Introduction
Estuaries and coastal lagoons generally provide ideal conditions for the development of many marine organisms. They are extensively used as nursery grounds by different fish and crustacean species, and also provide a vast source for feeding and shelter against predators (Boesch & Turner, Reference Boesch and Turner1984; Rozas & Minello, Reference Rozas and Minello1997). In the south and south-east regions of Brazil, the planktonic post-larvae of the pink shrimp Farfantepenaeus paulensis (Pérez Farfante 1967) enter these habitats carried by ocean water and develop as juveniles, following a pattern observed in other species of Farfantepenaeus. The cycle is completed when pre-adults return to the ocean, recruit to adult stock and reproduce (Garcia & Le Reste, Reference Garcia and Le Reste1981; D'Incao, Reference D'Incao1991; Lhomme, Reference Lhomme1992; Cházaro-Olvera et al., Reference Cházaro-Olvera, Winfield and Coria-Olvera2009).
The pink shrimp represents an important link in the local food web. It is well known, for instance, that they predate intensively on benthic invertebrates while also constituting the main food source for the blue heron Egretta caerulea (Linnaeus 1758) (Jorgensen et al., Reference Jorgensen, Bemvenuti and Hereu2009; Gianuca et al., Reference Gianuca, Gianuca and Vooren2012). Moreover, this species is largely targeted by artisanal fisheries. This is particularly evident in the Lagoa dos Patos, which is considered its largest nursery, and where it has become the main fishing resource since other fisheries started collapsing in the early 1980s (D'Incao et al., Reference D'Incao, Valentini and Rodrigues2002). The specimens enter the estuary as post-larvae, theoretically from September and harvest occurs in the summer and autumn (December–May) (D'Incao, Reference D'Incao1991). Pink shrimp harvests range from zero to more than 4000 tons with varying environmental conditions such as intensity and direction of the wind and the freshwater discharge from the lagoon system (Möller et al., Reference Möller, Castelo and Vaz2009; Pereira & D'Incao, Reference Pereira and D'Incao2012; Kalikoski & Vasconcellos, Reference Kalikoski and Vasconcellos2013).
In estuaries in the Indo-Pacific and the Gulf of Mexico, the highest abundance of post-larvae and juvenile penaeids is associated with higher salinity, increased temperature, the presence of submerged vegetation and debris-rich substrates (Sánchez, Reference Sánchez1997; Vance et al., Reference Vance, Haywood, Heales, Kenyon and Loneragan1998; Pérez-Castañeda & Defeo, Reference Pérez-Castañeda and Defeo2001, Reference Pérez-Castañeda and Defeo2004; Adnan et al., Reference Adnan, Loneragan and Connolly2002; Pérez-Castañeda et al., Reference Pérez-Castañeda, Blanco-Martínez, Sánchez-Martinez, Rábago-Castro, Aguirre-Guzmán and Vásquez-Sauceda2010; Noleto-Filho et al., Reference Noleto-Filho, Pucciarelli and Dumont2017). Higher salinities allow the Melicertus plebejus (Hess 1865) and Fenneropenaeus merguiensis (De Man 1888) post-larvae to reach the innermost areas of the estuaries (Young & Carpenter, Reference Young and Carpenter1977; Vance et al., Reference Vance, Haywood, Heales, Kenyon and Loneragan1998). Farfantepenaeus paulensis and Farfantepenaeus aztecus (Ives 1891) showed higher survival rates at salinities above 10, and increased mortality in scenarios with large saline variability, due to greater difficulty in osmoregulation (Tsuzuki et al., Reference Tsuzuki, Cavalli and Bianchini2000; Saoud & Davis, Reference Saoud and Davis2003). Additionally, shrimps find better survival conditions at temperatures above 25°C. This increased abundance due to higher temperatures causes seasonal growth in estuaries (D'Incao, Reference D'Incao1991; Branco & Verani, Reference Branco and Verani1998; Tsuzuki et al., Reference Tsuzuki, Cavalli and Bianchini2000; Pérez-Castañeda & Defeo, Reference Pérez-Castañeda and Defeo2001).
However, in the Brazilian estuaries, there is little information on the preference of the post-larvae of pink shrimp. Previous studies mainly analysed juvenile populations and concluded that their abundance varies with season and that they prefer salinity levels between 15 and 30 (D'Incao, Reference D'Incao1991; Branco & Verani, Reference Branco and Verani1998; Costa et al., Reference Costa, Lopes, Castilho, Fransozo and Simões2008; Lüchmann et al., Reference Lüchmann, Freire, Ferreira, Daura-Jorge and Marques2008; Ferreira & Freire, Reference Ferreira and Freire2009; Noleto-Filho et al., Reference Noleto-Filho, Pucciarelli and Dumont2017). D'Incao (Reference D'Incao1991) first studied the distribution of juveniles of pink shrimp in the Lagoa dos Patos estuary and highlighted the importance of protected shallow inlets for their growth, even without a quantitative analysis. Ruas et al. (Reference Ruas, Rodrigues, Dumont and D'Incao2014) conducted the only available study on the habitat preference of post-larvae pink shrimp in Brazil; they showed the importance of submerged seagrass meadows. The reach of post-larvae to the innermost regions of estuaries has not been studied, and the salinity influence is mainly associated with fishery production (D'Incao, Reference D'Incao1991; Costa et al., Reference Costa, Lopes, Castilho, Fransozo and Simões2008; Möller et al., Reference Möller, Castelo and Vaz2009; Pereira & D'Incao, Reference Pereira and D'Incao2012).
