INTRODUCTION
It is globally recognized that one of the most impactful human activities on the seabed is bottom trawling (Jennings & Kaiser, Reference Jennings and Kaiser1998; Agardy, Reference Agardy2000), which has increased in recent decades worldwide (Løkkeborg, Reference Løkkeborg2005). The impacts of bottom trawling can be divided into (1) impacts on the seafloor and water mass environment, including scraping and ploughing of the seabed, sediment re-suspension, increasing water turbidity, alteration of seabed bathymetry and seabed flattening (Løkkeborg, Reference Løkkeborg2005; Depestele et al., Reference Depestele, Ivanović, Degrendele, Esmaeili, Polet, Roche, Summerbell, Teal, Vanelslander and O'Neill2016); and (2) impacts on organisms, habitat-forming species and ecological processes, including direct damage to target and discarded species, changes in species composition, reduction of biomass, production and diversity, alteration of the functional diversity of communities, and modification of ecosystem functioning (Kaiser & Spencer, Reference Kaiser and Spencer1995; Bergmann et al., Reference Bergmann, Beare and Moore2001; Hiddink et al., Reference Hiddink, Jennings, Kaiser, Queirós, Duplisea and Piet2006; Tillin et al., Reference Tillin, Hiddink, Jennings and Kaiser2006).
Physical impacts of fishing gears are variable and depend upon size and weight of the gear as well as fishing practice and behaviour. Bivalve dredges with teeth mounted along their width are used to target species that stay on the bottom or that are partly buried in the upper few centimetres of the sediment (Pérez Martín, Reference Pérez Martín2003). Impacts on benthic communities caused by these gears have been widely documented (e.g. Currie & Parry, Reference Currie and Parry1996; Pranovi et al., Reference Pranovi, Raicevich, Franceschini, Torricelli and Giovanardi2001; Leitão et al., Reference Leitão, Range and Gaspar2014). Nevertheless, information regarding the impacts of certain fishing gears is still scarce due to the complexity and natural variability of benthic communities, as well as the difficulty related to conducting this type of study (Løkkeborg, Reference Løkkeborg2005); the case of artisanal dredges operating at shallow bottoms in the western Mediterranean is an example (Urra et al., Reference Urra, García, León, Gallardo-Roldán, Lozano, Baro and Rueda2017).
One of the main target bivalve species in southern Europe is the venerid Chamelea gallina (Linnaeus, 1758), which is distributed throughout the Black and Mediterranean Seas and along the Algarve coast of southern Portugal, inhabiting preferentially coastal well-sorted fine sand biocoenoses (Pérès & Picard, Reference Pérès and Picard1964; Gaspar et al., Reference Gaspar, Pereira, Vasconcelos and Monteiro2004; Gofas et al., Reference Gofas, Moreno and Salas2011). The artisanal fleet targeting this species in the Black Sea, along the Italian coast of the Adriatic Sea and in the Gulf of Cadiz (SW Spain), uses hydraulic blade dredges (Froglia, Reference Froglia and Caddy1989; Dalgıç & Okumuş, Reference Dalgıç and Okumuş2006; Silva et al., Reference Silva, Delgado, Cojan, Juárez, Peña, Terrón, Acosta and Sobrino2014); whereas the fleet targeting it in the Alboran Sea and along the Algarve coast uses mechanized dredges (also called mechanical dredges) (Gaspar et al., Reference Gaspar, Leitão, Santos, Chícharo, Dias, Chícharo and Monteiro2003a). Many studies have been carried out to analyse the rate of growth, life span, age/size at first maturity and gametogenic cycle of this species (Ramón & Richardson, Reference Ramón and Richardson1992; Arneri et al., Reference Arneri, Giannetti, Polenta and Antolini1995; Rodríguez de la Rúa et al., Reference Rodríguez de la Rúa, Prado and Bruzón2003; Gaspar et al., Reference Gaspar, Pereira, Vasconcelos and Monteiro2004). Despite the importance of the C. gallina fishery, very few studies have analysed the discard composition and the damage sustained by both undersized commercial individuals and non-target species in this fishery (Moschino et al., Reference Moschino, Deppieri and Marin2003; Dalgıç et al., Reference Dalgıç, Okumuş and Karayücel2010; Dalgıç & Ceylan, Reference Dalgıç and Ceylan2012).
