INTRODUCTION
Saline habitats are important natural assets of considerable economic, ecological, scientific and conservation value (Williams, Reference Williams2002). Most notably, their unique physical and chemical characterization and distinctive biota set them apart from other aquatic and/or semi-aquatic ecosystems (Major et al., Reference Major, Kirkwood, Major, McCreadie and William2005). The brine shrimp Artemia is a typical halophilic species of brackish, saline and hypersaline waters, found in a variety of harsh environments in many parts of the world except Antarctica (Van Stappen, Reference Van Stappen, Abatzopoulos, Beardmore, Clegg and Sorgeloos2002). This branchiopod has acquired adaptive mechanisms to survive and evolve in habitats with extensive and often abrupt fluctuations in salinity, UV irradiation, temperature and oxygen concentration (Persoone & Sorgeloos, Reference Persoone, Sorgeloos, Personne, Sorgeloos, Roels and Jaspers1980; Browne et al., Reference Browne, Sorgeloos and Trotman1991; Abatzopoulos et al., Reference Abatzopoulos, Beardmore, Clegg and Sorgeloos2002). Very few species tolerate high salinity for an extended time, however Artemia are found in natural habitats with salinities from 10 g l−1 to 340 g l−1 (Sorgeloos et al., Reference Sorgeloos, Lavens, Léger, Tackaert and Versichele1986), but are rarely found in waters with salinity lower than 45 g l−1. At these salinity levels, high ionic concentrations are a limiting factor due to the energy requirements for osmoregulation. Artemia populations are favoured by the absence of predators and food competitors: they develop successfully in these extreme biotopes often at high densities (Camargo et al., Reference Camargo, Ely, Duran and Sorgeloos2004).
The Tunisian climate is characterized by long dry summers and short rainy winters, which generally implies a lack of a well-developed permanent surface hydrographic network. For this reason, ephemeral and temporary habitats are the most common and representative natural superficial waters, such as Sabkhet El Adhibet (33°07′7.58″N–11°2′48.69″E), an inland site located in south-east Tunisia (Figure 1). Its total surface is 12,500 hectares, including 500 hectares occupied by industrial salt works. Maximum length and width are 8 and 7 km, respectively. Sabkhet El Adhibet is located in an upper-arid area. The saltern is generally filled by rain water from December to February. In the saltwork water is pumped from the underground brine reservoir with a salinity of approximately 280 g l−1 and directly administered in the crystallizer. Trenches delimiting saltworks were dug artificially to consolidate the dividing dykes of the basins with an average depth of 0.75 m; these artificial canals in the border of saltwork are filled by rain water accumulated during the rainy season until May or June, depending on the precipitation and evaporation rate and where Artemia occur. No rainfall data are available on that specific site. Yet, Table 1 shows the yearly average precipitation recorded for the period 1997–2004 in the closest climatological station, the town of Zarzis located at 50 km from Sabkhet El Adhibet. In Sabkhet El Adhibet Artemia was reported for the first time by Romdhane et al. (Reference Romdhane, Ben Chikh, Ghlala and Charfi2001). Furthermore, Romdhane et al. (Reference Romdhane, Ben Naceur, Hamrouni, Ben Rejeb Jenhani, El Cafsi, Agh and Sorgeloos2004) compared, using discriminant analysis, the morphometry of Artemia (males and females) with other Artemia populations, and they concluded that Artemia from Sabkhet El Adhibet belongs to the Artemia salina species.

Fig. 1. Sabkhet El Adhibet location with overview of sampling site.
Table 1. Weather characteristics average based on readings from 1997 to 2004 at Zarzis (33°30′N–11°07′E).

In this study, our objective was to characterize the Artemia biotope, such as physicochemical characteristics of the habitat, to determine Artemia population density, population composition, sex-ratio, fecundity and type of offspring.
