INTRODUCTION
Little is known of the behaviour or ecology of most tropical sea cucumbers such as Stichopus cf. horrens, despite their significant role in marine benthic ecosystems and their commercial importance (Wolkenhauer et al., Reference Wolkenhauer, Uthicke, Burridge, Skewes and Pitcher2010; Bordbar et al., Reference Bordbar, Anwar and Saari2011; Kiew & Don, Reference Kiew and Don2012; Mactavish et al., Reference MacTavish, Stenton-Dozey, Vopel and Savage2012; Purcell et al., Reference Purcell, Choo, Akamine and Fabinyi2014, Reference Purcell, Conand, Uthicke and Byrne2016). Information vital to effective conservation and culture efforts, e.g. activity patterns, behaviour, in situ growth rates and life history traits, among others, is lacking for many already threatened holothurian species (Lovatelli et al., Reference Lovatelli, Conand, Purcell, Uthicke, Hamel and Mercier2004; Conand, Reference Conand and Bruckner2006; Friedman et al., Reference Friedman, Eriksson, Tardy and Pakoa2010; Purcell et al., Reference Purcell, Hair and Mills2012a). Juvenile life stage habitat choice, ecological requirements and animal behaviour, in particular, remain largely undescribed. This is mainly due to an extremely cryptic behaviour and habitat or microhabitat choices differing from adult conspecifics (Cameron & Fankboner, Reference Cameron and Fankboner1989; Conand, Reference Conand1993; Wiedemeyer, Reference Wiedemeyer1994; Hamel & Mercier, Reference Hamel and Mercier1996; Mercier et al., Reference Mercier, Battaglene and Hamel1999, Reference Mercier, Battaglene and Hamel2000a, Reference Mercier, Battaglene and Hamelb; Shiell, Reference Shiell2004; Yamana et al., Reference Yamana, Hamano and Miki2006, Reference Yamana, Hamano and Goshima2010).
Knowledge of behavioural factors that influence sea cucumber survival at this early life stage is essential for understanding population dynamics and the evolution of life history characteristics. Survival of juvenile sea cucumbers is vital for re-establishment of adult populations in restocking and ranching efforts and release methods must be based on sound understanding of juvenile behavioural responses, juvenile nursery habitats, and the fitness of hatchery-reared compared with wild counterparts to ensure success (Tanaka, Reference Tanaka2000; Dance et al., Reference Dance, Lane and Bell2003; Bell et al., Reference Bell, Leber, Lee Blankenship, Loneragan and Masuda2008).
Limited observations of juvenile sea cucumbers in nature generally record juveniles underneath rocks, on boulders or in crevices, on vegetation such as seagrass or seaweeds or attached to or enclosed within shells (Sewell, Reference Sewell1990; Wiedemeyer, Reference Wiedemeyer1994; Hamel & Mercier, Reference Hamel and Mercier1996; Gutiérrez-García, Reference Gutiérrez-García1999; Mercier et al., Reference Mercier, Battaglene and Hamel1999, Reference Mercier, Battaglene and Hamel2000b; Shiell, Reference Shiell2004; James, Reference James2005; Yamana et al., Reference Yamana, Hamano and Miki2006, Reference Yamana, Hamano and Goshima2010; Slater et al., Reference Slater, Carton and Jeffs2010; Eriksson et al., Reference Eriksson, Jamon and Wickel2012; Purcell et al., Reference Purcell, Samyn and Conand2012b). These microhabitats serve as refuges or nursery areas for juveniles by lowering their risk of predation and providing a stable source of abundant or preferred food (Mercier et al., Reference Mercier, Battaglene and Hamel2000a; Palomar-Abesamis et al., Reference Palomar-Abesamis, Abesamis and Juinio-Meñez2017). These microhabitat preferences significantly shape the distribution and abundance of juvenile animals (Hereu et al., Reference Hereu, Zabala, Linares and Sala2005; Lürig et al., Reference Lürig, Best and Stachowicz2016).
Light avoidance and crypsis, also related to microhabitat choice, have been reported for temperate juvenile sea cucumbers (Slater, Reference Slater2006; Dong et al., Reference Dong, Dong, Wang and Tian2010; Sun et al., Reference Sun, Zhang, Pan, Lin, Wang, Kan and Yang2015). It has been suggested that this behaviour is related to predator avoidance when juveniles lack the speed or chemical defence mechanisms to avoid being consumed (Bakus, Reference Bakus1968; Cameron & Fankboner, Reference Cameron and Fankboner1989; Hatanaka et al., Reference Hatanaka, Uwaoku and Yasuda1994; Francour, Reference Francour1997; Kalinin et al., Reference Kalinin, Aminin, Avilov, Silchenko and Stonik2008). This is supported by evidence that the photonegative behaviour of juveniles weakens as the animals grow, e.g. Holothuria scabra juveniles shifted their activity rhythm and became slightly discordant with the natural light cycle as they grew in size (Mercier et al., Reference Mercier, Battaglene and Hamel1999), while medium- and large-sized juveniles of A. japonicus also developed a second feeding peak in the morning (Sun et al., Reference Sun, Zhang, Pan, Lin, Wang, Kan and Yang2015).
