Introduction
Cold-water corals (CWCs) are commonly defined as a heterogeneous group of habitat-forming species thriving below the continental shelf break (200 m) (Buhl-Mortensen et al., Reference Buhl-Mortensen, Vanreusel, Gooday, Levin, Priede, Buhl-Mortensen, Gheerardyn, King and Raes2010). The most relevant CWC facies are represented by white corals, dominated by the two scleractinian species: Lophelia pertusa (Linnaeus, 1758) and Madrepora oculata (Linnaeus, 1758) (Freiwald et al., Reference Freiwald, Beuck, Rüggeberg, Taviani and Hebbeln2009). These species have a worldwide distribution producing formations mainly found along the NE Atlantic continental margin (Rogers, Reference Rogers1999). Despite some scientific reports of scattered living white coral populations (Reyss, Reference Reyss1964; Bourcier & Zibrowius, Reference Bourcier and Zibrowius1973; Zibrowius, Reference Zibrowius1980; Vafidis et al., Reference Vafidis, Koukouras and Voultsiadou-Koukoura1997; Tunesi & Diviacco, Reference Tunesi and Diviacco1997) it was not until 2004 that the first characterization of a Mediterranean white coral assemblage, namely that of Santa Maria di Leuca, was made (Tursi et al., Reference Tursi, Mastrototaro, Matarrese, Maiorano and D'onghia2004). Today, an extent of living coral facies is known for the entire basin, from the Alboran Sea to the Aegean Sea, at depths of between 200 and 800 m (Tursi et al., Reference Tursi, Mastrototaro, Matarrese, Maiorano and D'onghia2004; Álvarez-Pérez et al., Reference Álvarez-Pérez, Busquets, De Mol, Sandoval, Canals, Casamor, Freiwald and Roberts2005; Schembri et al., Reference Schembri, Dimech, Camilleri and Page2007; Freiwald et al., Reference Freiwald, Beuck, Rüggeberg, Taviani and Hebbeln2009; Orejas et al., Reference Orejas, Gori, Iacono, Puig, Gili and Dale2009; Mastrototaro et al., Reference Mastrototaro, D'onghia, Corriero, Matarrese, Maiorano, Panetta, Gherardi, Longo, Rosso, Sciuto, Sanfilippo, Gravili, Boero, Taviani and Tursi2010; Taviani et al., Reference Taviani, Vertino, Lopez Correa, Savini, De Mol, Remia, Montagna, Angeletti, Zibrowius, Alves, Salomidi, Ritt and Henry2011; Gori et al., Reference Gori, Orejas, Madurell, Bramanti, Martins, Quintanilla, Marti-Puig, Lo Iacono, Puig, Requena, Greenacre and Gili2013; Angeletti et al., Reference Angeletti, Taviani, Canese, Foglini, Mastrototaro, Argnani and Macic2014; Fabri et al., Reference Fabri, Pedel, Beuck, Galgani, Hebbeln and Freiwald2014), with the most recently studied banks discovered off the southern Sardinian coasts (Taviani et al., Reference Taviani, Angeletti, Canese, Cannas, Cardone, Cau, Cau, Follesa, Marchese, Montagna and Tessarolo2017).
Even if in the Mediterranean basin CWC reefs are apparently of a smaller extent compared with their Atlantic counterparts (Duineveld et al., Reference Duineveld, Lavaleye and Berghuis2004; Álvarez-Pérez et al., Reference Álvarez-Pérez, Busquets, De Mol, Sandoval, Canals, Casamor, Freiwald and Roberts2005; Taviani et al., Reference Taviani, Remia, Corselli, Freiwald, Malinverno, Mastrototaro, Savini and Tursi2005), they still maintain their complex overall architecture. The specimens, initially settling on a rocky substrate, may overgrow one another resulting in an intricate net of biogenic ramifications and interstices. The living framework, growing at a rate of 1 cm per year, may exceed 1 m in height (Orejas et al., Reference Orejas, Gori and Gili2008).
The resulting three-dimensional structure is known to provide refuge, and a nursery area to numerous soft- and hard-bottom associated species, greatly enhancing the richness of these assemblages, properly defined as major deep-sea biodiversity hotspots (McCloskey, Reference McCloskey1970; Connell, Reference Connell1978; Jensen & Frederiksen, Reference Jensen and Frederiksen1992; Mortensen et al., Reference Mortensen, Hovland, Fosså and Furevik2001; Mortensen & Fosså, Reference Mortensen and Fosså2006; Mastrototaro et al., Reference Mastrototaro, D'onghia, Corriero, Matarrese, Maiorano, Panetta, Gherardi, Longo, Rosso, Sciuto, Sanfilippo, Gravili, Boero, Taviani and Tursi2010; D'Onghia et al., Reference D'Onghia, Maiorano, Carlucci, Capezzuto, Carluccio, Tursi and Sion2012). The richness associated with CWC reefs has been estimated to be around 1300 species for NE Atlantic Ocean reefs, while a recent review identified about 500 species for the CWC habitats of the Mediterranean Sea and adjacent areas (Rueda et al., Reference Rueda, Urra, Aguilar, Angeletti, Bo, García-Ruiz, González-Duarte, López, Madurell, Maldonado, Mateo-Ramírez, Megina, Moreira, Moya, Ramalho, Rosso, Sitjá, Taviani, Orejas and Jiménez2018). Among the most represented taxa there are sponges, cnidarians, polychaetes and bryozoans, more or less strictly associated to the reef ecosystem, in particular to the dead coral matrix and coral rubble (Mastrototaro et al., Reference Mastrototaro, D'onghia, Corriero, Matarrese, Maiorano, Panetta, Gherardi, Longo, Rosso, Sciuto, Sanfilippo, Gravili, Boero, Taviani and Tursi2010).