MATERIALS AND METHODS
Since, sites are dried up from May to October, sampling and monitoring took place monthly from November 2005 to April 2006 and from November 2006 to April 2007. All data were taken in the morning between 07:00 and 11:00 am. Three stations (Figure 1) were chosen in the artificial canals in the border of saltwork (Artemia is absent in the crystallizers). Geographical coordinates (S1: 33°05′42″N–11°24′29.8″E; S2: 33°06′31″N–11°25′28.7″E and S3: 33°05′54.3″N–11°25′42.8″E) were obtained by a GPS receiver (Garmin GPS 12). During these visits, water temperature, salinity and pH were measured in situ using portable multiprob (WTW, Multi/340i/SET). Dissolved oxygen concentration was carried out following the chemical method of Winkler. Water samples were stored for laboratory analysis of orthophosphate, nitrites, nitrates and ammonium (colorimetric method). For phytoplankton composition, water samples were collected from the surface water. Samples were preserved (with Lugol and a neutralized formaldehyde solution) and used for the identification and the quantification of microalgae in Sabkhet El Adhibet following sedimentation chambers and an inverted microscope (Leitz) observation.
Artemia samples were collected after filtration of 100 l of water at each station with plankton net (120 µm mesh size) and fixed in situ with neutralized 5% formalin solution to determine the Artemia population density expressed by individual per litre and population composition (nauplii, metanauplii, juveniles and adults) expressed as per cent of total number. Sex-ratio, fecundity and type of offspring output (ovigerous sacs were dissected under a magnifying glass and the number of cysts or nauplii was counted) were also analysed.
RESULTS
Abiotic conditions
Physicochemical water parameters are shown in Table 2. The surface water temperatures ranged between 12.1°C (March 2006) and 25.4°C (April 2006). The variability of the salinity between the three stations was limited except January 2007, were the difference between Stations 1 (S1) and the two other Stations (S2 and S3) is 73 and 117 g l−1, respectively. The mean values of minimum and maximum salinity obtained were 32.2 and 281.7 g l−1. During the two periods (November 2005–April 2006 and November 2006–April 2007), salinity peaked and exceeded 250 g l−1 in March–April. Large seasonal variations of dissolved oxygen were observed especially during the second period. Average values obtained varied from 3.4 to 17.5 mg l−1. Monthly average pH values ranged from 7.6 to 9.
Table 2. Physicochemical parameters (mean±SD) for the three stations.

DS, dry season.
The nutrients analysis (Figure 2) showed that:
– The mean values of orthophosphate (PO42−) ranged between 0.027±0.01 mg l−1 in November 2005 and 0.293±0.18 mg l−1 in January 2006.
– The mean values of nitrite (NO2−) ranged between 0.001±0.001 mg l−1 for December 2005 and 0.195±0.04 mg l−1 for November 2006.
– The nitrate (NO3−) minimum concentration was recorded in February 2007 (0.25±0.05 mg l−1) and the maximum in December 2006 (0.633±0.12 mg l−1).
– The ammonium (NH4+) concentrations ranged between 0.137±0.02 mg l−1 in November 2005 and 0.617±0.05 mg l−1 in November 2006.

Fig. 2. Mean values of nutrient (mg l−1) of Sabkhet El Adhibet (A, orthophosphate; B, nitrite; C, nitrate; D, ammonium).
Nitrate was the most frequently abundant nutrient during the investigation period contrasting with low concentrations of nitrite.
Phytoplankton density
Phytoplankton abundance was dominated by diatoms and chlorophytes were well represented at various times (Figure 3) with a maximum of 97.8% (November 2006) and 95.7% (January 2007), respectively. Seasonal successions were varied with a distinct seasonal periodicity occurring between different periods. Microalgal density level reached a maximum in December 2005 and January 2006, when salinity did not exceed 40 g l−1. The decline during spring and early summer resulted from the minimal values at the end of the sampling period. The comparison between the density of Artemia and those of microalgae showed that the phytoplankton density reached its maximum when the Artemia population was nearly absent or decreased. Cell concentration varies between 188,136 cells l−1 in November 2006 to 14,582,880 cells l−1 in January 2006.

Fig. 3. Seasonal fluctuations of microalgal density (103 cellule l−1).
Artemia population
In Sabkhet El Adhibet, the average Artemia density was measured between 0.22±0.12 and 38.57±15.07 individuals per litre. In each station, Artemia showed a patchy distribution and composition. The number of individuals collected monthly was the lowest in April of both years, except in December 2005 when the site was completely flooded by rain water (beginning of the rainy season), and this may have caused a severe dilution of the population (Figure 4).