At present, a total of 247 boats are involved in the mechanized dredge fishery in the northern Alboran Sea, being part of a multi-target artisanal shellfish fleet. These boats vary in length from 7 to 11 m, with engines of an average ~35 Hp. The shellfish fleet targets mainly bivalves (e.g. wedge clam D. trunculus, striped venus clam C. gallina, smooth clam Callista chione and rough cockle Acanthocardia tuberculata), but it can focus its fishing effort on other target species (e.g. cephalopods) in certain seasons, which demand other gears. Mean total catches for C. gallina over the last 10 years are of ~24 (±4 SE) tons year−1, and worth 172,000 (± 25,400) € year−1, with a mean price of ~7.8 (±0.4) € kg−1 (http://www.juntadeandalucia.es). This fleet usually works with (i) dredges consisting of a rigid iron frame (~1 m length) with 40–50 round iron teeth (length: 10–15 cm; width: 8–10 mm) that rake the seabed and a plastic or metallic grid (mesh size: 17–20 mm) to hold the catch, and/or (ii) with dredges having a net bag (mesh size: 22 mm) and a lower number of iron teeth (~20) that are set further apart (up to 20 mm) (Baro et al., Reference Baro, Ramos, Camiñas and Núñez1992). Each fishing operation takes about 30–40 min (including dredging at ~10 m min−1, sorting and discarding), and is repeated several times in a circle around the ‘gavilán’ anchor (Figure 1). Marketable venus clams (>25 mm length) are retained and the rest, including undersized clams and non-target species (discards) are released to the sea.

Fig. 1. Fishing technique using mechanized dredges targeting venus clam (Chamelea gallina) in the northern Alboran Sea. Inset: diagram of the fishing operation making concentric lines around the ‘gavilán’ anchor.
Data regarding discard composition and discard rate for the mechanized dredging fishery of the southern Iberian Peninsula are scarce, and the information on the damage caused by these fishing gears on non-target species is very limited (Urra et al., Reference Urra, García, León, Gallardo-Roldán, Lozano, Baro and Rueda2017). This kind of information is crucial for the improvement of management of fisheries at an ecosystem level, as it is established in the EU Marine Strategy Framework Directive (2008/56/CE) and in the Common Fisheries Policy (EU Regulation No. 1380/2013), among other directives. This study analyses for the first time (i) the discards generated in the venus clam mechanized dredging fishery in the northern Alboran Sea (western Mediterranean), (ii) its seasonal and spatial variability, and (iii) its impact on the benthic and demersal fauna inhabiting the fishing grounds where the fleet operates in the northern Alboran Sea.
MATERIALS AND METHODS
Data collection
The discard composition/structure and the damage assessment were evaluated from discard samples collected at two fishing sites 29.7 nautical miles apart, Fuengirola and Caleta de Vélez (hereafter Caleta), along the northern margin of the Alboran Sea (Figure 2), as these areas represent very important fishing grounds for the artisanal fleet. Soft bottoms here are composed of terrigenous sediments with a dominance of fine sands and a high bioclastic (e.g. empty shells) content in Fuengirola, with mud content ranging between 2–21% and values for percentage of organic matter between 1–3%; whereas in Caleta, bottoms are composed of fine and medium sands, with higher values of both mud (2–77%) and organic matter content (1.6–4%) (García Raso et al., Reference García Raso, Salas Casanova, Gofas, Manjón-Cabeza, Urra, Mateo-Ramírez and Marina2007; Sanz et al., Reference Sanz, Tello, Hermida, Fernández-Salas and Pastor2007; Urra et al., Reference Urra, Gofas, Rueda and Marina2011).

Fig. 2. Map of the study area in the northern Alboran Sea (western Mediterranean Sea), showing the location of the artisanal shellfish hauls analysed (black circles).
Samples were collected on board five similar commercial vessels (‘El Lele’, ‘Hermanos Urda’ and ‘Nuevo Marielva’ in Caleta; ‘Nuevo Hermanos Madueño’ and ‘Nuevo Pelao’ in Fuengirola) from 101 hauls carried out between March 2013 and March 2014. These were based on fishermen availability; N = 46 hauls for Caleta (spring: 15; summer: 15; autumn: 9; winter: 7), N = 55 hauls for Fuengirola (spring: 13; summer: 14; autumn: 9; winter: 19). Data regarding the fishing operation and the commercial catch were obtained onboard (catch was weighed (i.e. total wet weight) using hanging analogue scales). Normal fishing practices were adopted during the study. Overall, hauls were performed at a mean depth of 4.2 m and lasted 14.4 min, covering a mean area of 473.7 m2. Random samples of a standardized weight of 5 kg were collected from the caught material and handled carefully, once the target individuals were separated by fishermen and before the non-commercial specimens were discarded at sea. Discard samples were then stored at −20°C until further processing in the laboratory, following the protocol used by Pranovi et al. (Reference Pranovi, Raicevich, Franceschini, Farrace and Giovanardi2000, Reference Pranovi, Raicevich, Franceschini, Torricelli and Giovanardi2001) for damage assessment to non-target species.
Once defrosted, inorganic (e.g. bioclasts, pebbles) and plant remains (e.g. seagrass, macroalgae) were separated from each sample and quantified, whereas individuals were handled carefully in order to avoid additional damage to the discarded specimens. Collected individuals were identified to species level (when possible) and quantified (abundance and biomass (±0.1 g wet weight)). Individual damage was assessed on a three-level scale depending on the morphology of the taxon (intact, intermediate and severe damage, as detailed in Table 1), and expressed in a standardized form for each species as percentage of individuals within each damage category (Bergmann et al., Reference Bergmann, Beare and Moore2001). The experimental protocol considered the possibility of autotomy of arms and appendages by some echinoderms and crustaceans, respectively, as part of the fishing effects (e.g. physical damage, air exposure and acute temperature changes). Additionally, freezing/thawing can alter the body consistency, especially in crustaceans (i.e. loss of limbs) and echinoderms (i.e. loss of arms), therefore further loss of arms and appendages was expected. Nevertheless, a low amount of them were detected in the sorted samples. Scientific names for all taxa followed the nomenclature of the World Register of Marine Species (WoRMS) (http://www.marinespecies.org/).