An analysis of habitat preference could help develop management and conservation measures for pink shrimp as a resource. However, little information is available on the variability of post-larvae and juvenile abundances, which could help identify the patterns of distribution and occupation in estuaries. This study investigated the distribution and spatial-temporal variability in the relative abundance of post-larvae and juveniles of F. paulensis in the Lagoa dos Patos estuary. We analysed the effect of salinity and temperature on the abundance of these organisms.
Materials and methods
Study area
Lagoa dos Patos is the largest choked lagoon in the world (Kjerfve, Reference Kjerfve and Wolfe1986). Its estuarine portion (32°00′S 52°04′W) of 971 km2 lies to the south of the lagoon. A channel that measures 20 km in length and 0.5–3 km in width, allows ocean–estuary water exchanges (Asmus, Reference Asmus, Seeliger, Odebrecht and Castelo1998). The strength and direction of winds, and freshwater discharge control the hydrodynamics of the lagoon (Fernandes et al., Reference Fernandes, Dyer and Moller2005; Möller et al., Reference Möller, Castelo and Vaz2009). The main environments of this estuary are the protected shallow inlets (here termed P), with submerged prairies of phanerogams, small variability in salinity and current velocity, and an unprotected central water body (here termed U) with greater depth and higher variability of salinity and flow velocity (Asmus, Reference Asmus, Seeliger, Odebrecht and Castelo1998; Fernandes et al., Reference Fernandes, Monteiro and Moller2007; Martins et al., Reference Martins, Dias, Fernandes and Muelbert2007; Copertino & Seeliger, Reference Copertino, Seeliger, Seeliger and Odebrecht2010; D'Incao & Dumont, Reference D'Incao, Dumont, Seeliger and Odebrecht2010).
Field sampling and laboratory procedures
Samplings occurred monthly from September 2010 to January 2013, at eight sampling sites in the protected shallow inlets (P1–P8) and four sites on the margins of the unprotected central area (U1–U4) of the estuary (Table 1), during the day and at depths less than 1 m in all areas. Due to previous knowledge of species occurrence in the estuary, a one-year period was considered as starting in September and ending in August.
Table 1. Geographic coordinates of the sampling sites at Patos Lagoon Estuary, Brazil. Protected areas P1 to P8, and unprotected areas U1 to U4

The samples were collected with a trawl net according to Renfro (Reference Renfro1963). The net had a mesh size of 5 mm knot to knot, a codend of 500 µm, and an non-variable opening of 1.8 m. Trawling extended 40 m, with two hauls at each collection site. To minimize the effect of the boat, the engine was switched off close to trawling locations. The boat was moved by rowing, where the fishing net was then placed in the water and the cable stretched to reach the 40 metre mark. After that, the boat was anchored to prevent variability in the trawling distance, and the trawl net was drawn manually. The salinity level and temperature were checked during each trawl through a mini portable probe YSI 556 MPS model. The collected material was stored in labelled plastic bags, containing 4% formaldehyde in fresh water.
In the laboratory, the sampled material was washed under running water in a sieve of 500 µm and shrimp were separated and identified according to D'Incao (Reference D'Incao, Buckup and Bond-Buckup1999). The post-larvae pink shrimp (PL) were identified according to Calazans (Reference Calazans1993). In this study, shrimp were classified as post-larvae at up to 3 mm in carapace length (CL) (Haywood et al., Reference Haywood, Vance and Loneragan1995), and shrimps above this size were classified as juveniles (JU). The relative abundance was determined for PL and JU by counting the number of organisms from each trawl.
The CL measurements of the PL and JU pink shrimp were obtained in millimetres from the orbital angle to the dorsal edge of the carapace, with a stereomicroscope equipped with an ocular micrometer and a caliper (0.1 mm).
Data analysis
Descriptive analysis (means) of the data collected at all points was performed. The calculated means have a confidence interval of 95%.The size structure of the shrimps was analysed using frequency distribution by size class (CL), grouped into 1 mm intervals.
Generalized Linear Models (GLMs) (Nelder & Wedderburn, Reference Nelder and Wedderburn1972) were used to evaluate the effect of environmental, spatial and temporal predictors on PL and JU abundances for sites P1–P5 and U1–U4. GLMs extend the classic framework of linear models in the sense that the response variable can be any member of the exponential probability distribution family (McCullagh & Nelder, Reference McCullagh and Nelder1989). Thus, they fit well for modelling ecological data as they are not always restricted to a Gaussian distribution (Guisan et al., Reference Guisan, Edwards and Hastie2002).