Sponges have proven to be among the most important components of the fauna associated with Mediterranean white coral reefs (Longo et al., Reference Longo, Mastrototaro and Corriero2005; Freiwald et al., Reference Freiwald, Beuck, Rüggeberg, Taviani and Hebbeln2009; Mastrototaro et al., Reference Mastrototaro, D'onghia, Corriero, Matarrese, Maiorano, Panetta, Gherardi, Longo, Rosso, Sciuto, Sanfilippo, Gravili, Boero, Taviani and Tursi2010; Calcinai et al., Reference Calcinai, Moratti, Martinelli, Bavestrello and Taviani2013; D'Onghia et al., Reference D'Onghia, Capezzuto, Cardone, Carluzzi, Carluccio, Chimienti, Corriero, Longo, Maiorano, Mastrototaro, Panetta, Rosso, Sanfilippo, Sion and Tursi2015; Rueda et al., Reference Rueda, Urra, Aguilar, Angeletti, Bo, García-Ruiz, González-Duarte, López, Madurell, Maldonado, Mateo-Ramírez, Megina, Moreira, Moya, Ramalho, Rosso, Sitjá, Taviani, Orejas and Jiménez2018), however, their species diversity has often been poorly considered. This resulted in an unbalanced understanding of CWC provinces depending on experts' availability and sampling effort. In particular, apart from the pioneer study by Vacelet (Reference Vacelet1969) on the canyons of the Gulf of Lion and Corsica (Fourt et al., Reference Fourt, Goujard, Pérez and Chevaldonné2017), the most relevant studies to date, for the amount of taxonomic and ecological information, are those carried out in the Santa Maria di Leuca area (Longo et al., Reference Longo, Mastrototaro and Corriero2005; Mastrototaro et al., Reference Mastrototaro, D'onghia, Corriero, Matarrese, Maiorano, Panetta, Gherardi, Longo, Rosso, Sciuto, Sanfilippo, Gravili, Boero, Taviani and Tursi2010). Other studies dealing with sponges associated with the coral framework have been conducted in the Bari Canyon (D'Onghia et al., Reference D'Onghia, Capezzuto, Cardone, Carluzzi, Carluccio, Chimienti, Corriero, Longo, Maiorano, Mastrototaro, Panetta, Rosso, Sanfilippo, Sion and Tursi2015) and the Strait of Sicily (Calcinai et al., Reference Calcinai, Moratti, Martinelli, Bavestrello and Taviani2013), while additional works in the Alboran Sea mainly dealt with the description of massive sponges (Pardo et al., Reference Pardo, Rubio, García and Ubero2011; de la Torriente et al., Reference de la Torriente, Aguilar, Serrano, García, Fernández, García Muñoz, Punzón, Arcos and Sagarminaga2014). Preliminary information on the sponge fauna of a recently discovered living M. oculata reef off the SE coast of Sardinia (Nora Canyon) is also available (Taviani et al., Reference Taviani, Angeletti, Canese, Cannas, Cardone, Cau, Cau, Follesa, Marchese, Montagna and Tessarolo2017; Cardone et al., Reference Cardone, Pansini, Corriero and Bertolino2019).
This study aims principally to fill the knowledge gap relative to the sponge fauna associated with the deep coral framework in the Tyrrhenian basin and to compare the species composition of the known sponge assemblages in order to establish possible connectivity patterns over a large geographic scale. A second target is comparing the deep reef assemblages with those thriving in the coralligenous bioconcretion, a shallow, coastal, structurally similar ecosystem, in order to establish if there is a potential bathymetric connectivity.
Materials and methods
Samplings were carried out in two sites along the Sardinian coast (Tyrrhenian Sea), namely Capo Coda Cavallo Canyon (Dive 17) situated along the NE coast of Sardinia close to Tavolara Island (40°54,75′N 9°54,9′E), and Nora Canyon (Dive 26) situated in the SE side of the island, close to Cagliari (38°42,49′N 8°54,55′E) (Figure 1A). The first site is characterized by a M. oculata reef extending between 256 and 264 m, while the second site hosts a M. oculata and L. pertusa framework between 357 and 408 m (Figure 1B). In each site, one block of partially alive M. oculata, about 2000 cm3, was collected respectively at 264 and 408 m depth (Figure 1C, D).