Fig. 4. Mean value of seasonal fluctuation of Artemia density.
The maximum Artemia density was observed in November 2005 (60.27 individuals per litre in Station S1), and the minimum in December 2005 (0.1 individual per litre in Station S3). For the population composition, nauplii made up a very high fraction of the samples in November of the two periods (more than 60%). This fraction declines progressively and remains at a minimum level during March and April. Furthermore, metanauplii and juveniles presented the most important fraction of the population in December 2005 (37.4%) and in January and February 2007 (35.5 and 38.7%). Moreover, adult dominance was different between the two periods (November 2005–April 2006 and November 2006–April 2007): adults made up a very high fraction of the samples in March 2006 with 75.5% during the first period and in April 2007 with 66.9% during the second period (Figure 5).
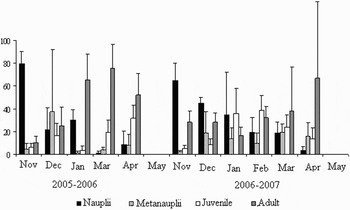
Fig. 5. Mean values (%) of monthly fluctuations of Artemia population composition (averages of all sampling stations).
Sex-ratio showed that males predominated with a maximum (1:0.52 males:females) in February 2007 (Table 3).
Table 3. Sex-ratio (males:females) and number of cysts or nauplii per females collected of Artemia from Sabkhet El Adhibet.

DS, dry season.
It is obvious that Artemia females in Sabkhet El Adhibet depict oviparous reproduction. It is the end of each period when ovoviviparity shows its minima (Figure 6). The average fecundity showed that: for cyst production, the average number of cysts per female ranged from 29.9 to 70.2 (December 2005 and April 2007, respectively) and 17.8 to 69.8 (December 2005 and April 2006, respectively) for the average nauplii per female (Table 3).

Fig. 6. Monthly fluctuation of oviparous and ovoviviparous reproduction (%).
DISCUSSION
Many saline lakes in endorheic basins in arid or subarid regions are very shallow and usually temporary. They are strongly dependent on the hydrological budget, and then limnological, ecological characteristics and physicochemical parameters have a large interannual variability and behaviour from one year to another (Garcia et al., Reference Garcia, Garcia-Ruiz, Rendon, Xavier Niell and Lucena1997). These lakes are likely to undergo complete desiccation and they are permanent only occasionally. This makes it difficult to distinguish regularities linked to this hydrological forcing. Thus, although there are some data in the literature on the seasonality of saline lakes, there are fewer studies on interannual variability (Stephens, Reference Stephens1990). Sabkhet El Adhibet is a typical example of these shallow saline lakes, having some infrequent episodes of permanence but usually becoming dry in the summer. However, despite the simplicity of the trophic structure (short food chain) and the limited biodiversity of these ecosystems, it is difficult to assess the consequences of abiotic fluctuation and shifts in phytoplankton concentration and composition on Artemia population (Gliwicz et al., Reference Gliwicz, Wurtsbaugh and Ward1995).
Abiotic conditions
Several studies have estimated environmental and genetic components of variance for life span and reproductive traits of Artemia (Vanheacke et al., Reference Vanheacke, Siddall and Sorgeloos1984; Wear et al., Reference Wear, Haslett and Alexander1986; Triantaphyllidis et al., Reference Triantaphyllidis, Poulopoulou, Abatzopoulos, Pérez and Sorgeloos1995; Browne et al., Reference Browne, Moller, Forbes and Depledge2002; Abatzopoulos et al., Reference Abatzopoulos, El-Bermawi, Vasdekis, Baxevanis and Sorgeloos2003). The majority of them have focused on temperature and salinity which are of the most important physical parameters affecting the life history in hypersaline organisms. Vanheacke et al. (Reference Vanheacke, Siddall and Sorgeloos1984) studied the combined effect of temperature and salinity on the survival of Artemia of various geographical origins (with two Artemia salina populations from Spain and Cyprus) and showed that temperature is the most important factor that affected Artemia survival, and that the A. salina and A. parthenogenetica strains did not survive at temperatures exceeding 30°C and A. franciscana at 34°C. On the other hand, studies of temperature effects on reproductive characteristics of clonal Artemia carried out by Abatzopoulos et al. (Reference Abatzopoulos, El-Bermawi, Vasdekis, Baxevanis and Sorgeloos2003), showed that 30°C seemed to be the extreme value affecting significantly nearly all of the studied variables. In our survey, variation in water temperature showed a difference between the two sampling periods (12.1–25.4°C during the first period and 15.2–18.7°C during the second period). These values were measured in the morning and probably the water temperatures could have been higher in the afternoon. However, lethal temperatures of 30–34°C were never recorded at the water surface in Sabkhet El Adhibet. Vanheacke & Sorgeloos (Reference Vanheacke and Sorgeloos1989) reported that the maximum growth and biomass production takes place in the range 20–27°C. Once again, the impact of temperature on growth varies from one strain to another, but for most strains, growth is limited below 15°C. If these data are extrapolated to Artemia from Sabkhet El Adhibet, the season for optimal biomass production is confined to December–March.
