INTRODUCTION
If copulation is limited to a particular female reproductive stage, males can increase their reproductive success by catching and keeping receptive females, i.e. exhibit precopulatory mate guarding behaviours. In many crustaceans, particularly amphipods and isopods, precopulatory mate guarding is common (Ridley, Reference Ridley1983; Conlan, Reference Conlan1991; Jormalainen, Reference Jormalainen1998; Johnson et al., Reference Johnson, Stevens and Walting2001), perhaps because the receptivity of females is limited to a short period just after moult in many species (Ridley, Reference Ridley1983; Johnson et al., Reference Johnson, Stevens and Walting2001).
Some theoretical models clearly predict that precopulatory mate guarding is an effective strategy for gaining receptive females in some situations, such as when low encounter rates with receptive females occur, when the cost of guarding is smaller than the cost of searching (Parker, Reference Parker1974; Grafen & Ridley, Reference Grafen and Ridley1983; Yamamura, Reference Yamamura1987). Therefore, the duration of guarding is affected by the numbers of competitors and receptive females (i.e. the operational sex-ratio (OSR)) and/or population density, with guarding starting earlier, resulting in longer guarding durations in the male-biased group (Manning, Reference Manning1980; Ward, Reference Ward1983; Dick & Elwood, Reference Dick and Elwood1996; Jormalainen, Reference Jormalainen1998; Wada et al., Reference Wada, Tanaka and Goshima1999).
Only a few experimental examinations of guarding duration have been performed in crustaceans. In Gammarus pulex (Linnaeus, 1758), for example, Ward (Reference Ward1983) reported that guarding duration was prolonged when the number of competitors increased and the number of potential mates decreased. Such guarding prolongation has also been shown in other crustaceans (Manning, Reference Manning1980; Wada et al., Reference Wada, Tanaka and Goshima1999; Kamio et al., Reference Kamio, Matsunaga and Fusetani2003).
In precopulatory mate guarding, a male's competitive abilities for retaining or taking over a mate are important, and both influence a male's reproductive success. Until now, two competitive advantages have been reported in precopulatory mate guarding, i.e. a size advantage and an owner advantage. In G. pulex, large males often take over females from small males (Ward, Reference Ward1983). A size advantage was also confirmed in the hermit crab Pagurus middendorffii (Brandt, 1851), but some smaller guarding males continued to hold receptive females against larger competitors, showing an owner advantage (Wada et al., Reference Wada, Tanaka and Goshima1999). Also, in the amphipod Hyalella azteca (Saussure, 1858), most mate guarding males could retain their females, even when facing larger competitors (Strong, Reference Strong1973). However, the generality of the owner advantage remains controversial.
In the genus Caprella, two types of precopulatory mate guarding, Type O and Type I, have been reported (Aoki, Reference Aoki1996). The female's posture resembles a horseshoe in Type O guarding, because the male folds the female into a circular shape to facilitate carrying, whilst the male and female are positioned in parallel in Type I guarding. Aoki (Reference Aoki1996) suggested that these guarding types might be determined by habitats and phylogeny, but they may also be related to social factors, such as the specific mating system and parental care (Aoki, Reference Aoki1997, Reference Aoki1999).
In the genus Caprella, males often engage in violent battles for females. In Caprella scaura typica Mayer, 1890, males are often killed by attacks from competitors with 2nd gnathopods (Lim & Alexander, Reference Lim and Alexander1986; Schulz & Alexander, Reference Schulz and Alexander2001). Lewbel (Reference Lewbel1978) also reported violent male–male competitions, with poison spine on the 2nd gnathopods, for females in Caprella gorgonia Laubitz & Lewbel, 1974. Despite such strong male competition in caprellid species, few detailed studies have been reported on their reproductive behaviours and the effects of precopulatory guarding.
Caprella penantis Leach, 1814, is a cosmopolitan species inhabiting various substrata, such as red algae and bryozoans, from temperate to arctic regions worldwide. Bynum (Reference Bynum1978) reported that in the North Carolina population of C. penantis, population density fluctuated greatly but the sex-ratio remained approximately equal and ovigerous females were abundant throughout the year. However, no other reports of C. penantis are available. Type O mate guarding has been reported in this species (Aoki, Reference Aoki1996); however, we found that two types of mate guarding occurred, Type O and a second type that is similar to Type I, and that individuals conducted both forms of guarding.