Stichopus cf. horrens is a commercially important tropical sea cucumber found in the Philippines and many other countries throughout the western and central Pacific (Purcell et al., Reference Purcell, Samyn and Conand2012b). It is a major species in the sea cucumber trade (Akamine, Reference Akamine2002; Choo, Reference Choo, Toral-Granda, Lovatelli and Vasconcellos2008; Purcell, Reference Purcell2014) and in the growing nutraceutical and pharmaceutical industries (Eriksson et al., Reference Eriksson, Friedman, Solofa and Mulipola2007; Bordbar et al., Reference Bordbar, Anwar and Saari2011). It is often misidentified as S. monotuberculatus and has been found to be part of a species complex (Byrne et al., Reference Byrne, Rowe and Uthicke2010). This species is the focus of efforts in South-east Asia (Zaidnuddin, Reference Zaidnuddin2009; Edullantes, Reference Edullantes2015) and China (Hu et al., Reference Hu, Li, Xia, Zhang, Luo, Fan, Peng, Yang and Wen2013) to assess its potential for culture and restocking. In the Philippines, adult S. cf. horrens are generally found on the outer reef flat and reef slope while juveniles are found within the seagrass habitat. The goal of this study was to better understand the activity patterns and behaviour of the juvenile stage of S. cf. horrens. Specifically, the aims were to describe the diel activity and movement rates of wild juveniles in situ, examine the influence of light and microhabitat on juvenile feeding and sheltering behaviour, and compare the activity and behaviour of wild and hatchery-reared juveniles in response to these two factors.
MATERIALS AND METHODS
Juvenile activity pattern in situ
Observations of the activity of juvenile Stichopus cf. horrens were conducted at a seagrass site off Cangaluyan Island in Anda, Pangasinan, north-western Philippines (16°21′15.3″N 119°59′49.3″E). The site is part of an extensive shallow sub-tidal seagrass bed with depth ranging from 0.6 to 1.5 m at low tide. Characterization using the Braun–Blanquet (BB) method revealed seagrass cover to be dominated by Thalassia hemprichii (mean BB score 4.40 ± 0.13 SE), with a few shoots of Halodule uninervis (0.74 ± 0.08 SE) and Enhalus acoroides (0.37 ± 0.04 SE). Grain size analysis of surface sediments showed the predominance of medium and coarse sand (0.25–1 mm). Stichopus cf. horrens juveniles were heterogeneously distributed at the site with a mean density of 6.50 ± 2.72 SE individuals per 100 m2 as determined by transect surveys (Palomar-Abesamis et al., Reference Palomar-Abesamis, Abesamis and Juinio-Meñez2017).
Movement of individual juveniles was monitored every 2 h over a 9 h period (5:00–14:00 h) and a 12 h period (16:00–4:00 h) on two separate days with similar tidal regime and lunar phase. At the start of each monitoring period, two observers each searched a 50 m long × 2 m wide area for juveniles. The initial location of each observed juvenile was marked by placing a uniquely numbered wooden stake about 1 cm from the posterior end of the animal. The time of first marking was then recorded. Subsequent marking and time recording of each juvenile's location were done in the same manner during each monitoring period. Juveniles were not touched or displaced during marking to minimize disturbance. Minimum movement rate was estimated from the linear distance between consecutive markings per time interval expressed in cm per hour. Estimates of movement rate were averaged for juveniles that were observed within 2 h blocks. Juveniles were searched for exhaustively at the start of each monitoring period and within a radius of 1 m from the last known position in subsequent markings. The juveniles being tracked were also at least 1 m apart at all times during monitoring. These suggested that the likelihood of encountering a different individual during movement tracking was low.
Position, visibility and general behaviour of the juvenile were also noted during the monitoring period. Position of the animal was recorded as either on bare sediment, the base of seagrass, seagrass leaf, macroalgae or sponge. Visibility (exposed or sheltered) and general behaviour (feeding or inactive) were based on the visual criteria and behavioural descriptions described in Table 1. At the end of the monitoring period, the length and width of each juvenile were measured underwater to the nearest 5 mm. Juvenile weight was estimated using fitted equations that were based on prior measurements of basal area for wild juveniles. Juveniles observed in situ were then classified as either large (>25 g) or small (≤25 g).
Table 1. Observed behaviours of juvenile Stichopus cf. horrens and criteria used to assess visibility in the wild and under laboratory conditions.

a Behaviour was only observed in the laboratory.
Collection and culture of juvenile animals
Wild juveniles (mean wet weight = 23.7 g ± 10.9 SD) were gathered in the seagrass areas of Cangaluyan Island and transported back to the laboratory in covered 40 l buckets with seawater within an hour after collection. Individuals were then held in opaque, covered 90 l bins with aeration and at ambient temperature for at least 6 h (but not exceeding 24 h) before any experiment. Wild juveniles were returned to the field soon after testing. Hatchery-reared juveniles (mean wet weight = 18.5 g ± 11.4 SD) were obtained from the University of the Philippines Bolinao Marine Laboratory. Larvae were reared in 250 l circular tanks and fed with monocultures of Isochrysis galbana and Chaetoceros calcitrans. After settlement, juveniles were provided with extract from blended, fresh Sargassum fronds as food until they were about 5–10 mm in size. These early juveniles were then transferred to an ocean nursery and reared in enclosed nets (1 mm × 1 mm mesh size) for about 30–45 days or until they reached about 5 g in weight. The juveniles were then returned to the hatchery and held in tanks with only surface-associated biofilm as food, but with running seawater, sand and dead coral boulders as shelter until they were used in experiments. Juveniles were never directly exposed to predators throughout the larval rearing and juvenile grow-out phases.