Salinity, as a crucial factor for Artemia presence, is linked to the occurrence of its predators at lower and intermediate salinity (Van Stappen, Reference Van Stappen, Abatzopoulos, Beardmore, Clegg and Sorgeloos2002). Triantaphyllidis et al. (Reference Triantaphyllidis, Poulopoulou, Abatzopoulos, Pérez and Sorgeloos1995) studied the salinity effects on survival, maturity, growth, biometrics, reproductive and lifespan characteristics of bisexual and a parthenogenetic population of Artemia. This evaluation revealed that the two examined populations exhibited significant differences in their response against high salinity levels. The same authors showed that survival is affected by salinity and both species showed high mortality when nauplii were directly transferred to high salinities. However, when salinity is gradually increased, bisexual Artemia (A. franciscana) exhibits better adaptation. D'Agostino & Provasoli (Reference D'Agostino and Provasoli1968) and Sorgeloos (Reference Sorgeloos, Personne, Sorgeloos, Roels and Jaspers1980) showed that brine shrimp nauplii can resist to sudden shifts in salinity, e.g. instar-I nauplii may be transferred from 5 g l−1 to 150 g l−1 without increased mortality. In this study, most important Artemia densities were observed at different salinity concentration. In the first period, the maximum density (30.58±29.24 individuals per litre) was obtained at salinity 57.3 g l−1. In the second period, the maximum density (39.57±15.03 individuals per litre) was observed at 156.7 g l−1. On the other hand, the rapid increase of salinity observed in March and April showed a stern reduction of Artemia density. Persoone & Sorgeloos (Reference Persoone, Sorgeloos, Personne, Sorgeloos, Roels and Jaspers1980) reported that Artemia are rarely found in waters with salinity lower than 45 g l−1. However, in this survey, the brine shrimp was present (in small numbers) at salinity between 32.2 and 38.7 g l−1.
Artemia is found in neutral to alkaline waters. According to Vos (Reference Vos1979), nauplii growth decreases and the overall appearance of adults deteriorates with pH values below 7.0, and they concluded that the optimum pH for Artemia growth ranges from 8.0 to 8.5. In addition, Sato (Reference Sato1967) determined that cysts hatching efficiency was greatly compromised at pH below 8. In Sabkhet El Adhibet, pH values ranged from 7.6 to 9. The high O2 levels registered at Sabkhet El Adhibet could be attributed to low salinity level or/and high phytoplankton density. The nutrient analysis showed that nitrate and ammonium represent the major nutrients. In addition, nitrate rate seems to be proportional to the salinity. Camargo et al. (Reference Camargo, Ely, Duran and Sorgeloos2004, Reference Camargo, Duran, Rada, Hernandez, Linero, Muelle and Sorgeloos2005) after analysing biological and physiological parameters of Artemia in the Colombian Caribbean, found the same results. They explained this by the fact that in saltworks, numerous organisms are trapped and slowly die when salinity increases progressively. Thus, organic matter accumulates and decomposes, both of animal (i.e. crustaceans, fish and insects) and vegetal origin (i.e. leaves and phytoplankton). Consequently, the concentration of the nitrogenous compounds, nitrite and nitrate (through the process of nitrification), increases through time as salinity increases. On the other hand, water in Sabkhet El Adhibet is originated in its major part from precipitation. Consequently, weak input of detritus is reported in the site explaining the low level of different nutriment.