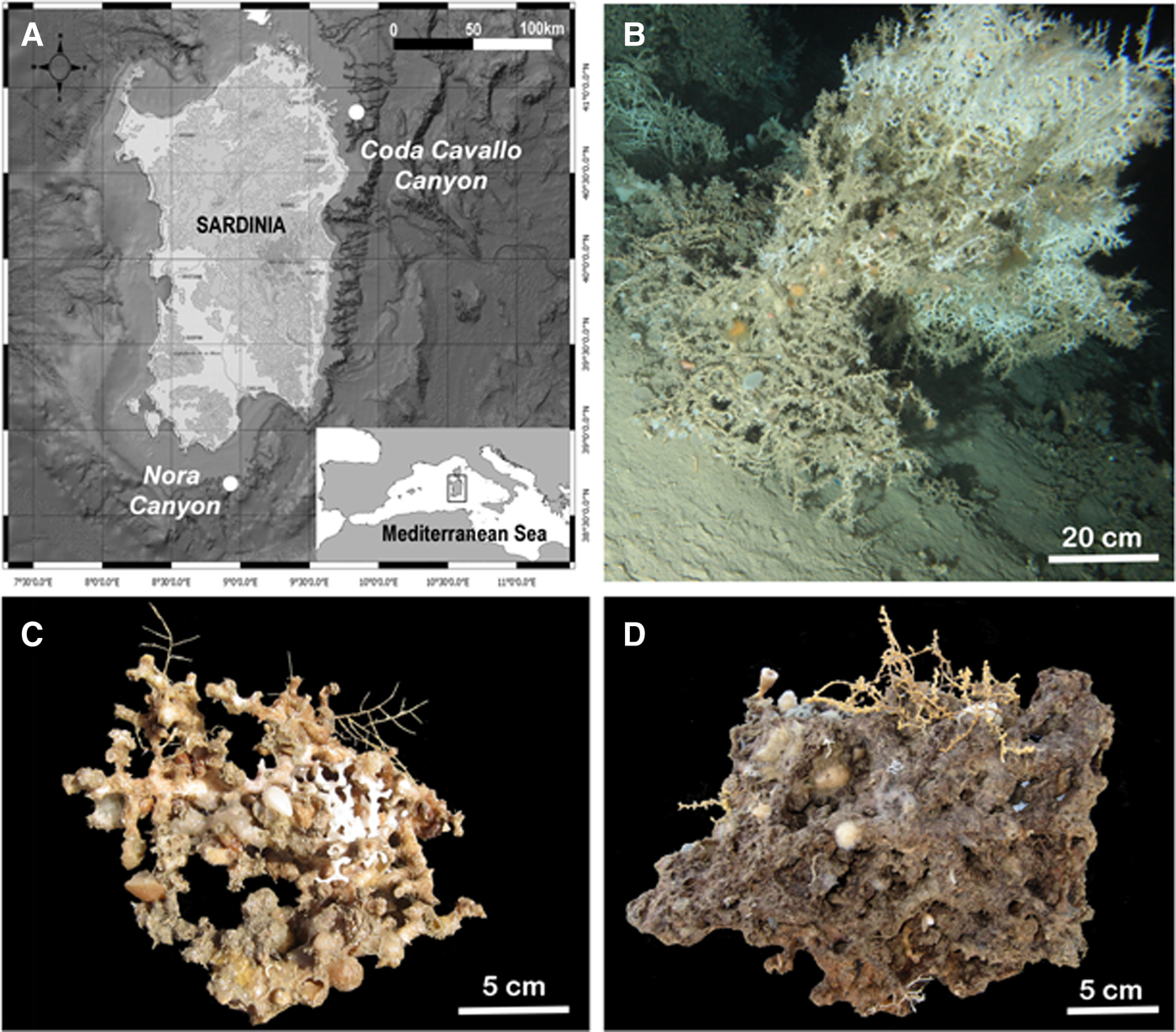
Fig. 1. Sampling characteristics: (A) location of the sampling sites; (B) underwater ROV image of the coral framework; (C) sample collected from the Coda Capo Cavallo Canyon; (D) sample collected from the Nora Canyon.
The material used in this study was collected by means of a ROV ‘Pollux’ during an oceanographic survey carried out in August–September 2013 onboard the RV ‘Astrea’ (ISPRA). The ROV was equipped with a digital camera (Nikon D80, 10 megapixel), a strobe (Nikon SB 400), a high definition video camera (Sony HDR-HC7), and a navigation camera (1/3-inch SONY CCD, focal length 4–9 mm). The vehicle also hosted a depth sensor, a compass, and three laser beams providing a 10 cm scale for the measurement of the frame areas and size of organisms. Collection was carried out by means of a grasping steel basket.
Samples were fixed onboard in 4% formaldehyde in filtered seawater and then preserved in 70% ethanol. Spicule complement and skeletal architecture were examined under light microscopy following Hooper (Reference Hooper2000). Length and width of at least 30 spicules per type were measured. Minimum, mean (in parentheses) and maximum values are reported. Dissociated spicules and tissues for scanning electron microscope (SEM) analysis were transferred onto stubs, sputter coated with gold and observed under a SEM Vega3 _TESCAN Microscope type LMU (DISTAV, UniGe).
In the systematics section, we provide a detailed description of seven less known species. The classification proposed by the World Porifera Database, implemented by Morrow & Cárdenas (Reference Morrow and Cárdenas2015), was followed for the taxonomic identification.
The sponge samples studied are deposited in the first author's personal collection at the Department of Sciences of the Earth, the Environment and the Life (DISTAV), Genoa University.
Results
SYSTEMATICS
From the two collected coral blocks, 112 specimens were sampled, identifying in total 28 sponge taxa: 27 at species level and one at genus level (Table 1). Additionally, three conspicuous species, Poecillastra compressa, Pachastrella monilifera and Phakellia robusta, were observed in the ROV footage over the coral concretions. In the following section the description of the most interesting species is supplied. One species, Rhabderemia profunda is a new record for the Mediterranean Sea, while Forcepia (Leptolabis) megachela was recorded for the first time after its original description; eight species were recorded for the first time in the white coral assemblage and four are new records for the Italian fauna. The geographic distribution as well as the bathymetric range was updated for numerous species, some of which were pictured in situ for the first time (Table 1, Figure 2).

Fig. 2. Living sponges in the coral framework of the Sardinia Canyons: (a) Oopsacas minuta Topsent, 1927; (b) Hamacantha (Hamacantha) johnsoni (Bowerbank, 1864); (c) Hamacantha (Hamacantha) lundbecki Topsent, Reference Topsent1904; (d) Plocamionida tylotata Brøndsted, Reference Brøndsted, Jensen, Lundbeck and Ragnar Sparck1932.