Effect of light on activity pattern and behaviour
The activity of wild (mean wet weight = 23.1 g ± 10.1 SD) and hatchery-reared (mean wet weight = 21.4 g ± 13.1 SD) juveniles was observed under three light cycle treatments: 12 h light + 12 h dark (LD), 24 h light (LL) and 24 h dark (DD). The bases of glass aquaria (35.5 × 20 × 25.5 cm) were covered uniformly with medium to coarse sand up to 4 cm depth and T. hemprichii seagrass shoots were placed in half of the aquarium (total leaf surface area ~400–600 cm2). Sediment and seagrass were collected from the field and any associated macrofauna were sieved out or manually removed. The aquaria were provided with running seawater (temperature range of 26.6–28.5°C and salinity range of 35–36 ppt) and conditioned for at least 48 h to allow a biofilm (~2–3 mm thick) to form on the sediments before the experiment. All animals were exposed to the natural light cycle with about 11 h and 40 min of daylight prior to experimentation. Under the LD treatment, animals were exposed to the natural light cycle with ambient light intensities in the hatchery ranging from 10–12,400 lx during the day. For the LL treatment, LED bulbs placed above the aquaria provided a mean intensity of 11,500 lx during the experiment reflecting high ambient-light conditions. Under the DD treatment, aquaria were covered completely with two layers of black plastic so light intensity was 0 lx throughout the day. Relative light levels in the hatchery were measured with a HOBO Pendant data logger. Each treatment had 10 replicate aquaria. These were arranged such that the microhabitats (seagrass or bare sediment) in each aquarium were oriented alternately.
One juvenile was introduced in the middle of each aquarium with a random orientation at 16:00 h. Observations began at 19:00 h and were conducted every 2 h for the first 24 h, then every 4 h for the second 24 h. Red light was used to observe animals under the LD treatment at night and animals under the DD treatment. Visibility and behaviour of each juvenile were recorded during every observation as described in Table 1. Position was noted as being on the walls of the aquaria, bare sediment or seagrass. The number of juveniles feeding or sheltering was evaluated for the day (5:00–15:00 h) and at night (17:00–23:00 and 1:00–3:00 h). Mean hours of feeding and sheltering were calculated using circular statistics (Zar, Reference Zar1998).
Two experimental runs were conducted over a week. The first run used wild individuals while the second run used hatchery-reared individuals. In between runs, sediments were mixed, seawater was fully exchanged, seagrass shoots were rinsed and all setups were conditioned for another 48 h.
Shelter preference among multiple microhabitats
Individual wild (mean wet weight = 25.5 g ± 13.1 SD) and hatchery-reared (mean wet weight = 18.3 g ± 12.2 SD) juveniles were able to access four alternative microhabitats in an experimental arena: bare sand (0.25–1 mm grain size; SA), dead coral boulder (>256 mm diameter; DC), seagrass (T. hemprichii; SG) and macroalgae (Sargassum sp.; MA). In nature, juveniles of S. cf. horrens were found to associate with these microhabitats in varying degrees (Palomar-Abesamis et al., Reference Palomar-Abesamis, Abesamis and Juinio-Meñez2017). All materials were collected from the field and any associated macrofauna were removed before being placed in the arena. The experiment was conducted in opaque, circular plastic containers (0.8 m diameter, 0.6 m height) with sand up to 4 cm depth, running seawater and aeration, and exposed to the natural light cycle. Each container was divided into four equal sections made up of the four microhabitats. Microhabitats were arbitrarily assigned to a section and all containers were conditioned for at least 48 h. Two experimental runs were conducted with 30 replicate arenas utilized for each run. The first run used wild individuals and the second run used hatchery-reared individuals.
One juvenile was placed in the middle of each arena with a random orientation to the different microhabitats at 17:00 h and allowed to acclimate to the experimental environment overnight. The position and visibility of each juvenile was observed from 18:00 h and then every 6 h after that for 18 h. Red light was used to observe animals at night. Position was noted as being on one of the four microhabitats. Presence or activity in neutral space, like the walls of the container, was included in the data analysis. Visibility was based on visual criteria described in Table 1. Most juveniles were sheltered by 05:00 h and did not change their position thereafter, so the microhabitat in which the juvenile was positioned from 06:00–12:00 h was considered the microhabitat chosen for shelter.
Shelter preference between seagrass and macroalgae
Results obtained from the previous shelter preference experiment indicated that wild and hatchery-reared juveniles sheltered preferentially in seagrass and macroalgae. To further investigate shelter preference, individual wild (mean wet weight = 22.5 g ± 9.2 SD) and hatchery-reared (mean wet weight = 15.9 g ± 8.1 SD) juveniles were subjected to a paired choice experiment. A choice-arena was created similar to the setup used to describe activity patterns, however half of the aquaria had T. hemprichii seagrass while the other half had Sargassum sp. macroalgae. Two experimental runs (one for wild and another for hatchery-reared juveniles) were conducted with 30 replicate arenas used for each run. The two microhabitats were oriented alternately in replicate arenas. All arenas were set up at least 48 h before experimentation.
A juvenile was placed in the middle of each arena with its anterior and posterior ends facing the glass walls at 16:00 h and allowed to acclimate to the experimental environment overnight. Based on field and laboratory observations, juveniles of S. cf. horrens were most active in their surroundings from 17:00–01:00 h. Thus the experiment began about 30 min before sunrise or ~05:00 h the next day. The position of the juvenile was monitored at 15, 30, 45, 60, 90, 120, 180 and 240 min from the start of the experiment and was recorded as being on seagrass, macroalgae or neutral space (bare sediment or walls of aquaria).