Table 1. Criteria used for damage assessment of different taxa discarded in the northern Alboran Sea mechanized dredge fisheries targeting venus clams.

Data analysis
Catch abundance, biomass and damage data were standardized to 15 min fishing operations (average hauling duration; N = 101). The abundance, weight, dominance (percentage of individuals/biomass of a species from the total catch) and frequency index values (percentage of samples in which a species is present) were calculated for every discarded species. The discard ratio of the fishery was calculated as σ discards/σ total catch (excluding debris). Comparisons between catch fractions and damage in different fishing grounds and seasons, as well as in frequently discarded species, were performed by means of one and two-factor analysis of variance (ANOVA). Transformation of data (i.e. arc-sine transformation for data expressed as percentages) was applied to satisfy the assumptions of parametric statistics (normality using Kolmogorov–Smirnov test, homoscedasticity using Levene test). Post hoc Tukey tests (P < 0.05) were used for a posteriori multiple comparisons.
The identification of similarities in the composition and structure of discards according to fishing ground was done using multivariate methods based on the Bray–Curtis similarity index. A fourth-root transformation pre-treatment was applied on the quantitative (abundance, biomass) data in order to minimize the contribution of the most abundant species to the analysis (Clarke & Warwick, Reference Clarke and Warwick2001). Distance-based permutational multivariate analysis of variance (PERMANOVA) was carried out on both biomass and abundance data to test for statistical differences in composition and structure between discards analysed in each site. The SIMPER procedure was used to identify those species that contributed most to the dissimilarity between discards.
The BIOENV procedure and distance based linear models (DistLM procedure) were used to test the linkage between damage (intermediate and severe data were pooled together) data and fishing (tow duration, trawled area, speed and depth), haul (amount of gravel in the catch, remains of shells, total wet weight (total catch + inert material)) and gear characteristics (number of iron teeth, length, width, distance among them, mesh size). Moreover, a Spearman rank correlation was run to test the relationship between damage data and fishing gear and haul characteristics. Univariate statistical analyses were performed using SPSS© statistical software, whereas multivariate analyses were executed using the software PRIMER6 and PERMANOVA+(Clarke & Warwick, Reference Clarke and Warwick2001).
RESULTS
Discard analysis
Considering all components of the total collected material in the 101 hauls analysed, marketable venus clams represented 17.7% of the total weight, 47.2% corresponded to discards and 34.9% to debris (24.7% to dead shells, 8.9% to gravels and 1.3% to algae). The catch of the target Chamelea gallina (hereafter commercial catch) displayed an overall significant decreasing temporal pattern from spring (highest values) to winter (lowest values) (one-factor ANOVA: F = 9.13, P < 0.005), and the opposite trend (maxima in winter) was observed for the discarded biomass of both the target species and the total discard (one-factor ANOVA: C. gallina discard, F = 7.78; total discard, F = 10.69, in both cases P < 0.005). Significant spatial differences were observed for the mean commercial catch, being significantly higher in Caleta (one-factor ANOVA: F = 5.63, P < 0.05), and for the discarded fraction, with maxima in Fuengirola (one-factor ANOVA: F = 30.2, P < 0.005) (Table 2). The discard/total catch ratio (excluding debris) showed high fluctuations between hauls (minima: 0.1; maxima: 0.98), displaying significant spatial and temporal differences (two-factor ANOVA: factor site: F = 17.9, P < 0.005; factor season: F = 3.2, P < 0.05; interaction: F = 0.85, P = 0.47) with maxima in spring for Caleta (0.63 ± 0.03) and in winter for Fuengirola (0.91 ± 0.01).
Table 2. Fishing characteristics and catch composition (mean biomass ± SE) recorded in 101 hauls analysed in the venus clam fishery with mechanized dredges of Fuengirola and Caleta de Vélez (northern Alboran Sea).

Information regarding the percentage of individuals with intermediate (%Ni) and severe (%Ns) damage for different faunistic groups is detailed. Proportions are given as percentages of the total number (N). Catch composition data are provided as standardized to 15 min haul duration. Analyses of variance (ANOVA) were carried out for testing spatial differences in the fishing characteristics. Significant differences (P < 0.005) are indicated with*.