Table 1. Summary of the taxonomic analysis of the sponges associated with deep Sardinian coral frameworks

For the species in bold the taxonomic description is presented in the text. The depth ranges in bold are those extended with respect to literature.
a First record for the white coral assemblage.
b First record for the Central Tyrrhenian sector.
c First record for the Italian fauna.
d First record for the Mediterranean Sea.
e Pictured in situ for the first time.
Class DEMOSPONGIAE Sollas, 1885
Subclass HETEROSCLEROMORPHA Cárdenas, Pérez & Boury-Esnault, 2012
Order AXINELLIDA Lévi, 1953
Family RASPAILIIDAE Nardo, 1833
Subfamily RASPAILIINAE Nardo, 1833
Genus Acantheurypon Topsent, 1927
Acantheurypon pilosella (Topsent, Reference Topsent1904)
(Figures 3 & 4; Table 1)
Hymeraphia pilosella Topsent, Reference Topsent1904

Fig. 3. Acantheurypon pilosella (Topsent, Reference Topsent1904): (A) specimen encrusting on branches of Madrepora oculata; (B) magnification of the sponge surface; (C) skeleton.

Fig. 4. Acantheurypon pilosella (Topsent, Reference Topsent1904): (A) large styles, with magnification of the head; (B) auxiliary styles, with magnification of the end; (C) acanthostyles.
Material examined
Sard 10b (7); dive 17; 29 August 2013; Capo Coda Cavallo Canyon; 40°54,75′N-9°54,9′E; 256–264 m; on Madrepora oculata reef.
SAR15a (2, 4, 8, 23, 24) and SARD 15 g (2c); dive 26; 02 September 2013; Nora Canyon; 38°42,49′N-8°54,55′E; 357–408 m; on Madrepora oculata and Lophelia pertusa reef.
DIAGNOSIS
Thinly encrusting sponge, about 2 mm thick, covering small portions, about 3 cm long, of the dead branches of M. oculata. The surface is hispid, due to acanthostyles protruding from the thin dermal membrane. The consistency is flaky. The colour is white, both in alcohol and in dry specimens.
The skeleton consists of a basal layer of spongin from which the spicules arise, perpendicular to the substrate. The styles protrude through the sponge surface making it hispid.
Spicules. Large styles, slightly curved, with microspined heads and rare spines long the shaft, 490 (1044) 1555.5 × 20 (26.2) 35 µm.
Auxiliary styles straight or slightly sinuous with microspined heads, 320 (425) 550 × 5 (7.8) 10 µm.
Acanthostyles straight or slightly curved, characterized by acuminate and blunt spines mainly concentrated on the head. The spines on the head are bent towards the acanthostyles tip while the spines of the shaft bend towards the head. They measured 130 (204.5) 335 × 12.5 (17.7) 22.5 µm.
Distribution and ecology
The species has been reported from: Azores Islands, between 550 and 1740 m depth (Topsent, Reference Topsent1904, Reference Topsent1928); Strait of Gibraltar, between 450 and 580 m; Alboran Sea, between 924 and 1378 m (Topsent, Reference Topsent1894; Vacelet, Reference Vacelet1969; Boury-Esnault et al., Reference Boury-Esnault, Pansini and Uriz1994); Tyrrhenian Sea, associated with white coral (Taviani et al., Reference Taviani, Angeletti, Canese, Cannas, Cardone, Cau, Cau, Follesa, Marchese, Montagna and Tessarolo2017). This is the second record for the white coral assemblage and for the Italian coasts. Its bathymetric range is extended upwards until 256 m depth.
Order BIEMNIDA Morrow, 2013
Family RHABDEREMIIDAE Topsent, Reference Topsent1928
Genus Rhabderemia Topsent, 1890
Rhabderemia profunda Boury-Esnault, Pansini & Uriz, Reference Boury-Esnault, Pansini and Uriz1994
(Table 1)
Rhabderemia profunda Boury-Esnault, Pansini & Uriz, Reference Boury-Esnault, Pansini and Uriz1994
Material examined
Sard 15a (48); dive 26; 02 September 2013; Nora Canyon; 38°42,49′N 8°54,55′E; 357–408 m; on Madrepora oculata and Lophelia pertusa reef.
Thin and very small incrustation on dead M. oculata. The spicule size is similar to that reported by van Soest & Hooper (Reference Van Soest and Hooper1993); however our specimens showed also unusually short microstyles: 30 (96.5) 147.5 × 1 (1.8) 2.5 µm.
Distribution and ecology
The species was described from the Atlantic coast of Morocco at 1367 m depth (Boury-Esnault et al., Reference Boury-Esnault, Pansini and Uriz1994) and from the Azores (Topsent, Reference Topsent1904, Reference Topsent1928 as R. minutula) between 1331 and 1360 m depth. The species is new for the white coral assemblage and also for the Mediterranean Sea since all the Rhabderemia records of the basin (generally regarded as belonging to R. minutula) are to be referred to different Rhabderemia species (van Soest & Hooper, Reference Van Soest and Hooper1993). The bathymetric range of R. profunda is extended up to 408 m depth.
Order CLIONAIDA Morrow & Cárdenas, 2015
Family CLIONAIDAE d'Orbigny, 1851
Genus Spiroxya Topsent, 1896
Spiroxya pruvoti (Topsent, Reference Topsent1900)
(Figure 5; Table 1)
Cliona pruvoti Topsent, Reference Topsent1900

Fig. 5. Spiroxya pruvoti (Topsent, Reference Topsent1900): (A) large and small oxeas with scattered spirasters; (B–C) spirasters.
Material examined
Sard 10 (b5-p1, b6-p1, b20-p3, b21-p1, b35-p2); dive 17; 29 August 2013; Capo Coda Cavallo Canyon; 40°54,75′N 9°54,9′E; 256–264 m; on Madrepora oculata reef.