Data analysis
The effects of time of day and size on juvenile movement rates in situ were analysed using non-parametric Kruskal–Wallis tests because data could not be normalized even after transformation. To determine if juvenile activity in the laboratory displayed diel periodicity, the numbers of feeding and sheltering individuals were subjected to non-parametric Rayleigh tests (Zar, Reference Zar1998). For cases where the null hypothesis of uniform distribution was rejected, the mean hours of feeding and sheltering were calculated for each combination of juvenile type and light cycle using circular statistics. The individual effects of time, light cycle and juvenile type (wild or hatchery-reared) on feeding and sheltering behaviour were tested with Fisher's exact test of independence.
For the multiple choice experiment, the possible effects of time and juvenile type on the number of juveniles observed per microhabitat were tested using a Poisson regression model for count data (Zeileis et al., Reference Zeileis, Kleiber and Jackman2008). To allow for possible overdispersion, a negative binomial model was also fit to the data. Overdispersion of data was evaluated by comparing the residual deviance to the residual degrees of freedom and using the ‘odTest’ function from the pscl library (Jackman, Reference Jackman2015). Akaike's information criterion (AIC) and Vuong statistics were used to compare goodness of fit between models.
For the paired choice experiment, preference was inferred from attraction (p s and p m) and leaving (μs and μm) rates of juveniles for seagrass and macroalgae, respectively. The rates were estimated using a continuous-time, likelihood-based model developed by Zeilinger et al. (Reference Zeilinger, Olson and Andow2014) and differences between these rates were tested using model selection. Four models of likelihood were compared: a Fixed model representing a null model of no preference where both the attraction rates and the leaving rates were equal to each other (p s = p m, μs = μm), a Free Leaving model where attraction rates were equal but leaving rates were allowed to vary (p s = p m, μs ≠ μm), a Free Attraction model where attraction rates were allowed to vary and leaving rates were equal (p s ≠ p m, μs = μm), and a Free model where all four parameters were allowed to fit independently (p s ≠ p m, μs ≠ μm). Akaike's information criterion corrected for small sample sizes (AICc) were used for model selection. Parameter estimates and variances were averaged for all models with ΔAICc < 7 following Burnham et al. (Reference Burnham, Anderson and Huyvaert2011). Predicted long-term probabilities, at equilibrium, that juveniles will be located on either of the two choice microhabitats were calculated using parameter estimates. All analyses for the multiple and paired choice experiments were run using R version 3.3.1 (R Core Team, 2013).
RESULTS
Juvenile activity pattern
Wild juveniles of Stichopus cf. horrens displayed a clear nocturnal activity pattern based on observations in situ. Wild juveniles in the seagrass habitat were most active at night, decreased activity at dawn, and became inactive during the day. This diel activity pattern was exhibited by both small and large juveniles (Figure 1). The movement rates of 51 juveniles in total were observed in the field (dawn: N = 51, day: N = 30, night: N = 18–30). The variable number of individuals observed at night was due to some juveniles not being relocated at certain hours of monitoring. Movement rate was significantly influenced by time (Kruskal–Wallis test, H(11) = 189.34, P = 0.0001) and size to a lesser extent (Kruskal–Wallis test, H(1) = 6.94, P = 0.01). Movement was greatest from 16:00–05:00 h and minimal to absent from 09:00–14:00 h.
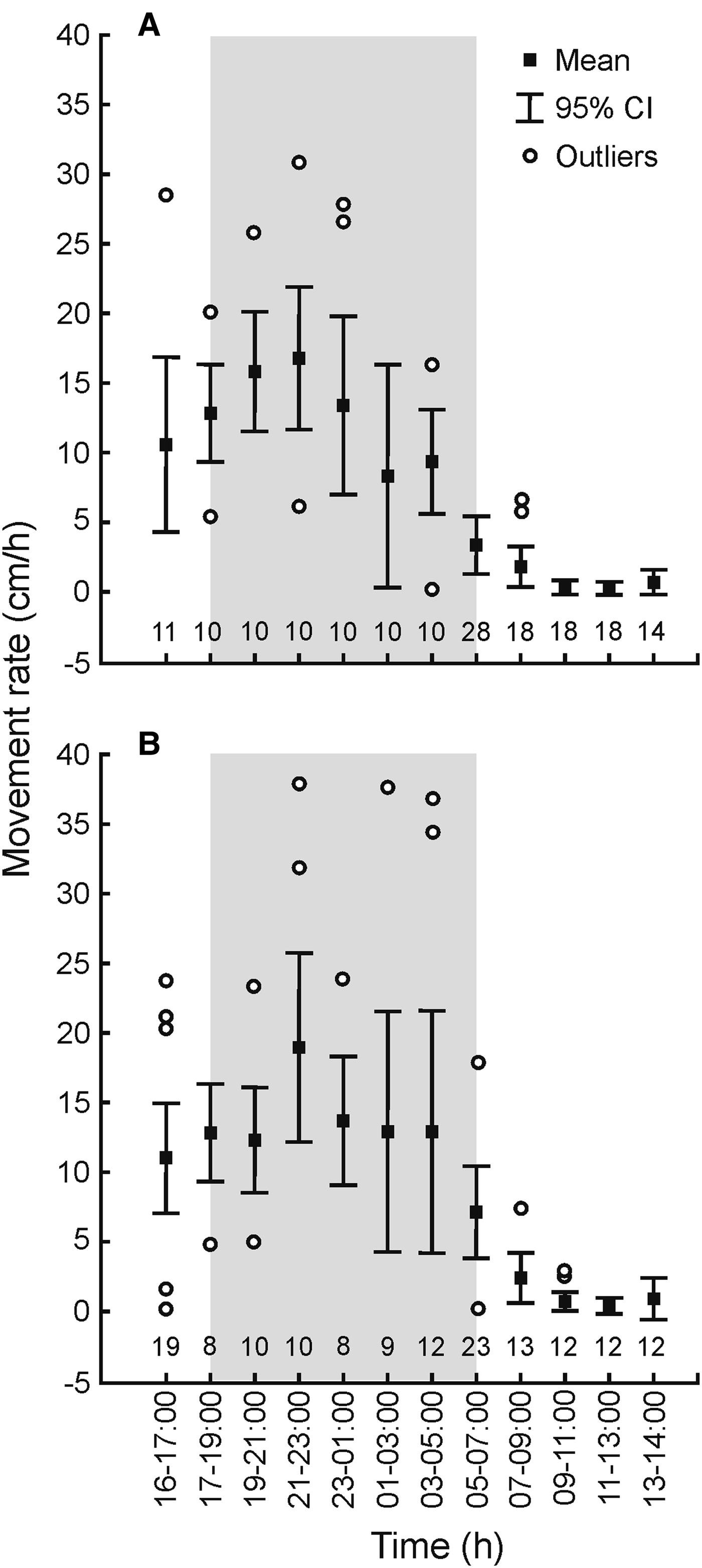
Fig. 1. Diel activity of small (3–18 g; A) and large (19–56 g; B) Stichopus cf. horrens juveniles in a seagrass habitat. Shaded area represents night. Numbers indicate sample size.