GLMs describe the relationship between the response variable Y i (i = 1,…,n) and the predictors x i through a linear predictor η = ![]() $\sum\nolimits_{j = 1}^k {x_j{\rm \beta} _j} $, where x j is a known function for k, the predictor variable, and βj is an unknown parameter to be estimated from the data. The linear predictor η is linked to the mean of the response E(Y) = μ by a known link function g, which is commonly expressed as η = g(μ). In cases where the response variable consists of discrete events, such as abundance of PL and JU, a Poisson distribution would be appropriate. However, this kind of distribution requires that the mean (μ) is equal to the variance (σ 2), which does not necessarily correspond to biological reality. In most cases, however, the variance is usually greater than the mean (also known as overdispersion), and may be caused by the spatio-temporal heterogeneity present in the data (Lindén & Mäntyniemi, Reference Lindén and Mäntyniemi2011).
$\sum\nolimits_{j = 1}^k {x_j{\rm \beta} _j} $, where x j is a known function for k, the predictor variable, and βj is an unknown parameter to be estimated from the data. The linear predictor η is linked to the mean of the response E(Y) = μ by a known link function g, which is commonly expressed as η = g(μ). In cases where the response variable consists of discrete events, such as abundance of PL and JU, a Poisson distribution would be appropriate. However, this kind of distribution requires that the mean (μ) is equal to the variance (σ 2), which does not necessarily correspond to biological reality. In most cases, however, the variance is usually greater than the mean (also known as overdispersion), and may be caused by the spatio-temporal heterogeneity present in the data (Lindén & Mäntyniemi, Reference Lindén and Mäntyniemi2011).
Overdispersion can be handled in many different ways. Describing, for instance, the extra Poisson variance as a quadratic function of the mean is one of the most commonly used approaches which, in turn, defines a Negative Binomial distribution (McCullagh & Nelder, Reference McCullagh and Nelder1989; Lindén & Mäntyniemi, Reference Lindén and Mäntyniemi2011). Therefore, as both response variables were overdispersed, PL and JU abundances were fitted according to a negative binomial distribution by means of the glm.nb function from the MASS R-package (Venables & Ripley, Reference Venables and Ripley2002). The general formulation for both models can be summarized as follows:
 $$\eqalign{Y_{i\;} \sim \; NB\; ({\rm \mu} _i,k);E(Y_i) = & \,\, {\rm \mu} _i\;{\rm and}\;{\rm var}(Y_i) = {\rm \mu} _i + \; \displaystyle{{{\rm \mu} _i^2} \over k} \cr {\rm \eta} _i = & \,\, {\rm \beta} _0 + \mathop \sum \limits_m^M {\rm \beta} _mX_{m_i} + {\rm \varepsilon} \; \cr {\rm \eta} _i = &\, \, {\rm log}({\rm \mu} _i)} $$
$$\eqalign{Y_{i\;} \sim \; NB\; ({\rm \mu} _i,k);E(Y_i) = & \,\, {\rm \mu} _i\;{\rm and}\;{\rm var}(Y_i) = {\rm \mu} _i + \; \displaystyle{{{\rm \mu} _i^2} \over k} \cr {\rm \eta} _i = & \,\, {\rm \beta} _0 + \mathop \sum \limits_m^M {\rm \beta} _mX_{m_i} + {\rm \varepsilon} \; \cr {\rm \eta} _i = &\, \, {\rm log}({\rm \mu} _i)} $$where Y i is the number of PL or JU individuals for each sampling location i; ηi is the linear predictor expressed in logarithmic scale; β0 is the intercept; βM is a vector of the repressor's coefficient which quantify the effect of some variable predictors X m on the response; and ε represents the error term.
Based on available data, we used a total of six potential predictors possibly related to the variability of PL and JU abundances. The potential predictors were salinity (ppt), temperature (°C), sampling sites (P1–P5 and U1–U4), months, seasons and year. Year was based on the shrimp post-larvae entrance and harvest period (from September to August: I – 2010/2011; II – 2011/2012; III – 2012/2013). An exploratory data analysis was first applied to the database to detect possible outliers and assess statistical relationships between the variables. As multicollinearity among predictor variables may increase the probability of Type I errors, pairplots and Pearson's correlation coefficient ρ were used to check specifically for multicollinearity between environmental predictors. Provided that no high collinearity (ρ < 0.7) was detected among these predictors, they could be simultaneously included in the tested models.
In the null model, predictor variables were evaluated using the forward stepwise selection procedure, which introduces all predictors one at a time progressively. Some models were tested considering only the interaction between particular predictors, as well as the quadratic term for the environmental predictors. At each model stage, the Akaike's Information Criterion (AIC) (Akaike, Reference Akaike, Petran and Csàaki1973) and the maximum likelihood pseudo R 2 (coefficient of determination) provided by the pscl R-package (Zeleis et al., Reference Zeleis, Kleiber and Jackman2008) were computed as indicative of ‘goodness-of-fit’.