Soft, white sponge boring the scleraxis of M. oculata. Oxeas are smooth and slightly curved in two size categories. The larger oxeas measured 200 (285) 330 × 6.25 (8) 11.25 µm, while the smaller ones 100 (117.8) 150 × 2.5 µm. Spirasters measured 7.5 (8) 10 × 2.5 µm.
Distribution and ecology
It was recorded from Banyuls (southern France) boring into scleractinians at 500–600 m depth (Topsent, Reference Topsent1900). It was dredged in the North Atlantic Ocean off Ireland at 706–851 m depth (Stephens, Reference Stephens1915) and in the Adriatic Sea at ~500 m depth (Volz, Reference Volz1939). Our records extend upwards the depth range of the species. The species is new for the white coral assemblage and this record is the second for the Mediterranean Sea.
Order POECILOSCLERIDA Topsent, Reference Topsent1928
Family COELOSPHAERIDAE Dendy, 1922
Genus Forcepia Carter, 1874
Subgenus Forcepia (Leptolabis) Topsent, 1901
Forcepia (Leptolabis) megachela (Maldonado, 1992)
(Figure 6; Table 1)
Leptolabis megachela Maldonado, 1992

Fig. 6. Forcepia (Leptolabis) megachela (Maldonado, 1992): (A) specimen encrusting on branches of M. oculata; (B) tylotes, with magnification of the end; (C) acanthostyles; (D) acanthose symmetric forceps and details; (E) anchorate isochelae; (F) arcuate isochelae; (G) sigmas.
Material examined
Sard 15a (18); dive 26; 02 September 2013; Nora Canyon; 38°42,49′N 8°54,55′E; 357–408 m; on Madrepora oculata and Lophelia pertusa reef.
DIAGNOSIS
Thin, small (~1 cm2), white encrusting sponge on a dead branch of M. oculata.
Skeleton. Basal acanthostyles erect on the substrate in a hymedesmioid arrangement. Other spicule types were not detectable from the skeleton preparations.
Spicules. Tylotes straight or faintly curved, with slightly different extremities, 270 (287.4) 310 × 5 µm. Acanthostyles straight, conical, without swollen heads measuring 105 (176) 300 × 12.5 (12.7) 13 µm. Spines evenly distributed, slightly stouter on the head. Acanthose symmetric forceps with outwards median flexion of the legs ending in small, button-like swellings with toothed margin. They measured 30 (60.5) 57.5 × 2 µm and the distance between the legs at their extremity was 7.5 (12) 17.5 µm. Two types of chelae: (I) anchorate isochelae, with three teeth, 32.5 (69) 85 × 2.5 (6.6) 7.5 µm; (II) arcuate isochelae 17.5 (20) 22.5 µm. Sigmas ‘C’ shaped or more rarely ‘S’ shaped, 25 (58.6) 80 × 2.5 (4.4) 7.5 µm.
Distribution and ecology
The species, described from Alboran Island at 70–120 m depth, on colonies of Corallium rubrum (Linnaeus, 1758) (Maldonado, 1992), is here recorded for the second time in the Mediterranean basin at 408 m depth.
Family HYMEDESMIIDAE Topsent, Reference Topsent1928
Genus Hymedesmia Bowerbank, 1864
Subgenus Hymedesmia (Hymedesmia) Bowerbank, 1864
Hymedesmia (Hymedesmia) mutabilis (Topsent, Reference Topsent1904)
(Figures 7 & 8; Table 1)
Hymeraphia mutabilis Topsent, Reference Topsent1904

Fig. 7. Hymedesmia (Hymedesmia) mutabilis (Topsent, Reference Topsent1904): (A) specimen encrusting on M. oculata; (B) skeleton.

Fig. 8. Hymedesmia (Hymedesmia) mutabilis (Topsent, Reference Topsent1904): (A) large acanthostyles; (B) small acanthostyles; (C) anisotylotes; (D) anchorate chelae; (E) sigmas.
Material examined
Sard 10b (29, 31); dive 17; 29 August 2013; Capo Coda Cavallo Canyon; 40°54,75′N 9°54,9′E; 256–264 m; on Madrepora oculata reef.
SARD 15a (28, 32) and SARD 15 g (7); dive 26; 02 September 2013; Nora Canyon; 38°42,49′N 8°54,55′E; 357–408 m; on Madrepora oculata and Lophelia pertusa reef.
DIAGNOSIS
All the specimens were white greyish in colour, thinly encrusting on M. oculate branches, about 0.5 mm thick. The surface was hispid, due to the acanthostyles protruding through the thin dermal membrane. Consistency was soft.
Skeleton. In the ectosomal skeleton single tylotornotes were tangentially arranged together with scattered microscleres, without forming a discrete spicule layer.
The choanosomal skeleton was hymedesmioid, with the two types of acanthostyles erect on the substrate, with heads embedded in a thin layer of spongin. Microscleres were scattered also in the choanosome.
Spicules. Acanthostyles in two size categories; the larger ones were straight or slightly curved, with spines concentrated on the head and less numerous along the shaft. The spines were relatively long and acuminate. They measured 275 (380.2) 500 × 30 (31.8) 35 µm. The smaller acanthostyles were similar in shape to the larger ones, but more uniformly spined. They measured 90 (105) 115 × 10 (12.5) 15 µm. Anisotylotes straight or slightly curved with acuminate or rounded extremities. They measured 187.5 (226.7) 270 × 2.5 µm. Anchorate chelae with three spaced teeth at each extremity, measuring 20 (42) 55 × 5 (7) 10 µm. Sigmas ‘C’ shaped or twisted, 10 (16.7) 27.5 µm in length.