At night, most juveniles observed in the wild were feeding (70–100%) and found predominantly on the sediment (50–80%). Other juveniles were located at the base or on the leaves of T. hemprichii seagrass (10–30%) or on various macroalgae (10–30%). The proportion of active juveniles was considerably lower from 03:00–07:00 h and continued as daytime progressed. Most juveniles retracted their bodies and hid at the base of seagrass shoots (40–50%), under or within sponges (10–20%) and under macroalgae (10–20%) during the day. Once inactive, juveniles were highly cryptic and well camouflaged in their surrounding environment.
Effect of light on activity pattern and behaviour
In the laboratory, all juveniles exhibited a nocturnal activity pattern under simulated natural conditions (LD light cycle) (Figure 2). Wild and hatchery-reared juveniles showed a distinct diel periodicity in feeding behaviour (Rayleigh test, z = 19.11 and 16.72, P < 0.001), with significantly more individuals feeding at night (Fisher's test, P = 0.02 and 0.005). Mean hours of feeding occurred at 22:54 h (wild) and 22:40 h (hatchery-reared). However, onset and duration of feeding differed between juvenile types, with most wild individuals feeding slightly later and for a shorter period (19:00–07:00 h) than hatchery-reared individuals (17:00–09:00 h). Diel periodicity of inactive-sheltered behaviour was displayed by both wild and hatchery-reared juveniles (Rayleigh test, z = 19.97 and 17.15, P < 0.001), with significantly more individuals sheltering during the day (Fisher's test, P = 0.0001 and 0.03). Mean hours of sheltering were at 12:55 h (wild) and 13:09 h (hatchery-reared). Differences in the onset and duration of sheltering were likewise detected between wild and hatchery-reared juveniles. Most wild juveniles were sheltered from 05:00–15:00 h, while most hatchery-reared juveniles were sheltered from 11:00–15:00 h. Only hatchery-reared individuals exhibited the inactive-exposed behaviour during daytime under the LD treatment.
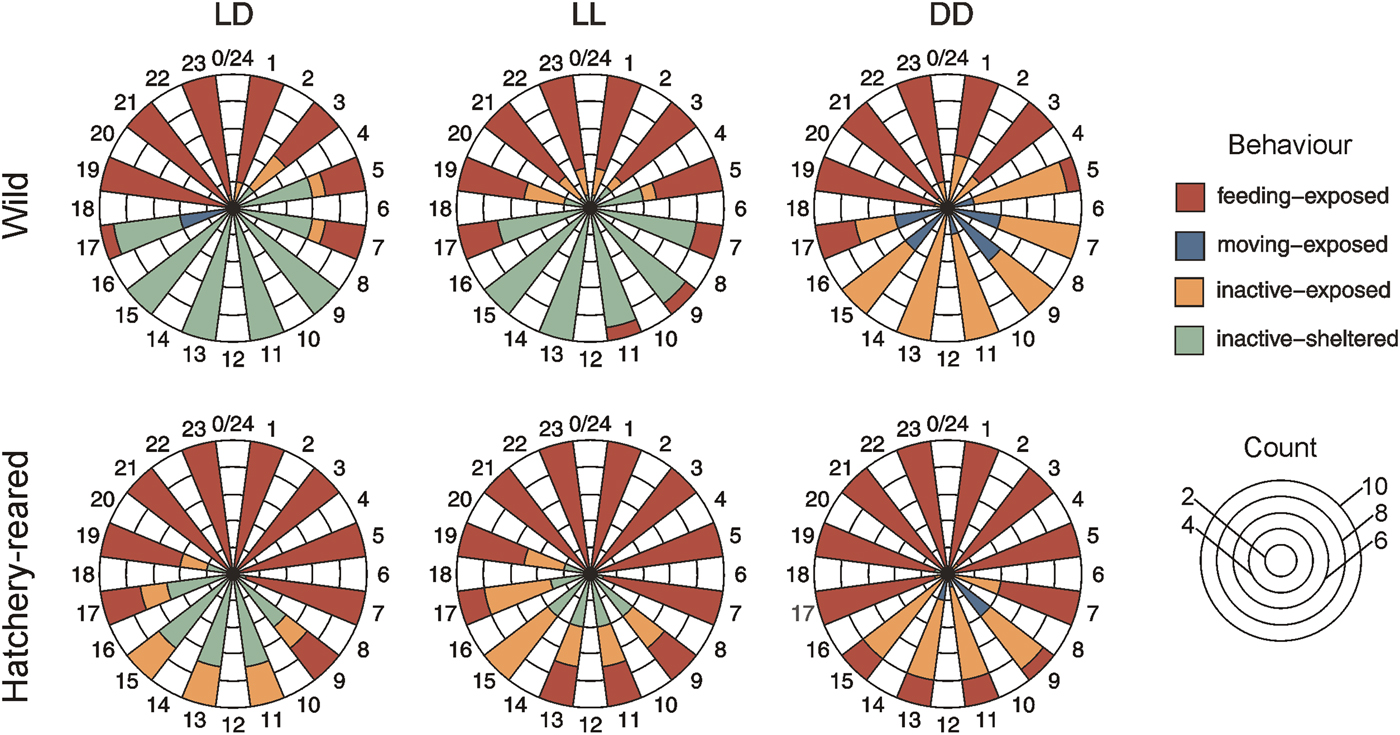
Fig. 2. Rose diagram representing the histogram of behaviours by wild (N = 30) and hatchery-reared (N = 30) Stichopus cf. horrens juveniles over one diel cycle and under three light cycle treatments (LD: 12 h light + 12 h dark; LL: 24 h light; DD: 24 h dark).