The AIC accounts simultaneously for the number of parameters used in the model as well as the residual deviance; the smaller the value, the better the model (Burnham & Anderson, Reference Burnham and Anderson2002). Furthermore, models with larger R 2 are better, as they express the percentage of variability in the response variable that was explained by the model. Thus, AIC and R 2 are inversely related to the compromise between fit and parsimony. It is noteworthy that in cases when two or more nested models competed with each other (AIC values smaller than 5 units), we also used the Deviance hypothesis test to evaluate if the additional predictor improved the model fit (Venables & Dichmont, Reference Venables and Dichmont2004).
When significant factors (P < 0.05) were detected in the selected models for both response variables, Tukey's post-hoc test was performed using the glht function from the multcomp R-package (Hothorn et al., Reference Hothorn, Bretz and Westfall2008) in order to test differences between factor levels. Finally, the quality of these models was assessed through residual diagnostic plots. While residual's normality was evaluated by means of Quantile-Quantile plots, homogeneity was assessed through a residual vs predicted values plot. Also, residuals independence was checked with the autocorrelation function. Moreover, since linearity is expected between the observed and predicted values, Pearson's correlation coefficient (ρ) was calculated and used for model validation purposes.
Results
Environmental conditions
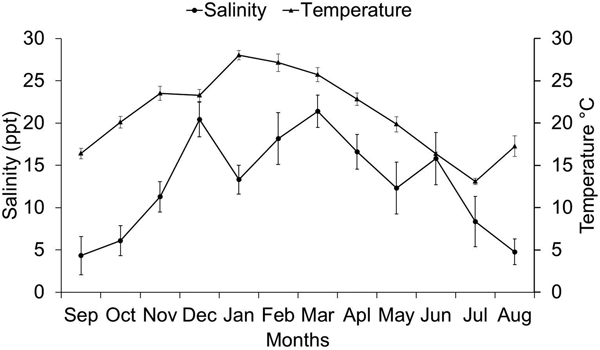
Salinity in the protected area of the estuary was similar at sites P1–P5 with means ranging from 10.43 ± 2.03 to 14.51 ± 2.33 (Figure 1). The lowest mean occurred in the northern part of the estuary (P6–P8), farthest from the estuary mouth that exchanges water with the ocean. Salinity in the unprotected area of the estuary decreased gradually toward the interior of the estuary, with the highest average salinity at U1 (17.99 ± 2.75) and the lowest at U4 (11.01 ± 2.56) (Figure 1). Average salinity increased from September (4.31 ± 2.25) to December, where average salinity was 20.42 (± 2.07). Salinity decreased slightly in January (13.30 ± 1.71), but increased on average afterwards to reach its highest value in March (21.39 ± 1.89). It followed a decreasing trend from April until the month of August (4.75 ± 1.52) (Figure 2).

Fig. 1. Mean salinity values (black dots) and relative abundance (bars) of post-larvae and juveniles of Farfantepenaeus paulensis at the sampling sites of the protected (P1–P8) and unprotected (U1–U4) areas throughout the study period, with their respective 95% confidence intervals. Abundance is expressed by the average number of individuals caught by haul instead of trawling.

Fig. 2. Mean salinity values during the months, with their respective 95% confidence intervals.
Temperature at sites U1–U4 ranged from 21.38 ± 1.09 to 22.72 ± 1.22. In the protected area, the lowest mean was 21.54 ± 1.04 at P1 and the highest was 23.53 ± 1.39 at P4. The lowest temperatures were recorded from June to September, with mean values occurring between 13.08 ± 0.36 and 16.40 ± 0.61. From October (20.09 ± 0.68) the temperature increased and in January (28.02 ± 0.57), February (27.13 ± 1.03) and March (25.71 ± 0.81) the highest values were recorded (Figure 2).
Overall captures
During the study period, a total of 9153 organisms were captured at all collection sites (2530 PL and 6623 JU). The lowest average abundance of PL and JU in the unprotected area occurred at site U1 (PL = 2.6 ± 1.66; JU = 0.84 ± 0.40), the site closest to the mouth of the estuary, and the highest averages were observed at U2 (PL = 11.45 ± 6.43; JU = 5.86 ± 2.48). In the protected area of the estuary, the lowest abundances of PL and JU occurred at P4 (PL = 0.98 ± 0.97, JU = 7.25 ± 2.81), whereas the highest abundances were observed at P5 (PL = 4.56 ± 5.28) and P1 (JU = 26.83 ± 17.76). If all sites were considered, P7 had the lowest average abundance (PL = 0.6 ± 0.82; JU = 1.85 ± 1.35) and P8 had the highest abundance of JU (32.35 ± 35.47). Additionally, highest abundance of JU and PL were registered in the protected and unprotected sites, respectively (Figure 1).
Size structure
Size structure analysis identified differences in the number of post-larvae and juveniles in each site. The largest juvenile measured 25.00 mm CL, however the major size classes were between 2.00 and 15.00 mm CL. Post-larvae were more common in sites U1–U4, with a unimodal trends frequency distribution, with peak occurrence in the 2.01–3.00 mm class interval, followed by a sharp drop in size classes above 3.00 mm (Figure 3). At sites P1–P8, the size distribution displayed a bimodal and multimodal trend. It can be seen in the protected area that mode can be identified in the size class over 3.01 mm (Figure 3).