Shape, colour and habit of the 15 specimens of H. mutabilis reported in the literature are very uniform. On the other hand the size of megascleres is variable also among sympatric specimens and this justifies the specific name mutabilis assigned by Topsent (Reference Topsent1904). Based on the occurrence of irregularities in the form of spines or undulations on the shaft of the arcuate isochelae in two specimens from the Azores (st. 3150 and 3293), Topsent (Reference Topsent1928) erected the variety costata. Vacelet (Reference Vacelet1969) reported slight undulations on the isochelae shaft just in a single specimen (st. 24) out of the four that he recorded from the Gulf of Lion. Neither Stephens' (Reference Stephens1921) nor our material showed such irregularities. We think that this character could be included within the range of spicule variability of the species and we propose to drop the variety costata (Topsent, Reference Topsent1928).
Distribution and ecology
Azores at 200–1360 m depth (Topsent, Reference Topsent1904), Ireland (Atlantic Ocean) at 450–1300 m depth on white corals (both Lophelia and Madrepora) (Stephens, Reference Stephens1921; van Soest et al., Reference Van Soest, Cleary, de Kluijver, Lavaleye, Maier and van Duy2007); Gulf of Lions and Cape Santa Maria di Leuca, offshore coral bank (Ionian Sea), Nora Canyon (Sardinia) (Mediterranean Sea) at 235–809 m depth (Vacelet, Reference Vacelet1969; Longo et al., Reference Longo, Mastrototaro and Corriero2005; Mastrototaro et al., Reference Mastrototaro, D'onghia, Corriero, Matarrese, Maiorano, Panetta, Gherardi, Longo, Rosso, Sciuto, Sanfilippo, Gravili, Boero, Taviani and Tursi2010; Taviani et al., Reference Taviani, Angeletti, Canese, Cannas, Cardone, Cau, Cau, Follesa, Marchese, Montagna and Tessarolo2017).
Genus Plocamionida Topsent, 1927
Plocamionida tylotata Brøndsted, Reference Brøndsted, Jensen, Lundbeck and Ragnar Sparck1932
(Figure 2D; Table 1)
Plocamionida tylotata Brøndsted, Reference Brøndsted, Jensen, Lundbeck and Ragnar Sparck1932
Plocamionida ambigua f. tylotata Brøndsted, Reference Brøndsted, Jensen, Lundbeck and Ragnar Sparck1932
Material examined
Sard 15a (4); dive 26; 02 September 2013; Nora Canyon; 38°42,49′N 8°54,55′E; 357–408 m; on Madrepora oculata and Lophelia pertusa reef.
DIAGNOSIS
Small, encrusting sponge (about 2 cm in diameter) on a dead branch of M. oculata. The surface was hispid and the colour grey.
Skeleton. The choanosomal skeleton consisted of a basal layer of acanthostrongyles of two dimensional categories of acanthostyles arranged perpendicular to the substratum. The large acanthostyles protruded through the sponge surface. The ectosomal skeleton had a layer of anisosubtylotes arranged more or less in bouquets with scattered isochelae.
Spicules. Acanthostyles in two categories. Acanthostyles I, slightly curved with spines concentrated on the head, 205 (527.6) 1045.5 × 12.5 (17) 25 µm; acanthostyles II, entirely spined, with a characteristic constriction under the head, 90 (115.6) 125 × 7.5 (8.4) 10 µm. Acanthostrongyles more or less curved, entirely spined, 75 (79.5) 90 × 7.5 (10.6) 12.5 µm. Anisosubtylotes, straight or slightly sinuous, with more or less discrete ends, 147.5 (188.4) 210 × 2.5 µm.Arcuate isochelae, 22.5 (25.3) 27.5 × 2.5 µm.
Distribution and ecology
This species has been recorded only from the Faroe Islands at 160 m depth (Brøndsted, Reference Brøndsted, Jensen, Lundbeck and Ragnar Sparck1932). In the Mediterranean Sea it was described once as Antho sp. by Longo et al. (Reference Longo, Mastrototaro and Corriero2005). Its depth range is extended to 408 m.
Order TETRACTINELLIDA Marshall, 1876
Suborder ASTROPHORINA Sollas, 1887
Family THENEIDAE Carter, 1883
Genus Annulastrella Maldonado, 2002
Annulastrella verrucolosa (Pulitzer-Finali, Reference Pulitzer-Finali1983)
(Figure 9; Table 1)
Sphinctrella verrucolosa Pulitzer-Finali, Reference Pulitzer-Finali1983

Fig. 9. Annulastrella verrucolosa (Pulitzer-Finali, Reference Pulitzer-Finali1983): (A) specimen; (B) small oxeas; (C) large oxeas; (D) microxeas, detail of the microspines; (E) large plesiasters; (F) small plesiasters; (G) spirasters.
Material examined
Sard 10b (11); dive 17; 29 August 2013; Capo Coda Cavallo Canyon; 40°54,75′N 9°54,9′E; 256–264 m; on Madrepora oculata reef.
SARD 15a (10) and SARD 15 g (6); dive 26; 02 September 2013; Nora Canyon; 38°42,49′N 8°54,55′E; 357–408 m; on Madrepora oculata and Lophelia pertusa reef.
DIAGNOSIS
Small sponge, 1.5 cm high and 0.5 cm wide, with an ovoid body and a single, apical, funnel-like, fringed oscule. Consistency compressible. Surface hispid, due to the oxeas protruding outward. The colour of alcohol-preserved specimens was white.