Juvenile S. cf. horrens likewise exhibited a nocturnal activity pattern when exposed to constant light or constant dark conditions over 48 h in the laboratory (Figure 2). Feeding behaviour still had a distinct diel periodicity for wild and hatchery-reared individuals under the LL treatment (Rayleigh test, z = 11.80 and 11.93, P < 0.001) or DD treatment (Rayleigh test, z = 20.18 and 9.80, P < 0.001). Mean hours of feeding were at 0:00 h (wild) and 22:10 h (hatchery-reared) under the LL treatment, and 22:19 h (wild) and 23:32 h (hatchery-reared) under the DD treatment. There was a decrease in the proportion of wild juveniles active at night under constant light, with only 70% of the animals feeding during peak hours. Conversely, there was an increase in hatchery-reared individuals feeding during real daytime hours under constant light and dark conditions. Diel periodicity of inactive-sheltered behaviour was only observed among wild and hatchery-reared juveniles under the LL treatment (Rayleigh test, z = 15.09 and 9.46, P < 0.001), with mean hours of sheltering at 12:14 h (wild) and 13:02 h (hatchery-reared). Sheltering behaviour was absent for all wild and hatchery-reared juveniles under the DD treatment (Figure 2). Instead, there was an increase in the proportion of juveniles that exhibited moving-exposed and inactive-exposed behaviours under constant dark.
Shelter preference among multiple microhabitats
Among animals exposed to the choice experiment using multiple microhabitats, the number of juveniles observed had significant positive associations (P < 0.001) with macroalgae and seagrass for both Poisson and negative binomial regression models (Table 2). A significant negative association (P < 0.05) between number of juveniles and bare sediment was detected by the Poisson model. No relationship was detected between time of day or juvenile type and the proportion of juveniles per microhabitat for both models. Due to overdispersion and a smaller AIC value, the negative binomial model showed a slightly better fit to the data than the Poisson model. A significantly greater number of juveniles used the microhabitats with vegetation more than bare sediment, dead coral or neutral space. Approximately 40% of the juveniles were located on dead coral, bare sediment and walls during the night, with 97% of them feeding by midnight. By 06:00 h, nearly 80% of the juveniles were sheltered and inactive within macroalgae or seagrass.
Table 2. Analysis results from Poisson and negative binomial models for number of juveniles exposed to different microhabitats.
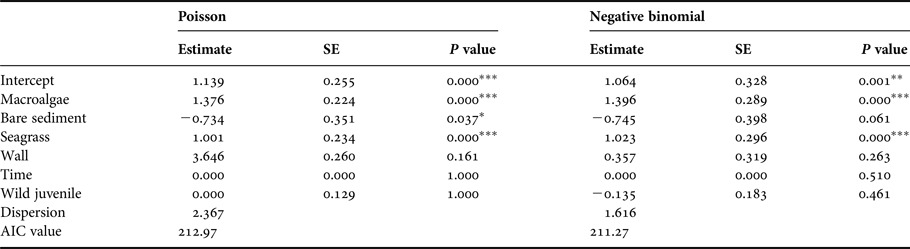
SE, standard error; AIC, Akaike's Information Criterion.
*P < 0.05, **P < 0.01, ***P < 0.001.
Shelter preference between seagrass and macroalgae
In the paired choice experiment with seagrass and macroalgae, all models were similar with ΔAICc < 7 but the Fixed model fit the data best (Table 3). Parameter values averaged from all four models (Table 4) indicated that S. cf. horrens juvenile attraction and leaving rates were equal between seagrass and macroalgae. Lack of convergence for the data of wild juveniles led to high confidence interval estimates and may be attributed to the predominance of zero values or no observations in neutral space. At equilibrium, the model predicted that the juvenile will be equally distributed between the two microhabitats (Figure 3). In the actual experiment, both wild and cultured juveniles were distributed nearly equally between seagrass and macroalgae by 06:15 h. Due to the nature of the paired choice data, the assumptions of the preference model on independent consecutive choices and constant attraction and leaving rates set by Zeilinger et al. (Reference Zeilinger, Olson and Andow2014) were violated, hence parameter estimates may be biased. However, because the Fixed Model was clearly the best model, and parameter estimates suggested little difference between choices, such bias is unlikely to affect interpretation of the results.