Fig. 3. Frequency distribution of carapace length (CL), grouped at intervals of 1 mm, for Farfantepenaeus paulensis in the protected and unprotected sites of the Patos Lagoon estuary.
Model selection and estimates of explanatory variables
Several models have been tested with respect to different combinations of variable predictors for PL and JU relative abundances. Although some similarities were shared among the selected GLMs, they highlighted that the relative importance of each predictor was different for each response variable.
The most relevant models with respect to the PL abundance are summarized in Table 2. Even though models 6–10 had the best fit qualities, the Deviance hypothesis test did not detect significant differences between these models. In this sense, model 8 was selected since it showed the best fit and parsimony, as well as the most reliable biological explanation. Thus, salinity, sampling sites, year and months were important to explain the variability in the PL abundances.
Table 2. Models to explain the variation in the relative abundance of post-larvae pink shrimp, containing the Akaike's Information Criteria (AIC), residual deviance (RD), degrees of freedom (DF) and maximum likelihood pseudo R 2

T, temperature; S, salinity; Ss, sampling sites; Y, year; M, month; Se, season.
Selected model is highlighted in bold.
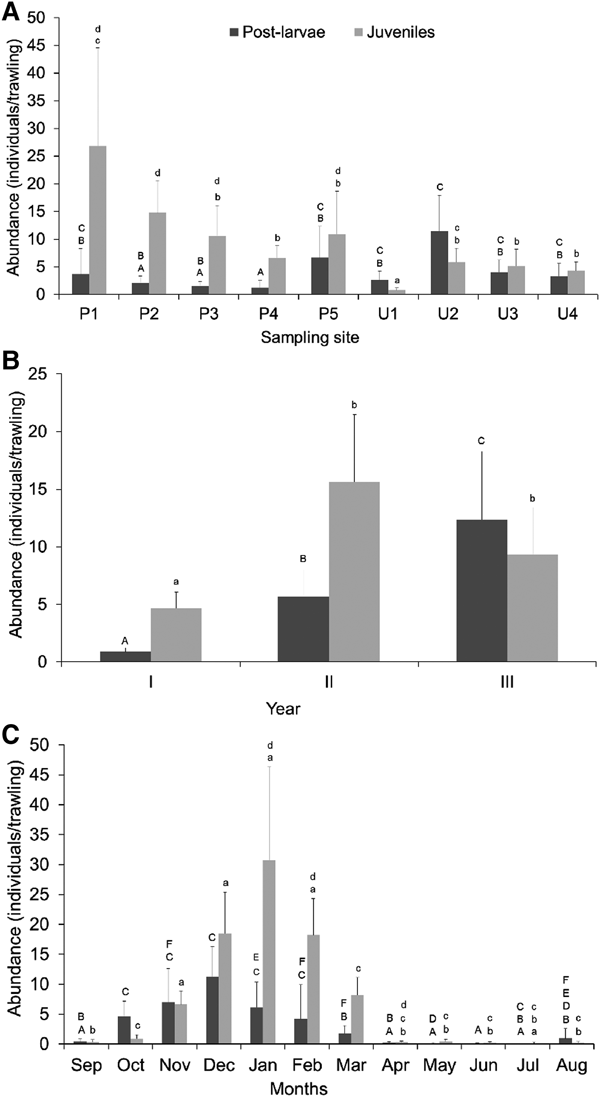
According to Table 3, all included co-variables were statistically significant (P < 0.05). Salinity showed a positive relationship with PL, indicating that a higher abundance of PL increases with salinity. Among the sampling sites, only P4 and U2 were statistically different with respect to the reference level (P1). Whereas lower PL abundances occurred in P4, higher abundances occurred in U2 when compared with the reference level. Moreover, according to the post-hoc analysis (Figure 4A), a more pronounced difference occurred when contrasting the sampling sites between protected and unprotected areas, than when contrasting the sampling sites within each area. The highest relative abundances were revealed at site U2, compared with P2, P3 and P4. No differences were detected when the abundances between U1, U2, U3, U4, P1 and P5 were compared (Figure 4A).

Fig. 4. Mean relative abundance of post-larvae and juveniles of Farfantepenaeus paulensis at the sampling sites (A), year (B) and months (C), with their respective 95% confidence intervals. Tukey's post-hoc analysis is indicated by letters above the bars, where different letters indicate significant differences (P < 0.05).
Table 3. Statistical summary of the selected model for post-larvae

NS, not significant.
Significant predictors (P < 0.05) are indicated by an asterisk (*).
Regarding the year, both year II and III were significantly different with respect to the reference level (year I) (Table 3). PL abundances increased slightly over the three years, where the last year showed the highest abundance values (Figure 4A). Additionally, the post-hoc results showed a significant difference between year II and III, revealing the highest relative abundances in year III. Post-larvae occurred in all months, and June, October, November, December, January, February and March were statistically significant when compared with the reference level (April) (Table 3). Except June, all other months listed above were positively related to PL abundance. December particularly showed the highest mean abundance values (Figure 4C) when compared to the reference level. The post-hoc analysis did not indicate substantial differences between months with higher mean PL abundances (October, November, December January, February and March) nor between months with lower mean PL abundances (April–September) (Figure 4C). However, significant differences were detected when comparing months with lower mean PL abundances (April–September) and those with higher mean PL abundances.
