Introduction
The family Dirivultidae Humes & Dojiri, 1981 (Copepoda: Siphonostomatoida) is endemic to deep-water hydrothermal vent fields and hydrocarbon seeps and the surrounding axial summit trough (Humes, Reference Humes1999; Gollner et al., Reference Gollner, Ivanenko, Martínez Arbizu and Bright2010; Ivanenko et al., Reference Ivanenko, Defaye, Segonzac, Khripounoff, Sarrazin and Ferrari2011). Currently, the family consists of 61 species of 12 genera. Many members have been found in residues after washings of terebellid polychaetes, gastropods, bivalves and decapods (Boxshall & Halsey, Reference Boxshall and Halsey2004; Gollner et al., Reference Gollner, Ivanenko, Martínez Arbizu and Bright2010), with few clearly defined hosts and sampling locations. Among this family, Stygiopontius Humes, Reference Humes1987 consists of 27 species that have been recorded from both Pacific and Atlantic Oceans (Humes, Reference Humes1987, Reference Humes1989, Reference Humes1990, Reference Humes1991, Reference Humes1996, Reference Humes1997; Ivanenko et al., Reference Ivanenko, Martínez Arbizu and Stecher2006, Reference Ivanenko, Defaye, Segonzac, Khripounoff, Sarrazin and Ferrari2011; Ivanenko & Ferrari, Reference Ivanenko and Ferrari2013; Uyeno et al., Reference Uyeno, Watanabe and Shimanaga2018; Lee et al., Reference Lee, Kim and Kim2020). These species are concentrated in the Mid-Atlantic Ridge and the East Pacific Rise and its neighbouring and connecting ridges. Recently, four species were described from the Central Indian Ridge, Indian Ocean (Lee et al., Reference Lee, Kim and Kim2020). From the Western Pacific, six species are recorded (i.e. S. brevispina Humes, Reference Humes1991 and S. lauensis Humes, Reference Humes1991 from Lau Basin; S. pectinatus Humes, Reference Humes1987 and S. stabilitus Humes, Reference Humes1990 from Mariana Back-Arc Basin; S. senckenbergi Ivanenko & Ferrari, Reference Ivanenko and Ferrari2013 from the New Ireland Fore-Arc; and S. senokuchiae Uyeno, Watanabe & Shimanaga, Reference Uyeno, Watanabe and Shimanaga2018 from Izu-Bonin Arc) (Humes, Reference Humes1990, Reference Humes1991; Gollner et al., Reference Gollner, Ivanenko, Martínez Arbizu and Bright2010; Ivanenko & Ferrari, Reference Ivanenko and Ferrari2013; Uyeno et al., Reference Uyeno, Watanabe and Shimanaga2018). In many species of the family, the morphology of adult stages was known, but the only developmental stages described were the nauplius I of S. pectinatus, the copepodid I of both S. horridus Lee, Kim & Kim, Reference Lee, Kim and Kim2020 and an unidentified species, and the copepodid V of Aphotopontius temperatus Humes, Reference Humes1997 (Humes, Reference Humes1997; Ivanenko, Reference Ivanenko1998; Ivanenko et al., Reference Ivanenko, Martínez Arbizu and Stecher2007; Lee et al., Reference Lee, Kim and Kim2020).
In this study, two species of the family Dirivultidae are described from hydrothermal vent fields in the Okinawa Trough, the western North Pacific. Adult females and two developmental stages (nauplius I and copepodid IV) of S. senckenbergi, which was originally described by Ivanenko & Ferrari (Reference Ivanenko and Ferrari2013) based on adult males from Papua New Guinea, are reported from the galatheoid squat lobster Shinkaia crosnieri Baba & Williams, Reference Baba and Williams1998 (Decapoda: Munidopsidae). Dirivultus kaiko sp. nov. is described based on adult females and males collected from the tentacular crown of the siboglinid tubeworm Lamellibrachia columna Southward, 1991.
Materials and methods
The copepod specimens were mainly collected during the research cruise (KR18-15), and also from other cruises (NT97-14, NT05-03 Leg. 2, NT10-E01, KR15-16, NT15-13, and KR16-16) all conducted in hydrothermal vent fields of the Okinawa Trough (Figure 1A–C), East China Sea, the western North Pacific operated by the R/V ‘Kairei’ with the ROV ‘Kaiko 7000’ and ‘7000II’ and the R/V ‘Natsushima’ with the ROV ‘Hyper-Dolphin’ belonging to Japan Agency for Marine-Earth Science and Technology (JAMSTEC), Yokosuka, Japan. Deep-sea squat lobsters and tubeworms were collected using a suction sampler (Slurp Gun) and a manipulator installed on the ROV, respectively (Figure 1B, C). Copepods were removed from hosts and fixed and preserved in 99% ethanol for morphological and future molecular studies.