In the present study, the precopulatory mate guarding behaviour of C. penantis is described. Moreover, the effects of body size, ownership and OSR on mate guarding were confirmed. This is the first report demonstrating body size and ownership advantages during mate guarding competition and the first demonstration of an OSR influence on guarding duration in C. penantis.
MATERIALS AND METHODS
Sample site and specimens
All specimens of Caprella penantis were collected with red algae Grateloupia filicina (Lamouroux) C. Agardh or sea moss Bugula neritina (Linnaeus, 1758), which were attached to a pier (32°31′N 130°26′E) in Amakusa, Kyushu, Japan. Afterwards, all samples were temporarily preserved with algae or sea moss in seawater-satisfied plastic bottles (500 ml) in the laboratory. In C. penantis, two morphological variations, coastal and estuarine types, have been reported (Bynum, Reference Bynum1978, Reference Bynum1980), but a recent study indicated that these two types were not genetically different (Guerra-García et al., Reference Guerra-García, Redondo-Gómez, Espina, Castillo, Luque, García-Gomez and Figueroa2006). In the present study, all specimens were of the coastal type described in Bynum (Reference Bynum1978, Reference Bynum1980).
To clarify the population structure of C. penantis, red alga G. filicina was collected randomly from the sampling site in March 2006. Afterwards, the red algae samples were washed with freshwater and all of the C. penantis specimens found on the red algae were preserved in 5% buffered-formalin seawater. The length of the 2nd pereonite was measured to the nearest 0.01 mm under a microscope and all specimens were classified into four categories: male, non-ovigerous female, ovigerous female and unsexable.
Mate guarding and male–male competition
Mate guarding behaviours of C. penantis were observed from March to May 2006. In the laboratory, all guarding pairs were sorted from the algae or sea moss and each pair was put into a seawater-satisfied plastic bottle (120 ml) containing an alga G. filicina that was cut to about 5 cm. Afterwards, the guarding posture of the pair was checked every 30 minutes until they had finished guarding; no food was supplied during guarding. After observation, all specimens were fixed in 5% buffered-formalin seawater. The specimens were photographed using a digital camera that was attached to a microscope and the body length between pereonites 1–7 was measured on a PC using Image J (http://rsb.info.nih.gov/ij/). Moreover, the number of eggs in each female's brood pouch was counted.
An experiment examining male–male competition during precopulatory mate guarding was conducted from May to June 2006. Two guarding pairs that demonstrated Type O guarding were randomly selected from the field sample in the laboratory. One guarding pair was separated and the male (the challenger) was put into the acrylic bottle (120 ml) with the other guarding pair (the owner and the female) that was attached to a 5 cm length of G. filicina substratum. The owners and challengers were selected to produce various size combinations. The owners were larger in 15 trials, and the challengers were larger in 19 trials. The male–male competition was observed in detail for the first 5 minutes of interaction. Afterwards, the positions of the males and the female were checked every 30 minutes until guarding stopped, and the last male that guarded the female was determined. After the experiment, the body length of each male was measured with Image J.
Sex-ratio and guarding duration
Sex-ratio manipulation experiments were conducted in July 2006 to confirm the influence of the sex-ratio on precopulatory mate guarding duration. Before the experiment, males and females were reared separately in plastic bottles (500 ml) containing a vinyl net (2 mm mesh, 30 mm × 40 mm) as a perch for the animals. Artificial powdered shrimp food was provided once per day. The plastic bottles provided flow-through seawater. Four days after rearing, the brood pouch of each female was checked and females that had no egg in their pouch were selected for the experiments because ovigerous females are seldom guarded by males.
Fifteen male-biased groups (2 males and 4 females) and 13 female-biased groups (4 males and 2 females) were placed in seawater-satisfied plastic bottles (500 ml) containing a vinyl net, and mate guarding was observed every 2 hours for 2 days to confirm guarding duration with food supply. When guarding pairs were temporarily detached, the longest guarding duration in each group was adopted.
RESULTS
Population structure
From the study site, 206 individuals of Caprella penantis (82 males, 69 ovigeous females, 27 non-ovigerous females and 28 unsexables) were collected (Figure 1). In this population, the sex-ratio was not significantly different from 1:1 (χ2 = 1.10, P > 0.05, Pearson's Chi-square test), but the OSR was male-biased because the proportion of ovigerous females was high. The average length of pereonite 2 was significantly longer in males (1.55±0.47 mm, mean±SD, N = 82) than in females (1.04±0.19 mm, N = 96; t = 9.37, P < 0.0001, t-test). Thus, a sexual size dimorphism was obvious in C. penantis.