Discards were composed of 96 taxa, with molluscs the best represented group with 53 species, followed by fish (17 spp.), decapods (12 spp.) and echinoderms (9 spp.). The number of species should be even higher because paguroid decapods and annelids could not be identified to species level. Taxa dominating discards in terms of biomass and abundance are indicated in Table 3. A large part of the fauna collected were filter feeders and deposit feeders (mainly bivalves), whereas about one third of the identified species correspond to carnivores and opportunistic scavengers (e.g. swimming crabs, hermit crabs, starfish, ophiurids, gastropods) of moribund discarded benthic fauna. In terms of biomass, infaunal bivalves dominated (~78% total biomass in Fuengirola; ~90% in Caleta), and the pattern was similar for the abundance, with a higher fraction of other faunistic groups in Fuengirola than in Caleta (Figure 3, Table 2). In fact, PERMANOVA revealed significant spatial differences in the discarded biomass (Pseudo-F = 16.1; P < 0.001) and abundance (Pseudo-F = 16.9; P < 0.001) among fishing grounds, with a higher discard for most quantitatively dominant taxa in Fuengirola according to SIMPER (average dissimilarity >52%). Overall, the mean biomass of discard caught per 500 g of target species (60 ind.) was 1334.3 g and includes 923.4 g of bivalves (106 ind.), 97.5 g of echinoderms (24 ind.), 73.4 g of decapods (54 ind.) and 33.7 g of gastropods (22 ind.), among other taxa.

Fig. 3. Relative composition of discards in the venus clam fishery in Caleta de Vélez (A, C) and Fuengirola (B, D) (northern Alboran Sea). Biv., bivalves; Gast., gastropods; Echin., echinoderms; Dec., decapods.
Table 3. Faunistic list of the top-25 dominant macrobenthic organisms in discards of the venus clam fishery in Fuengirola and Caleta de Vélez (northern Alboran Sea).

Mean abundance (N; ind·haul−1) and biomass (B; g·haul−1) (± standard error), as well as frequency index value (%F; percentage of samples in which a species is present) and percentage of individuals with severe damage (%Ns; proportions are given as percentages of the total number), are indicated for each taxa and site. Species belong to the following taxonomic groups: 1Class Bivalvia (Mollusca); 2Class Gastropoda (Mollusca); 3Order Decapoda (Crustacea); 4Class Echinoidea (Echinodermata); 5Class Ophiuroidea (Echinodermata); 6Class Asteroidea (Echinodermata); 7Class Actinopterygii (Chordata); 8Class Polychaeta (Annelida).
Damage assessment
The damage assessment for all species showed that 84.2% (±1.4) were undamaged individuals, 4.5% (±0.7) were individuals displaying intermediate damage and 11.3% (±1.1) displayed severe damage. The intermediate damage level was significantly higher in Caleta than in Fuengirola (two-factor ANOVA: factor site: F = 36.2, P < 0.001; factor season: F = 0.9, P = 0.43; interaction: F = 2.9, P < 0.05), and the pattern was similar for severely damaged individuals (two-factor ANOVA: factor site: F = 15.6, P < 0.001; factor season: F = 1.9, P = 0.13; interaction: F = 1.5, P = 0.21) (Table 2). The highest proportions of damaged individuals were observed in winter and spring in both Caleta and Fuengirola (Figure 4).