Phytoplankton density
Phytoplankton density, in Sabkhet El Adhibet, ranged from 0.19 to 14.59 106 cell l−1. The optimum density is observed during December and January of the first period, at a salinity of 32.2 and 38.7 g l−1, respectively. It is very important to underline that in this period the site was flooded with large volumes of fresh water. This may have caused a severe water dilution. Amat et al. (Reference Amat, Hontoria, Navarro, Gonzalbo and Varo1991) showed that salinity higher than 50 g l−1 hinders considerably primary productivity in hypersaline ecosystems perhaps because of an ionic complex formation of the dissolved macronutrients or because of a generic biological phenomenon of a drastic specific reduction of microalgae, also occurring at higher salinities. Nevertheless, Dolapsakis et al. (Reference Dolapsakis, Tafas, Abatzopoulos, Ziller and Economou-Amilli2005) revealed that microalgal densities were high in salinity of more than 100 ppt when inorganic-P concentrations were more than 0.2 mg l−1 within saltwork waters. In addition, in Sabkhet El Adhibet the maximum phytoplankton density corresponds to the minimum Artemia density. The same results were obtained by Dolapsakis et al. (Reference Dolapsakis, Tafas, Abatzopoulos, Ziller and Economou-Amilli2005) who showed that the density of Dunaliella salina populations decrease when the grazing by Artemia parthenogenetica and Fabrea salina was intense. Moreover, during our survey, the algal species composition in Sabkhet El Adhibet was very diverse with an important fraction of diatoms (such as Amphiprora, Cyclotella ocellata, Navicula and Nitzschia) and chlorophytes (such as Chlamydomonas and Dunaliella). This same microalgal composition was also reported by Van Stappen et al. (Reference Van Stappen, Fayazi and Sorgeloos2001) and Dolapsakis et al. (Reference Dolapsakis, Tafas, Abatzopoulos, Ziller and Economou-Amilli2005).
Artemia population
The results of this study demonstrated that variation in Artemia density and composition varies considerably from one month to another. In Sabkhet El Adhibet, Artemia density exceeds 39.57 ind l−1 with 32% of adults in February 2007 at a salinity of 156.7 g l−1. However, the two other months where we observed similar concentrations are November 2005 and March 2006 with a salinity of 57.3 and 76.7 g l−1, respectively. The relative low density observed on December 2005 (0.22 ind l−1) can be explained by the water dilution causing a rapid decrease of salinity (32.2 g l−1). Contrarily, the small number of Artemia collected in April is due to the beginning of the dry season and the increase of the salinity. Gliwicz et al. (Reference Gliwicz, Wurtsbaugh and Ward1995) summarize the population dynamics in Great Salt Lake as follows: in April–May, a first generation hatches from cysts and colonizes the environment rapidly. This generation reproduces ovoviviparously at high rate, illustrated by large broods of nauplii during late May. These animals produce a second generation of more slowly developing individuals, which do not reach maturity in the autumn, and only a small fraction of this second generation seems to survive and reach maturation, producing one or two smaller broods, which do not contribute significantly to the population density. In this study, cysts hatching may occur in November as a consequence of the first precipitation, resulting in highest density of nauplius. In fact, during the two study periods, nauplii composed the major part of the Artemia population with 79.3–64.7% in November 2005 and 2006, respectively. This level decreased proportionally to the increase of adults who present a reproductive mode dominated by oviparity implying a weak involvement at the level of the nauplii rate in water. It permitted us to suppose that population composition is not only controlled by abiotic conditions but also by the reproductive mode. In Sabkhet El Adhibet, adults represent their maximum density until March at a salinity of 76.7 g l−1 in 2006 and 276.3 g l−1 in April 2007.