Of the tested models for JU abundance, models 13 and 14 had the best fit quality, but the Deviance hypothesis test indicated that model 14 was significantly better than model 13 (Table 4). Model 14 included salinity, the quadratic term of salinity, sampling sites, year, months, and the interaction between month and salinity as fixed effect. Although the salinity and its quadratic term were not significant, all the other predictors were statistically significant.
Table 4. Models to explain the variation in the relative abundance of juveniles of the pink shrimp, containing the Akaike's Information Criteria (AIC), residual deviance (RD), degrees of freedom (DF) and maximum likelihood pseudo R 2

T, temperature; S, salinity; Ss, sampling stations; Y, year; M, month; Se, season.
Selected model is presented in bold.
Sampling site P4 and all sites from the unprotected area were significant with respect to the reference level (P1) (Table 5). All these sampling sites were negatively related to JU abundance, indicating that lower abundances occur in these areas. Also, the lowest JU abundances occurred specifically in unprotected areas. The post-hoc analysis indicated that greater differences in JU abundance occur when comparing the sampling sites from protected areas (P1–P5) to sampling sites from unprotected areas (U1–U4), rather than comparing protected areas and unprotected areas amongst themselves. The highest relative abundances were revealed at P1 and P2 when compared to unprotected sites. The lowest abundance were found at the mouth of the estuary: site U1 (Figure 4A).
Table 5. Statistical summary of the selected model for juveniles

Significant predictors (P < 0.05) are indicated by an asterisk (*).
Years II and III were significantly different to the reference level (year I), and both had a positive relationship with JU abundance (Table 5). The post-hoc analysis did not detect a significant difference between years II and III (Figure 4B). The lowest relative abundance was found in year I. Regarding the months, only September, November, December, January and February were significantly different when compared with the reference level (April). Except September, all other months listed above were positively related to JU abundance. The post-hoc analysis did not indicate substantial differences between months with higher mean JU abundances (November, December, January and February) nor between months with lower mean JU abundances (March–October). However, significant differences were detected when comparing months with lower mean JU abundances and those with higher mean abundances (Figure 4C).
The interaction term between salinity and months showed an influence in JU abundances. In particular, only salinity and the months September, October, January and March were significantly different when contrasted to the reference level (S: April). The positive relationship of all these levels indicates that JU abundances are greater at higher salinities detected in these months, and specifically in September.
Finally, with respect to the residuals diagnostic plots, both selected models obeyed the basic assumption of normality, homoscedasticity and independence. Two types of visual graphical checks were used to evaluate models' fit, namely: quantile-quantile plots and predicted vs observed values plots. Quantile-quantile plots showed a reasonable normal distribution for the residuals of each selected model (Figure 5A, C). Furthermore, the predicted vs observed values were positively and significantly correlated for both PL and JU abundance models (Figure 5B, D), indicating, therefore, that both models are suitable to explain the mean tendencies for each response variable.

Fig. 5. Evaluation of model performance. Left panels shows the quantile-quantile plots provided from the post-larvae (a) and juveniles (c) models. Right panels shows the observed vs predicted values for post-larvae (b) and juveniles (d) abundances.
Discussion
Spatial variability in abundance
This study shows that the pink shrimp Farfantepenaeus paulensis post-larval and juveniles stages are distributed throughout the Lagoa dos Patos estuary, extending from the mouth of the estuary, where salinity is higher, to the northernmost areas. Their distribution is marked by habitat preference, which can be recognized by the spatial variability of species abundance. D'Incao (Reference D'Incao1991) highlighted their wide distribution and the importance of shallow inlets for juvenile growth. Ruas et al. (Reference Ruas, Rodrigues, Dumont and D'Incao2014) analysed the abundance variability of the pink shrimp in two inlets and observed that post-larvae and juveniles prefer submerged vegetation and higher salinity.
In this study, the pink shrimp showed a distinct pattern between protected and unprotected sites. A unimodal distribution with large numbers of post-larvae occurred at the unprotected sites, where the early-stage juveniles were predominant at protected sites with bimodal and multimodal distribution. These patterns of habitat use are linked to habitat selection behaviour during the shrimp ontogeny in the estuary, which corroborates with the research of Noleto-Filho et al. (Reference Noleto-Filho, Pucciarelli and Dumont2017). Studies with other species of Farfantepenaeus show that spatial segregation by size occurs to reduce intraspecific competition and mitigate predation risks (Pérez-Castañeda & Defeo, Reference Pérez-Castañeda and Defeo2001).