Fig. 3. Predicted dynamics of wild (A) and hatchery-reared (B) Stichopus cf. horrens juveniles selecting seagrass (T. hemprichii) (solid line) and macroalgae (Sargassum sp.) (broken line), calculated using model-averaged parameter estimates.
Table 3. Information criterion corrected for small sample size (AICc) values, change in AICc (ΔAICc), relative marginal likelihoods and relative weights for each model variant in the paired choice experiment.

Table 4. Model-averaged parameter estimates and ±95% confidence intervals for attraction and leaving rates of Stichopus cf. horrens juveniles for seagrass and macroalgae microhabitats.

DISCUSSION
Animals have evolved multiple behavioural strategies to increase their chances of survival in nature. These often vary across life stages and give insight into the factors that are most influential to the animal within a particular space and at a given time. The present study described the activity pattern of wild and hatchery-reared juveniles of the sea cucumber Stichopus cf. horrens and specifically examined the influence of light and microhabitat on their feeding and sheltering behaviour. Results confirmed a distinct nocturnal activity that was strongly associated with feeding in juveniles of this species. This is analogous to the activity patterns observed in juveniles of the temperate stichopodids Australostichopus mollis (Slater, Reference Slater2006) and Apostichopus japonicus (Dong et al., Reference Dong, Dong, Wang and Tian2010; Sun et al., Reference Sun, Zhang, Pan, Lin, Wang, Kan and Yang2015). Wild and hatchery-reared juveniles of S. cf. horrens consistently fed at night and became inactive during the day. Wild adults of S. cf. horrens which are found in the reef flat and slope, likewise feed at night (21:00–0:00 h) when they are not spawning (Edullantes, Reference Edullantes2015), which indicates that the nocturnal feeding pattern is a characteristic of the species throughout its life.
The nocturnal feeding of both wild and hatchery-reared juveniles was maintained despite exposure to constant light or dark for 48 h. This indicates that the feeding rhythm of S. cf. horrens juveniles is controlled by an endogenous clock and is strongly entrained by natural light-dark cycles. The onset of night time (or decreased light levels) induces the feeding behaviour and, in the absence of this cue (or under constant light), the animal's internal clock takes control and ensures that feeding persists during true ‘night’ time. This was a short-term experiment and juvenile behaviour and activity pattern could change in longer-term exposure. Several studies have shown that endogenously controlled behaviour or activity patterns may undergo phase shifts, change or be lost (arrhythmia) depending on factors related to the zeitgeber that entrains it (Aschoff, Reference Aschoff and Aschoff1981; Kronfeld-Schor et al., Reference Kronfeld-Schor, Bloch and Schwartz2013). Although constant light conditions would never occur in nature, an internal control only underscores the importance of feeding to the juvenile stage of this species. An internal clock system that coordinates activities to environmental cycles is believed to provide a selective advantage to an organism by optimizing biological processes, enabling behavioural plasticity, and allowing for predictive homeostatic control in changing environments (Kronfeld-Schor et al., Reference Kronfeld-Schor, Bloch and Schwartz2013).
Unlike feeding, sheltering behaviour was controlled directly by light and not by an internal clock. This was confirmed when all the wild and hatchery-reared juveniles remained exposed over 48 h under constant dark conditions. The inactive-exposed behaviour exhibited by juveniles in the laboratory was never observed among juveniles in situ, and is likely an artefact of the experimental environment. This behaviour would evidently be detrimental to the animal in nature because it would submit itself to undue risk from daytime predators. Daytime inactivity among S. cf. horrens juveniles was strongly associated with sheltering and positive thigmotaxis. Juveniles immediately moved under or within various biotic (seagrass, macroalgae, sponges, live coral) and abiotic structures (dead coral, concrete blocks, polyvinyl chloride pipes, artificial vegetation) when exposed to light. A similar response was observed in juveniles of Actinopyga echinites, A. mollis and A. japonicus during the day (Wiedemeyer, Reference Wiedemeyer1994; Slater, Reference Slater2006, Reference Slater2009; Yamana et al., Reference Yamana, Hamano and Goshima2009). In the absence of shelter, S. cf. horrens juveniles always moved to darker or shaded areas and maintained contact with the corner walls of experimental aquaria or containers.
Light-mediated reflexive behaviours are common in echinoderms (Millott, Reference Millott1955; Hendler, Reference Hendler1984; Johnsen & Kier, Reference Johnsen and Kier1999; Adams, Reference Adams2001). They are elicited by dermal or neural photoreceptors in many invertebrates, including holothurians, and referred to as extraocular photoreception (Yamamoto & Yoshida, Reference Yamamoto and Yoshida1978; Cronin, Reference Cronin1986; Wolken, Reference Wolken1988). In sea urchins and brittlestars, negative phototaxis is believed to be a defensive response against damaging levels of UV light (Johnsen & Kier, Reference Johnsen and Kier1999; Adams, Reference Adams2001). Juveniles of S. cf. horrens in shallow waters probably avoid light for the same reason. Stichopodid sea cucumbers have body walls composed mainly of type 1 collagen (Cui et al., Reference Cui, Xue, Li, Zhang, Dong, Fu and Gao2007; Abedin et al., Reference Abedin, Karim, Ahmed, Latiff, Gan, Che Ghazali, Sarker and Zaidul2013; Zhong et al., Reference Zhong, Chen, Hu and Ren2015), that has been shown to undergo extensive damage under UV light (Miles et al., Reference Miles, Sionkowska, Hulin, Sims, Avery and Bailey2000; Jariashvili et al., Reference Jariashvili, Madhan, Brodsky, Kuchava, Namicheishvili and Metreveli2012). Negative phototaxis and pigmentation patterns in brittlestars are also thought to facilitate defensive shelter seeking and confer camouflage against predatory fish in varying light conditions (Hendler, Reference Hendler1984). Positive thigmotaxis, or the tendency to maintain contact with the surfaces, walls or boundaries of an environment, is likewise believed to facilitate the search for shelter, protection and/or escape routes for animals in nature (Sharma et al., Reference Sharma, Coombs, Patton and de Perera2009).