The quality or type of zygote is significant for the survival of brine shrimp populations since two reproductive modes (encystment and ovoviviparity) exist, and all Artemia strains combine both types with the ratio varying widely among them (Gajardo & Beardmore, Reference Gajardo and Beardmore1989). Oviparity is commonly seen in populations with a seasonal cycle of either temperature or salinity though factors such as brood number, hypoxia, photoperiod and availability and type of food can be also of importance. In this way, Rodriguez-Almaraz et al. (Reference Rodriguez-Almaraz, Zavala, Mendoza and Maeda-Martinez2006) mentioned that the different reproduction type for both periods could be attributed to temperature and salinity changes. Román & Rodríguez (Reference Román and Rodríguez1986) reported that cysts production in Cadiz saltworks (Spain) is given when the chlorophyll-a and Artemia density ratio decrease. Similarly, D'Agostino & Provasoli (Reference D'Agostino and Provasoli1968) affirmed that food quality and quantity could induce oviparity. According to Lenz & Browne (Reference Lenz, Browne, Browne, Sorgeloos and Trotman1991) and Lavens & Sorgeloos (Reference Lavens and Sorgeloos1996), the salinity may be considered as one of the main ecological factors affecting the reproduction mode in favour of ovoviviparity. In opposition, Gajardo & Beardmore (Reference Gajardo and Beardmore1989) suggested that encystment is under genetic control and is associated, at least in part, with the level of heterozygosity in the mother. Because of the number of generations produced per month, field data did not allow a definitive conclusion. Regarding the reproductive data we can affirm a predominance of oviparity. In fact, the maximum ovoviviparity mode was 30%, and the seasonal decline in percentage of ovoviviparous females coincides with the increase of salinity to more than 250 g l−1 and the decrease of phytoplankton density. On the other hand, Ben Naceur et al. (Reference Ben Naceur, Ben Rejeb Jenhani and Romadhane2005) showed that in laboratory conditions, females from Sabkhet El Adhibet were 54.75% oviparous. The comparison between these results (Ben Naceur et al., Reference Ben Naceur, Ben Rejeb Jenhani and Romadhane2005) and those obtained in this study permitted to suppose that the reproductive performance and encystment ratios are under double control: a genetic control associated with or triggered by the prevailing abiotic conditions.
Camargo et al. (Reference Camargo, Ely, Duran and Sorgeloos2004) mentioned that the results of the reproductive experiment (mean cysts production per female) do not entirely agree with the estimated cyst production potential at each site and may be due to a combination of certain parameters as salinity, per cent O2 saturation and starvation of the adult Artemia population after reaching a high density, particularly of the female portion. Lenz & Browne (Reference Lenz, Browne, Browne, Sorgeloos and Trotman1991) and Lavens & Sorgeloos (Reference Lavens and Sorgeloos1996) reported that the optimum reproductive performance recorded for American strains of Artemia ranged between 100 and 300 nauplii or cysts by female. Previous work with pond cultures demonstrated that cyst yields steadily decline with time (Camara & De Meideros Rocha, Reference Camara, De Meideros Rocha, Sorgeloos, Bengtson, Decleir and Jaspers1987). In our survey the maximum cysts and nauplii per female were 158 and 148, respectively. Nevertheless, the follow-up of reproductive performance of Artemia from Sabkhet El Adhibet showed that they decrease when salinity increases and microalgal density declines.
Gliwicz et al. (Reference Gliwicz, Wurtsbaugh and Ward1995) mentioned that in Great Salt Lake sex-ratio fluctuated from 4:1 in June to 1:1 in November/December. Van Stappen et al. (Reference Van Stappen, Fayazi and Sorgeloos2001) explained these fluctuations by the different physiological tolerance of adult males and females to variance in environmental conditions (temperature, salinity and/or oxygen). In Sabkhet El Adhibet, sex-ratio varies from one month to another. However, the monthly screening of adults revealed that males were more abundant than females, except for March 2006 and April 2007.
CONCLUSION
To summarize and after analysing the data collected during the two sampling periods, the influence of physical and chemical parameters as well as phytoplankton density over Artemia sampling was clearly noticed. Salinity, temperature and phytoplankton density seem to be the major abiotic and biotic factors affecting the life-cycle of Artemia population. Hatching of cysts may occur as early as November and the maximum of adults' density occurred between February and April. On the other hand, Artemia from Sabkhet El Adhibet showed a preference to oviparity mode and a sex-ratio in favour of males.