Habitat preference is a well-known behaviour for some species of penaeid. Pérez-Castañeda et al. (Reference Pérez-Castañeda, Blanco-Martínez, Sánchez-Martinez, Rábago-Castro, Aguirre-Guzmán and Vásquez-Sauceda2010) showed Farfantepenaeus aztecus and Farfantepenaeus dourarum (Burkenroad 1939) have a preference for submerged vegetation. Pérez-Castañeda & Defeo (Reference Pérez-Castañeda and Defeo2001, Reference Pérez-Castañeda and Defeo2004) studied species of the genus Farfantepenaeus in a coastal lagoon in the Gulf of Mexico. They noted intraspecific spatial segregation in shrimp distribution; the greatest abundance of recruits (CL <8.0 mm) and juveniles were associated with higher salinity and vegetated areas in the search for protection. According to Mohan et al. (Reference Mohan, Selvam and Azariah1995), a muddy substrate, rich in organic matter, is also an important factor in habitat selection for post-larvae and juveniles of Feneropenaeus indicus (H. Milne-Edwards 1837) and Farfantepenaeus merguiensis.
Increased abundance of post-larvae with higher salinity levels may indicate why these organisms enter the estuary. Post-larvae accompany the seawater inlet into Lagoa dos Patos. This process depends on a favourable combination of freshwater discharge and wind conditions for the entry of seawater (Möller et al., Reference Möller, Castelo and Vaz2009; Pereira & D'Incao, Reference Pereira and D'Incao2012).
Increased salinity levels may explain post-larval arrival in shallow inlets. Vance et al. (Reference Vance, Haywood, Heales, Kenyon and Loneragan1998) analysed F. merguiensis in two estuaries of north-eastern Australia and reported that an increased rainfall in wet seasons causes a decrease in salinity, inhibiting the post-larvae to reach the innermost parts of the estuary. These authors also emphasized that abundance varied due to a combination of hydrodynamic processes and behavioural changes associated with the development of the species. Staples (Reference Staples1980) observed that post-larvae of F. merguiensis moved from the substrate to the water column and migrated up the estuary by the influence of tidal flooding (of salt water). The same behaviour was observed for F. aztecus in their migration to estuaries in the Gulf of Mexico (Cházaro-Olvera et al., Reference Cházaro-Olvera, Winfield and Coria-Olvera2009). It can be concluded that if conditions are favourable, and salt water entry intense, a favourable scenario is created for the migration of post-larvae into the Lagoa dos Patos estuary. Higher salinities increase survival rates, as older pink shrimp post-larvae (late stages) are more susceptible to mortality when exposed to low salinity levels (<10) (Tsuzuki et al., Reference Tsuzuki, Cavalli and Bianchini2000), also limiting their spatial distribution.
The unprotected sites are the first contact that post-larvae have with low and highly variable salinity (Martins et al., Reference Martins, Dias, Fernandes and Muelbert2007; D'Incao & Dumont, Reference D'Incao, Dumont, Seeliger and Odebrecht2010). This area represents an acclimation space to salinity conditions in the estuary, because individuals enter this environment while undergoing their most important period of osmoregulatory development (Tsuzuki et al., Reference Tsuzuki, Cavalli and Bianchini2000).
The high abundance of post-larvae, at U1–U4 and P1 and P5, indicate the importance of these sites for pink shrimp recruitment, because they may represent a nesting area for individuals of ocean origin. The search for a substrate in marginal areas of the unprotected central area (mainly represented by U2) and shallow waters in protected areas at the mouth of the estuary as soon as they enter estuaries, may represent the attempt of post-larvae to remain in this environment, thus preventing the ebb tide from carrying them back to the ocean, as demonstrated for F. merguiensis, F. aztecus and Melicertus plebejus (Young & Carpenter, Reference Young and Carpenter1977; Adnan et al., Reference Adnan, Loneragan and Connolly2002; Cházaro-Olvera et al., Reference Cházaro-Olvera, Winfield and Coria-Olvera2009).
This study also showed that juvenile spatial distribution is uneven, with the largest abundances found in P1 and P2, showing the importance of these sites when compared with the unprotected area. The lower variability of salinity, the presence of submerged phanerogam prairies and lower current velocity are characteristic environmental factors of protected shallow inlets and can provide the ecological conditions necessary for the development of juveniles: increased food supply and protection against predators (D'Incao, Reference D'Incao1991; Mohan et al., Reference Mohan, Selvam and Azariah1995; Fernandes et al., Reference Fernandes, Monteiro and Moller2007; Martins et al., Reference Martins, Dias, Fernandes and Muelbert2007; Costa et al., Reference Costa, Lopes, Castilho, Fransozo and Simões2008; Copertino & Seeliger, Reference Copertino, Seeliger, Seeliger and Odebrecht2010; D'Incao & Dumont, Reference D'Incao, Dumont, Seeliger and Odebrecht2010; Pérez-Castañeda et al., Reference Pérez-Castañeda, Blanco-Martínez, Sánchez-Martinez, Rábago-Castro, Aguirre-Guzmán and Vásquez-Sauceda2010; Ruas et al., Reference Ruas, Rodrigues, Dumont and D'Incao2014).