Spicules. Megascleres were oxeas in two size categories. The larger ones were smooth, slightly curved, sometimes modified into styles, 790.5 (1799.16) 2805 × 12.5 (32.5) 67.5 µm; the smaller ones were thin and slightly curved, also modified into styles, 1310 (1864.8) 3090 × 5 (6.5) 7.5 µm. Microscleres were plesiasters in two size categories, small microspined oxeas and spirasters. The larger plesiasters, with 2–6 rays covered by spirally arranged microspines, measured 25 (88.7) 195 × 7.5 (12) 15 µm; the smaller plesiasters with 6–7 spined rays, measured 35 (48) 65 × 2.5 (3.43) 5 µm. The microxeas with spirally arranged microspines measured 120 (191.4) 320 × 10 (11) 15 µm. Spirasters with relatively thin axis and 11–12 actines, 12.5 (18.68) 22.5 µm. The rays are 5 (8.47) 12.5 µm long.
Apart from the present species, the other three are attributed to the genus Annulastrella: A. schmidti Maldonado, 2002 from the Gulf of Mexico, A. annulata (Carter, 1880) from the Gulf of Manaar, and A. ornata (Sollas, 1888) from the North Atlantic and Cape Verde Island. A. verrucolosa is here recorded for the second time and is new for the white coral assemblage and the Italian seas.
Distribution and ecology
The holotype was dredged off Calvi (Corse) between 123 and 147 m depth from a detrital bottom (Pulitzer-Finali, Reference Pulitzer-Finali1983). Specimens from Sardinia, all associated to M. oculata, extend the depth range of the species to 408 m.
Discussion
This study provides additional evidence supporting the dominant role of sponges, in terms of diversity and abundance, within the associate fauna of CWC frameworks in the bathyal Mediterranean Sea (D'Onghia et al., Reference D'Onghia, Capezzuto, Cardone, Carluzzi, Carluccio, Chimienti, Corriero, Longo, Maiorano, Mastrototaro, Panetta, Rosso, Sanfilippo, Sion and Tursi2015; Rueda et al., Reference Rueda, Urra, Aguilar, Angeletti, Bo, García-Ruiz, González-Duarte, López, Madurell, Maldonado, Mateo-Ramírez, Megina, Moreira, Moya, Ramalho, Rosso, Sitjá, Taviani, Orejas and Jiménez2018). While the biodiversity of these sponges has been explored for the Ionian and Adriatic seas, virtually no data are available for the Tyrrhenian Sea due to a substantial lack of records of living white coral reefs, for a long time reported only along the northern Sicilian arc (Freiwald et al., Reference Freiwald, Beuck, Rüggeberg, Taviani and Hebbeln2009, Reference Freiwald, Boetius and Bohrmann2011).
Living frameworks dominated by Madrepora oculata and Lophelia pertusa were recently found along the Sardinian margin in the area of Nora Canyon (Taviani et al., Reference Taviani, Angeletti, Canese, Cannas, Cardone, Cau, Cau, Follesa, Marchese, Montagna and Tessarolo2017). In the madreporian framework of this area we have recorded 28 species (31 considering the conspicuous species and a total of 36 considering also those found by Taviani et al., Reference Taviani, Angeletti, Canese, Cannas, Cardone, Cau, Cau, Follesa, Marchese, Montagna and Tessarolo2017) (Table 2). One of these species is new for the Mediterranean Sea and four are new for the Italian fauna, suggesting that this habitat withholds many potential discoveries. In addition, nine species were recorded for the first time associated to CWC reefs supporting the attractiveness of this ecosystem for bathyal sponges. Overall, at a Mediterranean level, the current species richness associated to this habitat (Rueda et al., Reference Rueda, Urra, Aguilar, Angeletti, Bo, García-Ruiz, González-Duarte, López, Madurell, Maldonado, Mateo-Ramírez, Megina, Moreira, Moya, Ramalho, Rosso, Sitjá, Taviani, Orejas and Jiménez2018) reaches 90 sponge taxa. The studied sponge assemblage includes 24 species (80% of the total) with an Atlantic boreal distribution confirming the general biogeographic affinity already stated for the Porifera living in this environment (Rueda et al., Reference Rueda, Urra, Aguilar, Angeletti, Bo, García-Ruiz, González-Duarte, López, Madurell, Maldonado, Mateo-Ramírez, Megina, Moreira, Moya, Ramalho, Rosso, Sitjá, Taviani, Orejas and Jiménez2018).
Table 2. Cold-water coral-associated sponge fauna of the Mediterranean Sea with indication of species habit and occurrence in the shallow-water coralligenous ecosystem

Code legend for habit: Ms, massive; Ec, encrusting; Br, boring; CD, cavity dwelling.
In terms of taxonomic composition the present analysis confirms Poecilosclerida as the dominant group associated with white corals, followed by Tetractinellida (Vacelet, Reference Vacelet1969; Longo et al., Reference Longo, Mastrototaro and Corriero2005; Calcinai et al., Reference Calcinai, Moratti, Martinelli, Bavestrello and Taviani2013; D'Onghia et al., Reference D'Onghia, Capezzuto, Cardone, Carluzzi, Carluccio, Chimienti, Corriero, Longo, Maiorano, Mastrototaro, Panetta, Rosso, Sanfilippo, Sion and Tursi2015). In terms of growing habitus, the majority of the species strictly associated with the coral framework are encrusting, with occasionally massive, fan-like species such as Pachastrella monilifera, Poecillastra compressa and the pedunculate-erect Phakelia robusta. Also the diversity of boring sponges is low; in Sardinia the most representative species are Spiroxya pruvoti and Siphonodictyon infestum. S. infestum, very common in the CWC reef off Bari (D'Onghia et al., Reference D'Onghia, Capezzuto, Cardone, Carluzzi, Carluccio, Chimienti, Corriero, Longo, Maiorano, Mastrototaro, Panetta, Rosso, Sanfilippo, Sion and Tursi2015), was never reported in other Mediterranean deep reefs.