Fig. 1. Size–frequency distributions of male and female Caprella penantis in May 2006. Unsexable individuals were classified as 50% females, 50% males, assuming a sex-ratio of 1:1.
Mate guarding behaviour
From the study site, 69 mate guarding pairs were collected. Each of these pairs was reared in an individual plastic bottle in the laboratory. Of the 69 pairs, 29 females died before egg production and 4 females produced no eggs although they survived throughout the experiment. The remaining pairs continued precopulatory mating behaviour for 363±175 minutes (range 30–600 minutes; N = 36) and brood production was confirmed for all females immediately after pair separation.
Almost all of the precopulatory guarding was of Type O (N = 67; Figure 2A), with the exception of two pairs. In Type O guarding, the male grasped the female between her 1st and 2nd pereonites with one of his 5th pereopods and between her 5 and 6th pereonites with another of his 5th pereopods, folded her into a horseshoe shape and carried her. In Type O guarding, the female could not move freely because her pereopods were detached from the substratum. In the two exceptions, however, the male only grasped the female's 5th pereonite using one of his 5th pereopods and held the female parallel to him (Figure 2B). The latter guarding was similar to the Type I guarding described by Aoki (Reference Aoki1996). In the latter guarding, some of the female's pereopods grasped the substratum and the male could not move her easily. Although we cannot confirm that this was Type I guarding, this alternative guarding differed considerably from Type O guarding in that the female was not held in a circular configuration. In the present study, the latter guarding was regarded as Type I for convenience. In C. penantis, the guarding forms were not fixed, even within an individual, and some Type O guarding males changed their guarding form to Type I-like guarding after detaching one of their 5th pereopods from the female; afterwards, however, some individuals returned to Type O guarding.
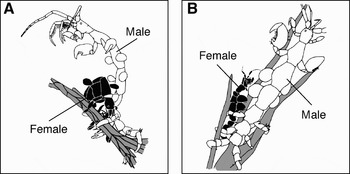
Fig. 2. Precopulatory mate guarding behaviours of Caprella penantis; Type O (A) and Type I-like (B) guardings are shown. The substratum is coloured grey.
In the guarding pairs, size assortative pairing was detected (N = 69; r 2 = 0.168, P < 0.0005; Figure 3). Females produced 23.9±13.0 eggs (1–48 eggs) in the laboratory and the number of eggs was correlated with female body length (N = 36; r 2 = 0.158, P < 0.01; Figure 4).