According to D'Incao & Dumont (Reference D'Incao, Dumont, Seeliger and Odebrecht2010) and Ruas et al. (Reference Ruas, Dumont and D'Incao2011), stable salinity levels are an important environmental factor associated with the greatest abundances of juveniles. Therefore, lower abundances of juveniles at unprotected sites may be due to more variable environmental conditions (Martins et al., Reference Martins, Dias, Fernandes and Muelbert2007; Costa et al., 2008; D'Incao & Dumont, Reference D'Incao, Dumont, Seeliger and Odebrecht2010). A wide range in salinity, for example, can impact the survival of the late stages of pink shrimp post-larvae (Tsuzuki et al., Reference Tsuzuki, Cavalli and Bianchini2000).
Pink shrimp artisanal fishing occurs both legally and illegally throughout the estuary and has detrimental effects on the benthic community and estuarine ecosystem (Benedet et al., Reference Benedet, Dolci and D'Incao2010). Pink shrimp fishing productivity has shown a downward trend in recent years (D'Incao & Dumont, Reference D'Incao, Dumont, Seeliger and Odebrecht2010). Shrimps currently living in the Lagoa dos Patos estuary do not contribute to the adult stock of shrimp, as they are prevented from returning to the ocean due to intense fishing pressure (D'Incao, Reference D'Incao1991). This study shows the spatial variability of post-larvae and juvenile pink shrimp and key areas of recruitment in the estuary. These sites deserve special consideration in fishery management, to reduce the impact of fishing and contribute to the conservation of areas of post-larvae recruitment and juvenile growth. The conservation of these areas can help juveniles return to the ocean.
Temporal variability in abundance
Annual variability in abundance of post-larvae and juvenile pink shrimp, presented in this work, should be considered characteristic of the Lagoa dos Patos estuary. Their annual variability is linked to environmental conditions and the availability of post-larvae pink shrimp in the coastal zone. The least productive catches are positively related to high rainfall in the Lagoa dos Patos drainage basin. In high rainfall conditions, a strong flow of fresh water through the narrow mouth that connects the estuary to the ocean impedes salt water from entering and compromises pink shrimp recruitment in the estuary (Möller et al., Reference Möller, Castelo and Vaz2009; Pereira & D'Incao, Reference Pereira and D'Incao2012). The overfishing of the adult stock also causes a reduction in the number of larvae available for recruitment in the estuary (D'Incao et al., Reference D'Incao, Valentini and Rodrigues2002; Teodoro et al., Reference Teodoro, Terossi, Costa and Mantelatto2015). The current fishery production of pink shrimp adult stock varies annually (Pezzuto & Benincà, Reference Pezzuto and Benincà2015), and possibly has a negative effect on the reproduction dynamics of the species and consequently, there is an annual variability in larvae density.
Post-larvae pink shrimp captured throughout the year during all months support the idea proposed by D'Incao (Reference D'Incao1991) that, based on growth studies, post-larvae enter the estuary throughout the entire year, which also occurs in estuaries in the north of Brazil. October to March (spring to summer) is characterized as the principal period of recruitment. D'Incao (Reference D'Incao1991), analysing the velocity and direction of marine currents and larval development to the youngest post-larvae stage in Lagoa dos Patos, suggests that post-larvae that inhabit the estuary may have originated from a spawning population off the coast of Santa Catarina State, in southern Brazil. The pink shrimp reproduces continuously but reproductive intensity varies with latitude. In south-east Brazil the adult stock of pink shrimp presents two reproductive peaks, while the principal reproductive period in Santa Catarina begins in spring (September) and extends to summer (D'Incao, Reference D'Incao1991; Costa et al., Reference Costa, Lopes, Castilho, Fransozo and Simões2008).
The variability in abundance of juveniles shows a defined monthly pattern, which covers the period of November to February. Although the model did not identify temperature as a significant factor, it was evident that juvenile abundance was higher during the hottest months of the year. This reinforces the findings of D'Incao (Reference D'Incao1991), which showed an increase in abundance with temperature. The same pattern was observed in the same species by Branco & Verani (Reference Branco and Verani1998) and Lüchmann et al. (Reference Lüchmann, Freire, Ferreira, Daura-Jorge and Marques2008) in Conceição Lagoon in Santa Catarina, and by Costa et al. (Reference Costa, Lopes, Castilho, Fransozo and Simões2008) in an estuary along the south-east coast of Brazil. However, it is possible that other factors not identified in the model may have influenced the observed pattern. Fishing, for example, starts at the beginning of February.
The monthly variability of salinity proved important for juveniles. The increase in juvenile abundance with an increase in salinity during September, October, January and March show juveniles’ preference for elevated salinities. These results corroborate previous results from Lagoa dos Patos, that seem to be linked to annual variability patterns and present greatest abundance during periods of high salinity (D'Incao, Reference D'Incao1991; Möller et al., Reference Möller, Castelo and Vaz2009; Ruas et al., Reference Ruas, Rodrigues, Dumont and D'Incao2014). According to Tsuzuki et al. (Reference Tsuzuki, Cavalli and Bianchini2000) the highest survival rates of this species are linked to salinities above 10.
Author ORCIDs
Vinicius Mendes Ruas 0000-0002-6995-2777
Financial support
The authors are grateful to the Post-Graduation Program in Biological Oceanography of the Federal University of Rio Grande – FURG and the Coordination for the Improvement of Higher Education Personnel – CAPES for granting the scholarship.