and lower beaks.

Fig. 3. Robsonella fontaniana: schematic drawing of hatchlings. (A) Arm suckers at the time of hatching; (B) dorsal view; (C) ventral view; (D) lateral view.
Except for the oral surfaces of the arms, the skin of the whole animal is covered with Kölliker's organs, which are seen as iridescent structures in live animals. At hatching these bristle-like structures are found with different degrees of expansion, ranging from new fascicles beginning to emerge to radiate fascicles of 35–40 µm length (Figure 2D & E). The rostrums of both upper and lower beaks are denticulate (Figure 2F).
The head is narrower than the mantle and a large muscular funnel extending to the base of the arms, comprised 41% of the ventral mantle. The web is poorly developed (Figures 3 & 4). Morphological measurements of Robsonella fontaniana paralarvae are summarized in Table 3.

Fig. 4. Robsonella fontaniana: newly hatched paralarvae anaesthetized by lowering the temperature to 3°C. (A) Dorsal view; (B) ventral view; (C) lateral view; (D) live animal directly illuminated, adhered to the glass bottom with the chromatophore fields of the dorsal head, perivisceral epithelium and (partially) arms expanded. Note that the chromatophore fields are very distinctive on the dorsal and ventral mantle, the dorsal and ventral head, and the aboral side of the arms.
Table 3. Robsonella fontaniana: morphological measurements (in mm) and indices of freshly dead hatchlings.