Fig. 3. Regression plot of male and female body sizes in guarding pairs (N=69) in Caprella penantis from March to May 2006. The broken line indicates the regression line (y = 0.332x+2.646; r 2 = 0.168).
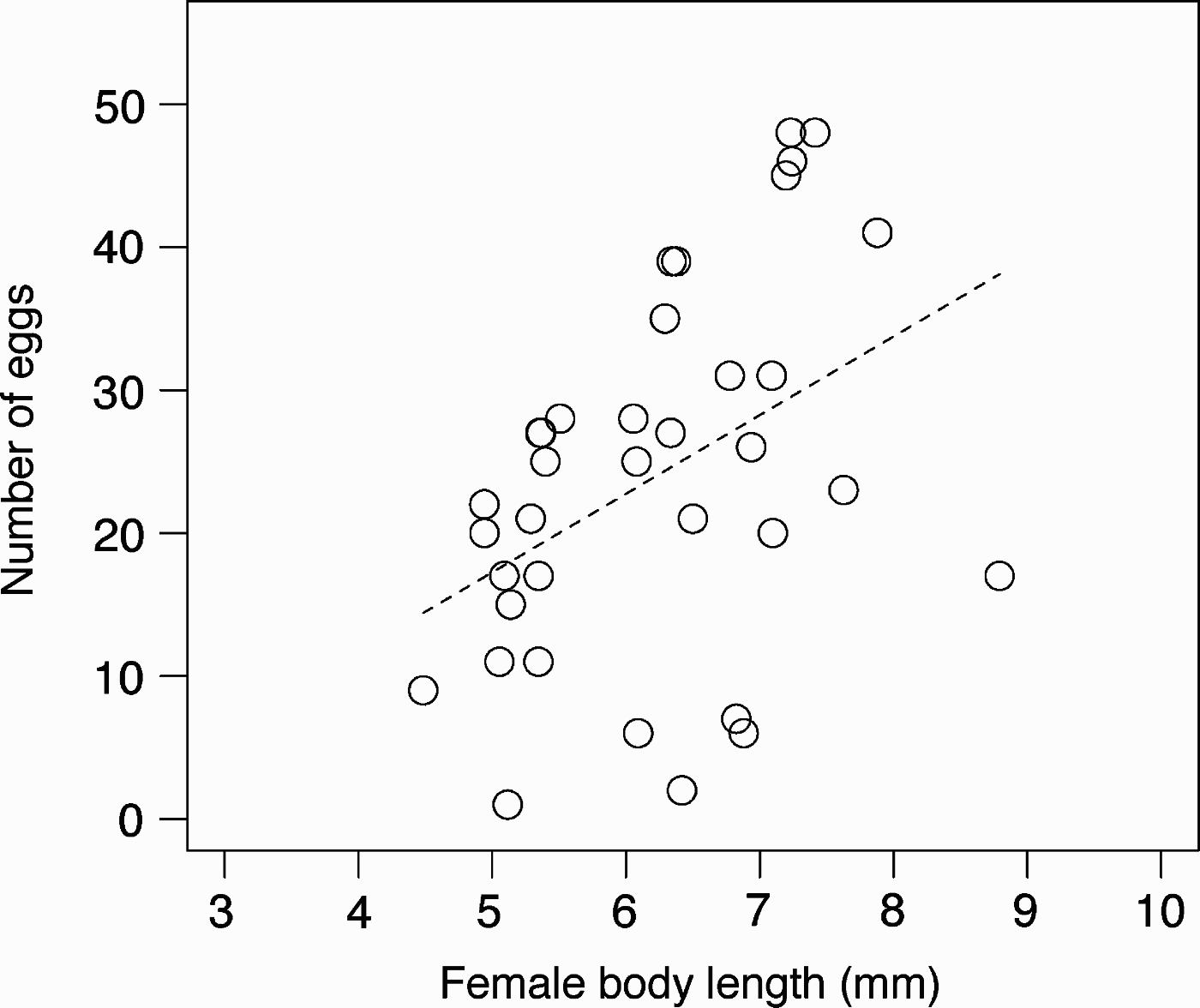
Fig. 4. Number of eggs in the brood pouch versus female body length in Caprella penantis from March to May 2006 (N = 36). The broken line indicates the regression line (y = 5.499x – 10.25; r 2 = 0.158).
Male–male competition
Within 5 minutes after starting each experiment, all challengers contacted the guarding pair. Five minutes after the start of each experiment, all of the larger owners (15/15) continued guarding but the partners of some of the smaller owners (7/19) had been taken over by the challenger. The size–frequency of winners was significantly different between owners and challengers (P < 0.05, Fisher's exact probability test). However, the final mate guarder was frequently different from the guarder at 5 minutes. All of the larger owners, except one individual who was similar in size to the challenger, continued guarding, but most of the smaller owners (15/19) had lost their partners. No significant difference was seen in the size–frequency of winners between owners and challengers (P > 0.05, Fisher's exact probability test).
In the competition between owners and challengers for receptive females, most challengers first attacked the owners using their 2nd gnathopods, and afterwards, the owners and challengers fought for several seconds using their 1st and 2nd gnathopods. In cases when the challenger succeeded in taking over the female, most of the challengers grasped the female with their 5th pereopods placed over those of the owner, and the owner detached and escaped. However, some owners escaped from the challenger with the female. Conversely, in cases when the challenger failed to take over the female, the owner behaved aggressively towards the challenger. In the latter cases, most of the challengers did not touch the female and either escaped or stayed beside the pair. Thus, the battles for females were very aggressive but no males died during the experiment.
Sex-ratio and guarding duration
In the sex-ratio manipulation experiment, mean guarding duration was 10.3±6.0 hours in the male-biased groups (N = 13) and 5.6±3.6 hours in the female-biased groups (N = 15). The mean duration was significantly longer in male-biased groups (Z = 2.31, P < 0.05, Mann–Whitney U-test).