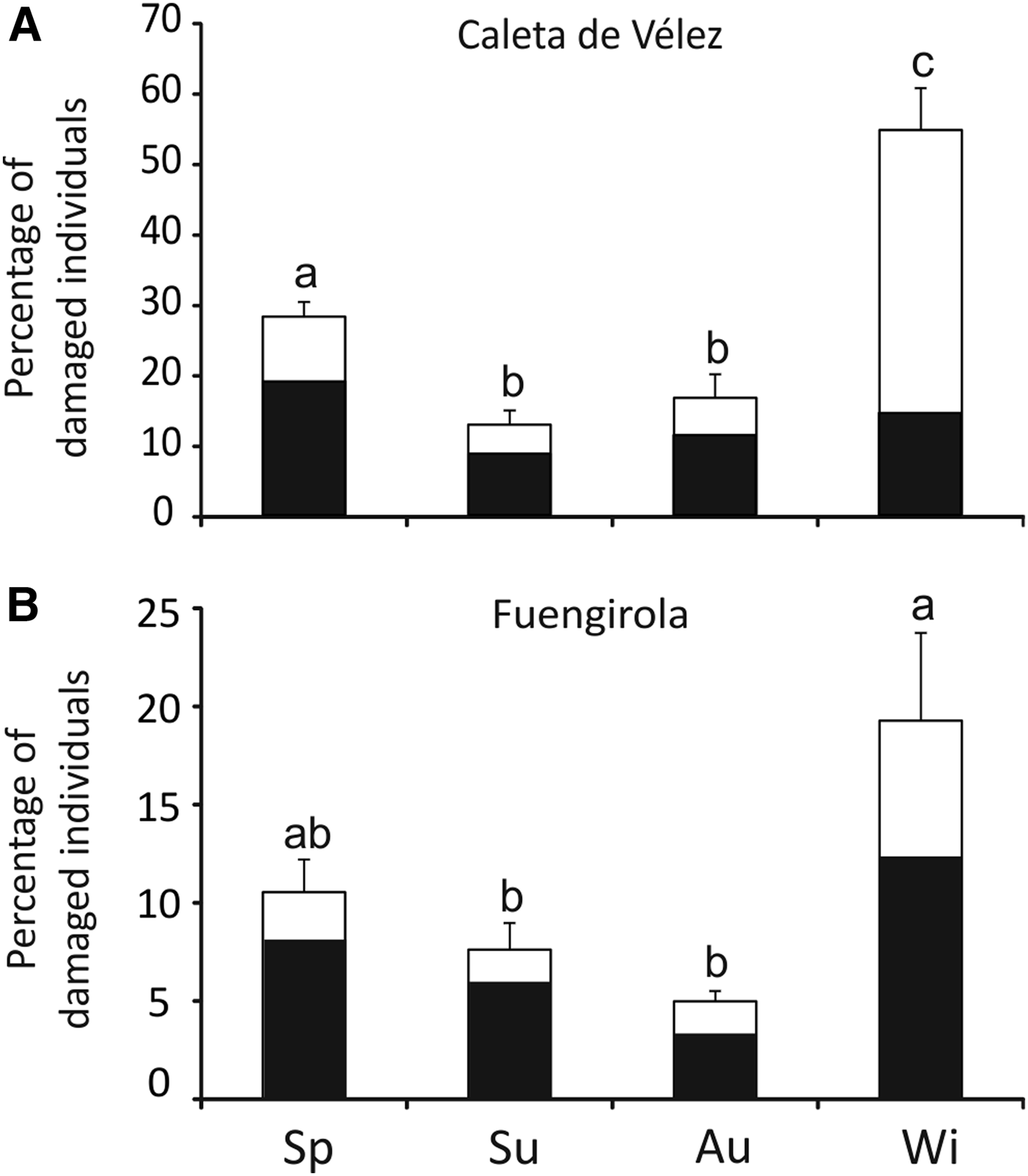
Fig. 4. Proportion of damaged individuals (% ind. haul−1) in discards of the venus clam fishery of Caleta de Vélez (A) and Fuengirola (B) (northern Alboran Sea). Shaded areas of bars indicate proportion with severe damage and empty areas indicate proportion with intermediate damage. Error bars represent standard error. Lower case letters above error bars display the results of post-hoc test; different letters distinguish significantly different means at P < 0.05. Sp, spring; Su, summer; Au, autumn; Wi, winter.
The analysed fishing, haul and gear characteristics displayed a low but significant correlation with individuals that had some sort of damage (intermediate and severe pooled together; hereafter total damage) (BIOENV: ρ = 0.36, P < 0.005). The best combination of variables through this correlation was, according to BIOENV analysis, amount of gravel–number of iron teeth–iron teeth width, whereas DistLM marginal tests also assigned some of the variation to tow duration and iron teeth length (P < 0.05). Significant but very low positive correlations were found between the proportion of damaged individuals and tow duration (R S = 0.39, P < 0.001), number of iron teeth (R S = 0.33, P < 0.001) and length of iron teeth (R S = 0.29, P < 0.005), whereas a significant negative correlation was found between the proportion of damaged individuals and width of iron teeth (R S = −0.55, P < 0.001). Figure 5 shows the distribution of analysed samples with damage data in relation to the analysed fishing, haul and gear characteristics, reflecting spatial differences in the level of damage caused to discards.

Fig. 5. Two-dimensional biplot showing the relationship of total damage data and environmental variables including fishing (TD, tow duration; TA, trawled area; S, speed; De, depth), haul (G, amount of gravel in the catch; B, remains of shells or bioclasts; TW, total wet weight (total catch + debris)) and gear characteristics (N, number of iron teeth; L, length; W, width; Di, distance among them; MS, mesh size).
Echinoderms showed the highest level of total damage within discards in both sites, with proportions of severely damaged individuals significantly higher in Fuengirola (Table 2; one-factor ANOVA: F = 5.6, P < 0.05). Crustaceans and bivalves also displayed high levels of intermediate and severe damage, respectively, in both sites (Table 2), with significantly higher proportions of damaged individuals in Caleta (one-factor ANOVA: crustaceans with intermediate damage, F = 7.8; bivalves with severe damage, F = 30.1, P < 0.005 in all cases). Some taxa with minor catch (cephalopods, cnidarians, nemerteans, fish, sipunculids and polychaetes) also showed high levels of damage (e.g. 100% damage for fish due to the high degree of maceration and/or fragmentation of their bodies), but they represented less than 1.5% of total damaged individuals. The observed pattern was similar regarding biomass, with echinoderms showing damage to 75.2% of their biomass. In contrast, none of the paguroids and <1% of gastropods were severely damaged.