In the Sardinian samples, not only the species richness but also the number of specimens (112) is relevant in relation to the volume of the analysed coral framework. The most abundant species is Hamacantha (Vomerula) falcula (12% of the recorded specimens), followed by Haliclona (Flagellia) cf. hiberniae, Acantheurypon pilosella and Siphonidium ramosum. As already highlighted by previous authors (Longo et al., Reference Longo, Mastrototaro and Corriero2005; Mastrototaro et al., Reference Mastrototaro, D'onghia, Corriero, Matarrese, Maiorano, Panetta, Gherardi, Longo, Rosso, Sciuto, Sanfilippo, Gravili, Boero, Taviani and Tursi2010; Taviani et al., Reference Taviani, Angeletti, Canese, Cannas, Cardone, Cau, Cau, Follesa, Marchese, Montagna and Tessarolo2017), these species have always been found associated with the dead coral matrix.
Even if the sampling effort has to be considered as a potential bias, some preliminary considerations can be made regarding the connectivity among the sponge assemblages associated with the Mediterranean CWC reefs studied so far, namely the Tyrrhenian banks (present study and Taviani et al., Reference Taviani, Angeletti, Canese, Cannas, Cardone, Cau, Cau, Follesa, Marchese, Montagna and Tessarolo2017), the Maltese reef (Calcinai et al., Reference Calcinai, Moratti, Martinelli, Bavestrello and Taviani2013), and the Apulian ones from Santa Maria di Leuca (Longo et al., Reference Longo, Mastrototaro and Corriero2005; Mastrototaro et al., Reference Mastrototaro, D'onghia, Corriero, Matarrese, Maiorano, Panetta, Gherardi, Longo, Rosso, Sciuto, Sanfilippo, Gravili, Boero, Taviani and Tursi2010), and Bari Canyon (D'Onghia et al., Reference D'Onghia, Capezzuto, Cardone, Carluzzi, Carluccio, Chimienti, Corriero, Longo, Maiorano, Mastrototaro, Panetta, Rosso, Sanfilippo, Sion and Tursi2015) (Table 2).
In terms of species richness, similar values are recorded comparing Sardinia (35 species) with Santa Maria di Leuca (37 species) and Bari Canyon (29 species), while the Maltese community shows the lowest biodiversity (13 species). Only one species, Desmacella inornata, is present in all the assemblages and three species (Hamacantha (Hamacantha) johnsoni, Pachastrella monilifera and Poecillastra compressa) are recorded in three out of four sites. The percentage of exclusive species (found only in one site) is 81% supporting an overall low similarity among the sponge assemblage occurring in geographically separated coral banks. When considering the four assemblages separately, the Bari Canyon shares about 38% of the species with at least one of the other assemblages, Santa Maria di Leuca 35%, Malta 54% and Sardinian canyons 34% (Figure 10A). It is relevant that the Maltese sponge fauna shows the highest percentage of shared species with the Apulian and Sardinian assemblages. Its geographic position, indeed, within the Strait of Sicily, supports the role of this biogeographic area as a crossroad for faunas of the two basins (Bianchi & Morri, Reference Bianchi and Morri2000; Pinardi & Masetti, Reference Pinardi and Masetti2000; Bianchi, Reference Bianchi2007).

Fig. 10. Sponge assemblages from the studied cold-water coral sites: (A) percentage composition of the four assemblages divided among exclusive (black) and shared (white); (B) percentage of species shared with the coralligenous environment. SML: Santa Maria di Leuca.
Additional considerations can be made from a bathymetric point of view. In fact, although the co-occurrence of sponge species in deep CWC reefs and inside the shallow coralligenous community has already been pointed out (Bertolino et al., Reference Bertolino, Cerrano, Bavestrello, Carella, Pansini and Calcinai2013), no formal comparison between these two assemblages has ever been conducted. A comparison of the present dataset with the lists of sponge species associated with coralligenous concretion of Bertolino et al. (Reference Bertolino, Cerrano, Bavestrello, Carella, Pansini and Calcinai2013) and Longo et al. (Reference Longo, Cardone, Pierri, Mercurio, Mucciolo, Nonnis Marzano and Corriero2017) shows that these two communities share 48 sponge species (55% of the sponges associated with CWC frameworks). A geographic variability exists in terms of percentage of shared species between the two bioconstructions, from 54% for the Strait of Sicily to 69% for the Bari Canyon (Figure 10B), plausibly suggesting different levels of bathymetric connectivity.
Moreover, the percentage of deep species living in the CWC reefs and found also in shallow-water coralligenous assemblages varies according to their habitus: 71% and 77% respectively of boring and cavity-dwelling species occupy the same habitat in the coralligenous cavities, followed by 54% of encrusting species and 37% of massive ones. These eurybathic species exploit the structural analogy between the deep coral framework and the coralligenous matrix, the latter also characterized by an intricate net of crevices, and potentially representing a dark, deep-like refuge in shallow waters. The ecological plasticity of sponges greatly enhances their ability to extend their distribution depth range variously adapting to different environments. Some species keep their cavity-dwelling habitus in both the habitats (for example Dercitus (Stoeba) plicatus, Jaspis incrustans, Calthropella (Calthropella) pathologica and Erylus discophorus), while others, such as Pachastrella monilifera, completely modify their erect habitus becoming cavity dwelling only when penetrating into the coralligenous cavities (Bertolino et al., Reference Bertolino, Cerrano, Bavestrello, Carella, Pansini and Calcinai2013).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed by the authors.