DISCUSSION
Guarding forms
In the genus Caprella, Aoki (Reference Aoki1996) distinguished two guarding forms, Type O in Caprella penantis and Caprella glabra Aoki, 1991 and Type I in Caprella decipiens Mayer, 1890, Caprella danilevskii Czerniavski, 1868 and several other species. Caine (Reference Caine1991) has already described the guarding form of Caprella laeviuscula Mayer, 1903, and it might be thought of as Type O guarding. Aoki (Reference Aoki1996) suggested that the guarding type was constant in each species and determined by habitat conditions and phylogeny. However, we found that C. penantis conducted two guarding types, Type O and Type I-like guarding, although the latter was rare. In addition, some pairs conducting Type O guarding plastically changed their guarding to a Type I-like one. These observations indicate that mate-guarding forms in C. penantis are not constant, even within the same individual, and guarding forms change according to circumstances. Moreover, male size was correlated with female size in the guarding pair, showing size assortative pairing (Figure 3).
In amphipod species, other classifications of precopulatory guarding forms (i.e. carrier and attender forms) were suggested by Conlan (Reference Conlan1991). According to her definitions, in carrier species, the male grasped the female's dorsum or lateral plates and held her until she moulted. The male was still capable of walking or swimming, but the female either curled her abdomen and remained passive or extended her body and swam with her partner. In contrast, in attender species, the male did not carry the female but maintained close proximity by placing his body over hers or sharing her tube. Unfortunately, the correspondence between these two classifications has not been reported. However, in C. penantis, Type O guarding is clearly carrier guarding because the male could carry the female, and Type I-like guarding may be equivalent to attender guarding because the male could not easily carry the female and only remained close to her.
OSR in the natural population and guarding duration
In the field population of C. penantis, the proportion of ovigerous females was very high and the OSR was strongly male-biased. Bynum (Reference Bynum1978) also reported that the proportion of ovigerous females remained very high year-round in a North Carolina C. penantis population. However, Lewbel (Reference Lewbel1978) reported that the adult sex-ratio of Caprella gorgonia in California was female-biased (male:female = 1:3 or 1:4) because of intraspecific male aggression for mates. Caine (Reference Caine1979) reported that the sex-ratio of C. laeviuscula in Washington was male-biased because of selective predation on females. Thus, in the genus Caprella, the sex-ratio and the OSR may differ among populations and species.
Grafen & Ridley (Reference Grafen and Ridley1983) indicated that the OSR greatly affected precopulatory mate guarding duration. In the present study, the guarding pairs sampled from the field continued guarding for an average of 350 minutes in the laboratory, indicating that guarding duration may be approximately 10 hours. However, the precopulatory guarding started earlier in the male-biased group, and consequently the guarding duration was longer. This result supports the prediction of Grafen & Ridley (Reference Grafen and Ridley1983) that the mate guarding decision is dependent on the sex-ratio. Similar results have been reported in Gammarus (Ward, Reference Ward1983; Dick & Elwood, Reference Dick and Elwood1996), the hermit crab (Wada et al., Reference Wada, Tanaka and Goshima1999) and isopods (Manning, Reference Manning1980). However, studies of other factors affecting the initiation of mate guarding may be required, such as the fertility of receptive females, predation pressure on guarding pairs (Cothran, Reference Cothran2004) and male–female interactions in guarding as sexual conflict (Jormalainen, Reference Jormalainen1998).
Size and ownership advantage
In precopulatory mate guarding, whether the male can guard his mate until the female moults and becomes receptive is a decisive problem. Therefore, determining which factors contribute to the success of female guarding and guarding duration is very important. Two factors have been suggested as being important, ownership (Strong, Reference Strong1973; Wada et al., Reference Wada, Tanaka and Goshima1999) and competitive abilities such as body size (Ward, Reference Ward1983). In this study, body size was the most important factor and ownership was secondary. This result agrees with the pattern observed in Gammarus pulex by Ward (Reference Ward1983). However, whether earlier precopulatory guarding is less advantageous for large males that can take over females remains controversial. If the most important factor in competition for females is body size, as is shown in this study, large males should invest their energy into searching instead of guarding a receptive female. In natural populations, however, the cost of taking over a female may be higher because of wave action and/or complex substratum structure. Hence, comparing the reproductive success of males among various size-classes, and measuring guarding costs, searching costs and takeover costs under natural conditions are important.
ACKNOWLEDGEMENTS
We thank M. Shimanaga, H. Shimasaki, T. Watanabe and T. Nojima for their generous contributions to this study.