Damage among the most abundant and/or frequently caught species was highly variable (Figure 6; Table 3). Regarding severe damage, the echinoid Echinocardium cf. mediterraneum (Forbes, 1844) was the most susceptible species with 84.9% of individuals displaying crushed exoskeletons. The decapod Liocarcinus vernalis (Risso, 1827) showed ~50% of individuals with crushed carapace and/or the loss of both chelae and many pereiopods, whereas the bivalve Mactra stultorum (Linnaeus, 1758) showed 34.9% of individuals with crushed valves or fatal chipping at the outer margin of the shell or even hinge displacement. Other echinoderms such as Ophiura ophiura (Linnaeus, 1758) and Astropecten irregularis (Pennant, 1777) showed ~18% of severely damaged individuals, with loss of all arms and/or damage to the disc. The target species C. gallina showed a low proportion of damaged individuals (4.9% ind.), probably due to their thick protective shell as also observed for other bivalves such as Callista chione (Linnaeus, 1758) and Glycymeris glycymeris (Linnaeus, 1758) (~3% ind.). Percentages of severely damaged individuals were generally higher in Caleta, with significantly higher values for abundant species including C. gallina, the bivalves Acanthocardia tuberculata (Linnaeus, 1758) and G. glycymeris, and E. cf. mediterraneum (Figure 6; Table 4). Regarding seasons, few of them showed significant seasonal differences in the proportion of severely damaged individuals, including C. gallina that showed the maxima in spring in both sites, and the bivalve Glycymeris nummaria (Linnaeus, 1758) and O. ophiura that showed maxima in autumn-winter (Table 4).

Fig. 6. Mean abundance of the most representative discarded species in the venus clam fishery of Caleta de Vélez (A) and Fuengirola (B) (northern Alboran Sea). Shaded areas of bars indicate proportion of individuals with any damage (intermediate and severe pooled together). Mean + standard error.
Table 4. Results of two-factor ANOVA analyses for testing differences in the proportion of damaged individuals of frequent discarded species in relation to site and season in the northern Alboran Sea mechanized dredge fisheries targeting venus clams.

DISCUSSION
This study provides the first complete assessment of the discards associated with the mechanized dredging fleet targeting venus clam (Chamelea gallina) in the western Mediterranean Sea, where the information regarding discards of this type of fisheries is very limited (Urra et al., Reference Urra, García, León, Gallardo-Roldán, Lozano, Baro and Rueda2017). The analysis of discards could provide a baseline for fisheries assessment, but also a useful tool for monitoring benthic biodiversity components such as native and exotic range-expanding species in this area where some marine protected areas have been recently declared for the benthic biodiversity conservation (e.g. Calahonda) (Urra et al., Reference Urra, Marina, Rueda, Mateo-Ramírez, García, Baro, Gofas, Salas and García Raso2015).
Our results suggest a poorly selective fishery, with the collection of more than double the biomass of discarded species than target species (~73% of total catch excluding debris). This would confirm that new more selective fishing gears should be developed in order to reduce high discard values, prompting further field experiments on the selectivity of traditional dredges towards improving their efficiency. Similarly, high percentages of discards are commonly reported in fisheries targeting bivalves in European coastal waters, such as in ‘Rapido’ trawling for scallops (Pranovi et al., Reference Pranovi, Raicevich, Franceschini, Torricelli and Giovanardi2001), in hydraulic dredging for venus clams (Morello et al., Reference Morello, Froglia, Atkinson and Moore2005), or in hydraulic blade dredging for razor clams (Hauton et al., Reference Hauton, Atkinson and Moore2003). Regarding this, Gaspar et al. (Reference Gaspar, Castro and Monteiro1999) concluded that tooth spacing did not have an effect on the selectivity of clam dredges of the southern Portuguese artisanal fleet, whereas an appropriate mesh size will result in a considerable reduction in the numbers of small- and medium-sized individuals in the catch.
Spatial differences observed for the commercial catch and the discarded fraction could be related to (i) the different sedimentary characteristics of the two fishing sites, which would determine differences in the composition and structure of the benthic community as reflected by the multivariate analyses, (ii) the patchy spatial distribution of the target species, and (iii) some minor different gear characteristics due to the artisanal character of this fishery. Hall-Spencer et al. (Reference Hall-Spencer, Froglia, Atkinson and Moore1999) and Pranovi et al. (Reference Pranovi, Raicevich, Franceschini, Torricelli and Giovanardi2001) reported high discard/commercial ratios in areas with high densities of non-target species for the Rapido trawling fisheries in the northern Adriatic Sea; and Gaspar et al. (Reference Gaspar, Dias, Campos, Monteiro, Santos, Chícharo and Chícharo2001) reported by-catch proportions of 25–48% in the Portuguese smooth clam fishery due to differences in gear specifications and structure and composition of benthic communities. Results provided here suggest that a spatial management plan based on the type of grounds being fished would be useful in order to improve efficiency of these fisheries and minimize their impact in soft bottoms with different commercial catches and biological communities.