Shelter or spatial refuge is essential for the survival of many soft-bodied animals as it provides temporary physical protection. This study showed that wild and hatchery-reared juveniles of S. cf. horrens sheltered preferentially in microhabitats composed of vegetation, such as seagrass and macroalgae, rather than coral boulders, bare sand or open space. These microhabitats probably provided the juveniles with sufficient protection from light under laboratory conditions. In the field, Palomar-Abesamis et al. (Reference Palomar-Abesamis, Abesamis and Juinio-Meñez2017) revealed that more juveniles are found in seagrass habitats than in Sargassum beds, probably due to less favourable conditions for juvenile growth and survival in the latter habitat. Sargassum sp. attach to hard substratum, have large, buoyant thalli that get detached by strong water motion, and regularly undergo senescence and die-off (Trono & Lluisma, Reference Trono, Lluisma, Lindstrom and Gabrielson1990). They also produce secondary metabolites with strong antifouling properties that inhibit the growth of epiphytic microorganisms (Bazes et al., Reference Bazes, Silkina, Douzenel, Faÿ, Kervarec, Morin, Berge and Bourgougnon2009; Cho, Reference Cho2013) which juveniles feed on. Reef flats and bare sediments would also be unsuitable for growth because they reportedly have lower microphytobenthic and bacterial biomass than seagrass meadows (Moriarty et al., Reference Moriarty, Roberts and Pollard1990; Garrigue, Reference Garrigue1998), and might therefore have limited food for deposit-feeding juveniles.
Growing in different environments leads to differential experiences in animals and these experiences are likely to generate behavioural differences. Although comparisons of behaviour have been made between wild and cultured individuals of some fish and invertebrate species, the present study is the first to compare activity patterns and behaviour between wild and hatchery-reared individuals of a commercially important echinoderm. Unlike wild juveniles, hatchery-reared individuals had longer feeding periods and sustained feeding behaviour when exposed to light. The inactive-exposed behaviour was also more prevalent among hatchery-reared juveniles. These strongly indicate acclimation to an artificial environment with minimal threats and a decreased sensitivity to light. Acclimation is commonly observed in animals raised in artificial conditions that lack spatial and temporal unpredictability and variability from factors normally present in nature. This leads to the development of animals with a diminished ability to detect, respond to or evaluate various types and levels of threats (Huntingford, Reference Huntingford2004; Brokordt et al., Reference Brokordt, Fernández and Gaymer2006). For hatchery-reared S. cf. horrens juveniles, this would certainly lead to decreased fitness and survival if they were to be released in nature.
The behaviour and shelter preferences of S. cf. horrens juveniles elucidated in this study have clear implications on the future culture and restocking of this species. It is common practice for aquaculture facilities to rear newly settled holothurians under reduced light or dark conditions (Hu et al., Reference Hu, Xu, Wen, Zhang, Fan and Su2010; Mercier & Hamel, Reference Mercier, Hamel, Allan and Burnell2013). This is done to minimize algal overgrowth and increase growth by inducing juveniles to feed all day. Since the behaviours of S. cf. horrens are strongly influenced by light, it may be possible to alter its activity pattern if juveniles are exposed to the dark for long periods, i.e. several days or weeks. Moreover, as this study revealed, rearing the juvenile under reduced light or dark conditions weakens the animal's light-induced reflexes which are essential for sheltering. To minimize the development of maladaptive behaviours, the rearing period of S. cf. horrens juveniles under reduced light and artificial conditions should be minimized or avoided as much as possible. It would be beneficial for juveniles to be exposed to some degree of natural light-dark cycles while being reared in the hatchery and to have shelter that provides shade and surfaces for contact. Juveniles should also be released in the wild preferably at dusk, to coincide with the onset of their activity, and initially into enclosures with natural or artificial shelter. This will facilitate the acclimatization of hatchery-reared juveniles to the release site. These methods evidently need to be further refined and optimized for S. cf. horrens considering the effects of juvenile size, stocking density and handling conditions on the survival of the animals after release.
ACKNOWLEDGEMENTS
We thank R. de Guzman, A. Abuan, R. Castro Jr., M. Sinsona, C. Edullantes and C. Diolazo for assistance in the field and laboratory; R. Abesamis for technical advice; A. Zeilinger and D. Boegner for statistical advice, and two anonymous reviewers whose comments greatly improved the paper. We also thank the local governments and communities of Anda and Bolinao, Pangasinan for supporting this research. All procedures performed on animals were in accordance with Philippine laws and regulations (RA 9147; FAO 233). This study was conducted in support of the sea cucumber research programme coordinated by the Philippine Council for Agriculture, Aquatic and Natural Resources Research and Development – Marine Resources Research Division of the Department of Science and Technology.
FINANCIAL SUPPORT
NPA was supported by grants from the Philippine Department of Science and Technology Accelerated Science and Technology Human Resource Development Program and the University of the Philippines Marine Science Institute Bolinao Marine Laboratory.