*, calculated as the size of each body dimension relative to dorsal mantle length.
CHROMATOPHORES SHAPE, COLOUR AND ARRANGEMENT
In freshly dead or fixed hatchlings, retracted chromatophores over the whole animal appear as a suite of oval to round dots. However, due to the nerve-controlled expansion and contraction of the chromatophore organelles, live paralarvae exhibit a variety of body patterns depending on the relative expansion of the chromatophore pigment sac. Paralarvae are nearly transparent (i.e. chromatophores retracted) when they are swimming, or a variable degree of expansion of the different chromatophore fields either under stress situations (e.g. direct illumination) or when they are resting (Figures 3 & 4).
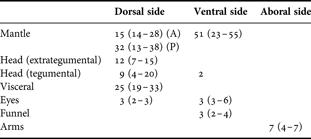
On the dorsal mantle, tegumental chromatophores are present on the anterior margin in two to three bands (5 to 7 across) and in a wide band (6 to 8 across) on the posterior margin but are absent in the midregion. Over the perivisceral epithelium, large tegumental chromatophores cover the dorsal surface of the visceral mass. They have a round to pentagonal shape according to the degree of expansion. This is the primary expanded chromatophore field in live animals (Figures 3B, 4A & D).
Over the dorsal head, three rows (2–6–4 pattern) of extrategumental chromatophores are present, with a similar size and shape to those of the dorsal visceral mass. The marginal chromatophores of the central row partially cover the eyes when expanded. Additionally, the dorsal head is spotted with a variable number (4 to 20) of smaller tegumental chromatophores extending to the dorsal arms, similarly to those of the anterior and posterior margins of the dorsal mantle (Figures 3B, 4A & D).
The aboral side of all arms has rectangular tegumental chromatophores in a single line decreasing in size towards the tip. In the first pair of arms, chromatophores appear continuous with those of the dorsal head (Figures 3B & 4D).
On the ventral mantle, tegumental chromatophores are uniformly and densely distributed (8–9 across) (Figures 3C & 4B), and are rectangular in shape when expanded. In many animals, these chromatophores extend up to the posterior mantle tip (Figures 3D & 4C).
Over the ventral head, two conspicuous tegumental chromatophores are present, one on each side adjacent to the funnel. They are oval in shape, and are the largest compared with those of other body regions (Figures 3C & 4B). In addition, in almost all cases, 2 to 4 chromatophores are observed dispersed or surrounding one or both eyes (Figures 3C, D & 4C). Small tegumental chromatophores of the funnel (2 or 3) are present on the ventral side, near the lip of the funnel orifice arranged in a single row. Chromatophores are not present over the ventral visceral mass (Figures 3C & 4B).
In R. fontaniana, two colour types of chromatophores are recognized. Over the ventral mantle, dorsal and ventral head, perivisceral epithelium and arms, expanded chromatophores are red and contracted chromatophores appear dark red. In addition, contracted and expanded chromatophores on the dorsal mantle and funnel, and small tegumental chromatophores over the dorsal head and arms range in colour from orange to somewhat yellow and are lighter than in all other chromatophore fields.
Mantle chromatophores are significantly more abundant ventrally than dorsally (t = 4.95, N = 15, P < 0.0002), but for the whole animal more chromatophores are found on the dorsal side than the ventral side (t = 7.10, N = 15, P < 0.0001). A summary of chromatophore numbers present in R. fontaniana hatchlings is shown in Table 4.
Table 4. Robsonella fontaniana: hatchling chromatophore numbers (mode and range) in different body areas (N = 17).
A, anterior margin; P, posterior margin.
DISCUSSION
Knowledge on the life history of Robsonella fontaniana is sparse. In particular, no previous information is available on any aspect of reproduction in wild populations. In this work, clutches of eggs and spent females were obtained in shallow waters and during a restricted period of three months (April, May and June) in different years and areas. In addition, hatchlings of this species were occasionally found in plankton samples taken in April at 10 m depth in Nuevo Gulf (42°07′S 65°03′W) (N. Ortiz, personal observation). These findings suggest that along the north and central Atlantic coasts of Patagonia the spawning and brooding season occurs at least from last summer to early winter, and that coastal shallow waters may act as spawning sites.
The hatching period from a single egg mass may reflect the extended time during which the eggs have been laid and could be influenced by incubation temperature and species. In consequence, a single egg mass can contain eggs in different stages of embryonic development (Boletzky & Hanlon, Reference Boletzky and Hanlon1983; Villanueva & Norman, Reference Villanueva and Norman2008). In this context, we observed different embryonic stages of development within a single clutch at the time of egg collection. But, we also detected embryos with differences in stages of development within a single egg string which would have been released at the same time (Boletzky, Reference Boletzky1986). Thus, in R. fontaniana other factors such as slight variations in the volume of yolk mass or the levels of yolk utilization on each egg (Boletzky, Reference Boletzky1989, Reference Boletzky2003; Laptikhovsky, Reference Laptikhovsky1999) could also explain the disparity in the embryonic developmental stages and the duration of hatching period that extended up to 25 days at 14°C from a single clutch. Besides, 3°C decreases in temperature over the duration of embryogenesis from stages VII–X resulted in a duplication of embryonic development time. This inverse relationship between environmental temperature and duration of embryonic development is expected to be non-linear. It may have a strong influence on the age structure of the population by causing differences in paralarvae settlement patterns (Boletzky, Reference Boletzky1974, Reference Boletzky1994; Katsanevakis & Verriopoulos, Reference Katsanevakis and Verriopoulos2006). This effect coupled with a hatching period from a single egg mass spread over weeks, could be pronounced in R. fontaniana whose recognized distribution comprises more than 14 latitudinal degrees in the south-western Atlantic Ocean, from Northern Patagonia to Tierra del Fuego, or even 49 latitudinal degrees in the south-eastern Pacific Ocean, from Peru to Tierra del Fuego.
Taxonomic characters of cephalopod hatchlings caught in different areas have been used for the development of identification keys within a specific geographical range (Kubodera & Okutani, Reference Kubodera and Okutani1981; Young et al., Reference Young, Hartman and Hochberg1989; Diekmann et al., Reference Diekmann, Piatkowski and Schneider2002; Bello, Reference Bello2004). At present, the other co-occurring coastal octopodid species recorded for the Patagonian Atlantic coast are Enteroctopus megalocyathus, Octopus tehuelchus and Eledone massyae Voss, 1964. For the latter species the eggs and hatchlings are still unknown. Both E. megalocyathus and O. tehuelchus have eggs with EL > 9–12 mm and hatchlings with ML > 6.5 mm. Based on nearly mature ovarian egg lengths (10–11.9 mm), E. massyae is expected to present similar EL and ML values (Iribarne, Reference Iribarne1991; Perez & Haimovici, Reference Perez and Haimovici1991; Ré, Reference Ré and Boschi1998; Ortiz et al., Reference Ortiz, Ré and Márquez2006). Since R. fontaniana showed EL < 5.2 mm and ML < 3.07 mm, their morphometric characteristics differ broadly from those of sympatric species.
However, eggs and hatchlings may undergo morphological changes with growth (Wells & Wells, Reference Wells, Wells, Giese and Pearse1977; Kubodera & Okutani, Reference Kubodera and Okutani1981; Young et al., Reference Young, Hartman and Hochberg1989; Hochberg et al., Reference Hochberg, Nixon, Toll, Sweeney, Roper, Mangold, Clarke and Boletzky1992) which may cause misclassification when using only these measures. Therefore, the amount of eggs in the clutches, the length of the chorion stalk, the way in which the chorion stalks are attached to a substratum (i.e. in festoons or individually) (Table 2) and chromatophore fields of hatchlings could be used to separate species in this area. In this sense R. fontaniana at hatching exhibited between 4 and 8 chromatophores in a single row on the aboral side of each arm (Table 4), whereas E. megalocyathus has between 14 and 23 with the same pattern (Ortiz et al., Reference Ortiz, Ré and Márquez2006). In contrast, the benthic hatchlings of O. tehuelchus have chromatophores densely covering the arms without a distinctive arrangement (Ortiz & Ré, personal communication). Thus, chromatophore fields on the arms could be used as a relatively quick and easy tool to differentiate species. Moreover, the patterns and positions of chromatophores remain unchanged throughout ontogenetic growth and are diagnostic in post-settlement animals (Villanueva & Norman, Reference Villanueva and Norman2008).
In this study, three clutches were found together with a brooding female. In addition, number of eggs per spawn, numerical and morphometric characteristics of strings, EL, EW, ML of hatchlings and duration of embryogenesis (at least for the clutch of April 2005, collected at earlier stages of development), were very similar to that recorded for R. fontaniana reared at 12 ± 2°C in captivity (González et al., Reference González, Arraigada, López and Pérez2008). These findings, as well as other differences with octopodid species presented in the region, allow assigning the clutches to R. fontaniana even in the absence of a brooding female, and could prevent against a potential misidentification of the paralarvae.
ACKNOWLEDGEMENTS
We express our gratitude to Mr Miguel Angel Hormiga Díaz and Ricardo Bebote Vera for their help with fieldwork and to Héctor Zaixso for sending the eggs from Malaspina Inlet. We are also grateful to Dr Pim Edelaar for careful reading and improvement of English language, to Jaime Groizard from Aluar for his assistance in obtaining SEM images, to Tomas Gimbernat for drawings of paralarvae and two anonymous referees for their useful suggestions and comments. Institutional support was given by Centro Nacional Patagónico (CONICET). Financial support was provided by Agencia Nacional de Promoción Científica y Tecnológica, PICT 2002 No. 12737.