Data collected in this study would suggest that mechanized dredges with a lower number of narrower iron teeth, and towed for a shorter time, would decrease the damage rate in discards of this fishery. Nevertheless, further experimental hauls should be undertaken in order to confirm if the decreased damage rate is observed in other independent studies. Moreover, the amount of gravels in the catch was also selected as a variable explaining some of the variation in damage data, therefore becoming a factor to be considered when selecting areas for fishing regarding the type of community inhabiting that ground. Many authors have reported that damage to benthic organisms is dependent on several factors acting together, including vessel, fishing gear and haul characteristics, which can differ according to depth and habitat type (Gaspar et al., Reference Gaspar, Castro and Monteiro1998, Reference Gaspar, Leitão, Santos, Sobral, Chícharo, Chícharo and Monteiro2002, Reference Gaspar, Leitão, Santos, Chícharo, Dias, Chícharo and Monteiro2003a; Bergmann et al., Reference Bergmann, Beare and Moore2001; Pranovi et al., Reference Pranovi, Raicevich, Franceschini, Torricelli and Giovanardi2001), as well as on-deck sorting procedures (Pranovi et al., Reference Pranovi, Raicevich, Franceschini, Torricelli and Giovanardi2001). Moreover, damage to benthic organisms is higher when fishing gears are towed on rocky, muddy or detritic bottoms because the net fills with different materials that considerably damage the catch during towing, hauling and sorting operations (Jennings & Kaiser, Reference Jennings and Kaiser1998; Pranovi et al., Reference Pranovi, Raicevich, Franceschini, Torricelli and Giovanardi2001), as perceived in the multivariate analyses of this study. Therefore, the impact of even small artisanal dredges on benthic communities is real but complex and difficult to assess, with a detailed analysis of each fishery being necessary towards understanding the extent of damage sustained by organisms.
Damage was generally higher in winter but the reasons for this are difficult to identify and are probably linked to a combination of factors associated with the catching and sorting process. According to the analysed data, this could be related to the higher amounts of discards in this season, mainly of hard shelled molluscs which accounted for ~75% of the total catch, including undersized target individuals. This hard material promotes the abrasion between organisms with weak exoskeletons (e.g. irregular echinoids, ophiuroids) and those with hard shells and with debris inside the metallic grid during towing and hauling (Pranovi et al., Reference Pranovi, Raicevich, Franceschini, Torricelli and Giovanardi2001; Broadhurst et al., Reference Broadhurst, Suuronen and Hulme2006). Other factors to be considered are the higher fragility of certain species during their moulting periods, for example of decapods (Raviv et al., Reference Raviv, Parnes, Sagi and Mente2008), that makes them more susceptible to damage from fishing activities, or the bathymetric migrations linked to reproductive strategies. Moreover, discarded organisms are also subject to additional cumulative stress linked with being brought to the surface, exposed to air for sorting activities and thrown from the vessel (Broadhurst et al., Reference Broadhurst, Suuronen and Hulme2006), which should be monitored in future studies to decrease potential negative effects. Furthermore, other potential factors would be related to the lower catch of the target species in cold months, together with the higher catch of discards, which would involve a higher number of fishing operations to get the daily catch. In this respect, the artisanal fleet concentrates its fishing effort in a narrow stretch of coastline, and thus the probability of capturing and damaging previously injured organisms is high.
Overall ~84% of total individuals collected were undamaged but every faunistic group displayed some sort of damage in the discards of this fishery, with echinoderms generally displaying the highest level of damage. Several authors have reported on the fragility of echinoids as an impact of fishing activities, linking their brittle and vulnerable exoskeletons with their high mortality (Hall-Spencer et al., Reference Hall-Spencer, Froglia, Atkinson and Moore1999; Pranovi et al., Reference Pranovi, Raicevich, Franceschini, Torricelli and Giovanardi2001). The echinoids Echinocardium spp. live buried in sandy sediments and move at a maximum speed of 6–8 cm per hour (Schultz, Reference Schultz2006), so mechanized dredges can collect many individuals of these species that are generally damaged due to rubbing and crushing with the collected hard material. A high percentage of collected asteroid, and especially ophiurid individuals, presented missing arms and arms that were regenerating, probably associated with previous fishing impacts (Gallardo-Roldán et al., Reference Gallardo-Roldán, Urra, García, Lozano, Antit, Baro and Rueda2015). Ophiuroids have been reported to be more susceptible to damage than asteroids, with higher mortality rates due to their fragile exoskeleton (Kaiser & Spencer, Reference Kaiser and Spencer1995; Bergmann et al., Reference Bergmann, Beare and Moore2001); although both groups have a high regenerative capacity (Candia Carnevali, Reference Candia Carnevali2006).
The loss of a chela or few pereiopods (many times with breaks extending into the body) was frequently observed in all decapod species, as well as crushed carapaces, which represent a major cause of death (Juanes & Smith, Reference Juanes and Smith1995; Bergmann & Moore, Reference Bergmann and Moore2001; Bergmann et al., Reference Bergmann, Beare and Moore2001). This is of importance because several authors have reported that the consequences of non-lethal injuries can increase the long-term mortality in discarded live damaged crustaceans (Smith & Hines, Reference Smith and Hines1991; Juanes & Smith, Reference Juanes and Smith1995; Hall-Spencer et al., Reference Hall-Spencer, Froglia, Atkinson and Moore1999; Bergmann & Moore, Reference Bergmann and Moore2001), especially in particularly vulnerable species such as the abundant L. vernalis. In contrast, almost all hermit crabs were in perfect condition due to the protection provided by gastropod shells. These organisms are important scavengers that can consume large amounts of discards, enhanced by their ability to quickly migrate and aggregate in recently trawled areas (Ramsay et al., Reference Ramsay, Kaiser and Hughes1996). This could partly explain why hermit crabs are among the most abundant taxa in total catches in areas that are frequently dredged such as those studied in the northern Alboran Sea.
Bivalves were much more susceptible to damage than gastropods, the latter being generally undamaged as a result of their strong shells and, in some cases, smaller sizes. Nevertheless, larger species (e.g. Cymbiun olla) showed some chipping of the outer lip, whereas some bivalve species characterized by relatively thin shells (e.g. M. stultorum, Pharus legumen, Ensis ensis and E. minor), displayed a high percentage of damaged individuals, as observed in other shallow dredging fisheries (Hall-Spencer et al., Reference Hall-Spencer, Froglia, Atkinson and Moore1999; Pranovi et al., Reference Pranovi, Raicevich, Franceschini, Torricelli and Giovanardi2001; Gaspar et al., Reference Gaspar, Santos, Leitão, Chícharo, Chícharo and Monteiro2003b). In contrast, thick-shelled bivalves are more resistant to fishing (Bergmann et al., Reference Bergmann, Beare and Moore2001; Gaspar et al., Reference Gaspar, Santos, Leitão, Chícharo, Chícharo and Monteiro2003b), as detected for the target species C. gallina or other commercial bivalves (e.g. C. chione), of which <5% of total individuals presented severe damage. This is of importance for commercial species (but also for non-commercial species) because it promotes the survival of discarded undersized target individuals, contributing to the sustainable exploitation of the resource.
In summary, mechanized dredging targeting venus clams in the northern Alboran Sea can cause considerable damage to some benthic and demersal species and groups in particular, but only corresponds to 15.8% of the total individuals collected in discards. The level and type of damage observed is dependent on species-specific characteristics such as morphology, body size and structure, and the extent of this damage is influenced by fishing gear and haul characteristics. Experimental hauls with modified mechanical dredges on different soft bottom types should be undertaken in order to investigate how to minimize their ecological impact. Considering the low proportion of damaged individuals observed in discards of this fishery, and that discards are released on suitable sedimentary habitats soon after on-deck sorting, a high potential survival rate for many organisms could be expected (Broadhurst et al., Reference Broadhurst, Suuronen and Hulme2006). This would further support the temporary derogation of the landed obligation that has recently been granted for certain fisheries in the Mediterranean Sea, including those targeting bivalves (mentioned as Venus spp. in the EU documents). Nevertheless and despite an apparent good condition for many discarded benthic invertebrates, long-term mortality of discards is likely to occur due to sub-lethal damage, internal injuries and physiological stress, as observed in different studies and experiments (Kaiser & Spencer, Reference Kaiser and Spencer1995; Gaspar & Monteiro, Reference Gaspar and Monteiro1999; Bergmann & Moore, Reference Bergmann and Moore2001; Pranovi et al., Reference Pranovi, Raicevich, Franceschini, Torricelli and Giovanardi2001). Therefore, a low proportion of damaged individuals would not necessarily imply a high survival, and post-trawling mortality of discarded organisms may have been underestimated in the past (e.g. for crustaceans: Bergmann & Moore, Reference Bergmann and Moore2001). Despite this, benthic communities inhabiting shallow and dynamic environments with soft sediments have shown a greater capacity to recover from fishing disturbance (Jones, Reference Jones1992; Currie & Parry, Reference Currie and Parry1996; Kaiser et al., Reference Kaiser, Edwards, Armstrong, Radford, Lough, Flatt and Jones1998), and could also benefit from the high local productivity of the northern Alboran Sea (Sarhan et al., Reference Sarhan, García Lafuente, Vargas, Vargas and Plaza2000), which could decrease the long-term impact of this artisanal dredging fishery on soft bottoms of the Alboran Sea.
ACKNOWLEDGEMENTS
We thank the captains and crew of the commercial vessels who have collaborated with the REMARAN team; to Ana Garrido, Alejandro J. Ibañez Yuste and Alejando Terrón Sigler (AGAPA-Junta de Andalucía) for the collection of faunistic samples and continuous interest; to Alba Rojas García, Blanca Orúe Montaner and Marina Gallardo Núñez for their help in processing the samples; to José Miguel Serna (Centro Oceanográfico de Málaga) for his help with the GIS facilities, and other members of the research team for their collaboration.
FINANCIAL SUPPORT
This study was developed under the collaboration agreement between Junta de Andalucía (Spain) and Instituto Español de Oceanografía (IEO) (Contract 126/2012-SEN), within the framework of the research project entitled ‘Estudio integral en zonas de protección pesquera y marisquera y otras áreas marinas protegidas del litoral andaluz: Estudio previo para la protección, ordenación y determinación de reservas marisqueras en el litoral mediterráneo de Andalucía’ (REMAN-REMARAN). This project was funded by the European Fisheries Fund.