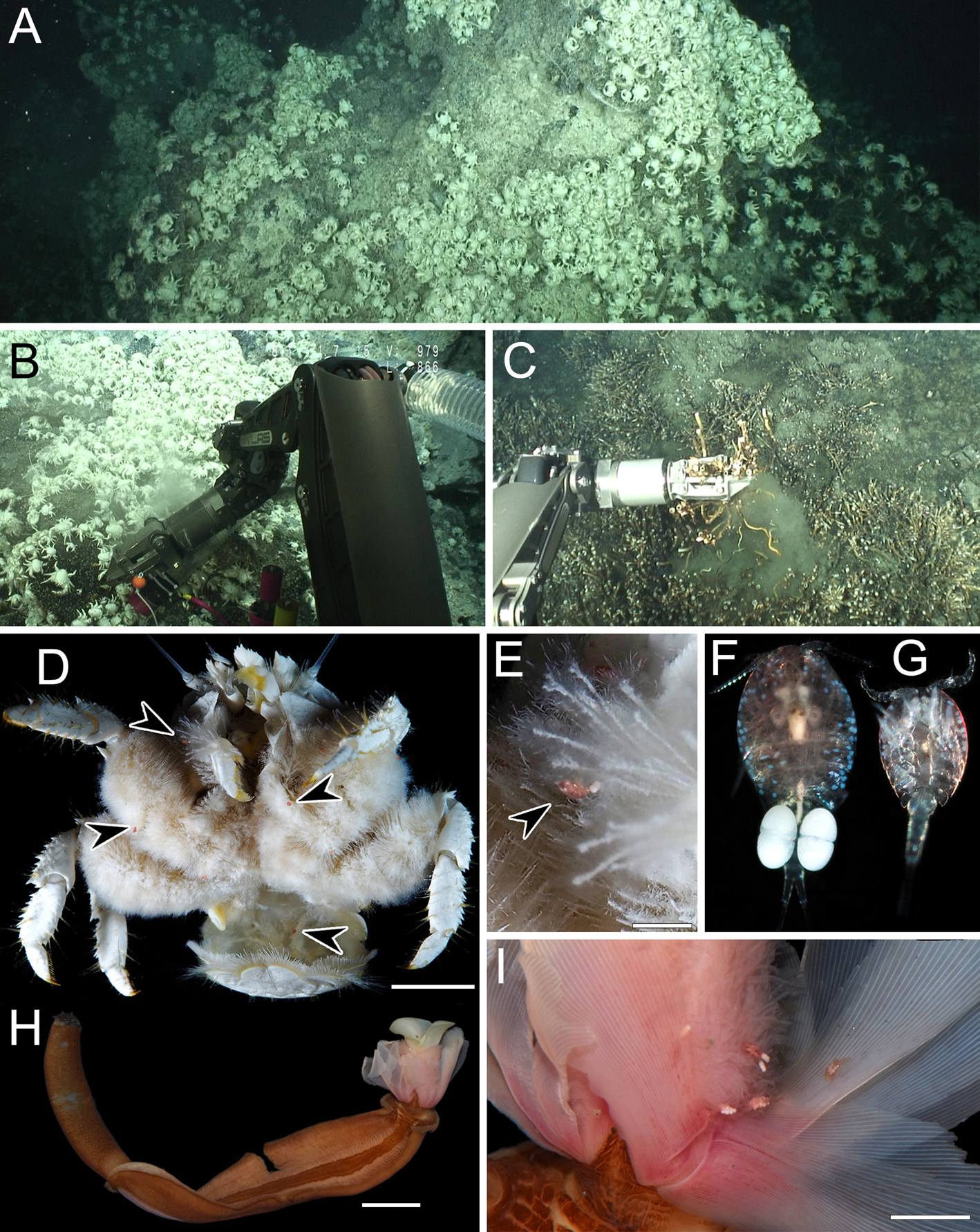
Fig. 1. Sampling sites at the Iheya North Knoll, Okinawa Trough and live colouration of animals. A, the sampling site of Stygiopontius senckenbergi Ivanenko & Ferrari, Reference Ivanenko and Ferrari2013 at a depth of 980 m. B, Shinkaia crosnieri Baba & Williams, Reference Baba and Williams1998 collected by a suction sampler (Slurp Gun). C, Lamellibrachia columna Southward, 1991 collected by a manipulator at a depth of 987 m. D, Sh. crosnieri harbouring S. senckenbergi. E, S. senckenbergi on the ventral seta of the host. F, fresh specimen of S. senckenbergi, adult female (~1.2 mm in body length). G, same, adult male (~0.7 mm in body length). H, fresh specimen of L. columna. I, Dirivultus kaiko sp. nov. attached on the tentacular crown of L. columna. Arrowheads indicate copepods. Scale bars: D, H, 10 mm; E, 2 mm; I, 3 mm.
For morphological study, selected copepod specimens were subsequently soaked in lactophenol for 24 h, dissected by a sharpened tungsten needle under a dissecting microscope, and examined based on the modified version of the wooden slide method of Humes & Gooding (Reference Humes and Gooding1964). Drawing were made with the aid of a drawing tube mounted on the biological microscope BX53 (Olympus, Tokyo, Japan) with differential interference contrast. Copepods were measured by an ocular micrometer, and measurements were given in μm as the range followed by the mean and standard deviation in parentheses. Types and other examined specimens are deposited in the crustacean collection of the National Museum of Nature and Science, Tsukuba, Japan (NSMT).
Results
Systematics
Dirivultidae Humes & Dojiri, Reference Humes and Dojiri1980
Stygiopontius Humes, Reference Humes1987
Stygiopontius senckenbergi Ivanenko & Ferrari, Reference Ivanenko and Ferrari2013
(Figures 1D–G, 2–9)
Stygiopontius senckenbergi Ivanenko & Ferrari, Reference Ivanenko and Ferrari2013, pp. 1807–1812.

Fig. 2. Stygiopontius senckenbergi Ivanenko & Ferrari, Reference Ivanenko and Ferrari2013, adult female (NSMT-Cr 28363). A, habitus, dorsal; B, urosome, dorsal; C, right caudal ramus, dorsal; D, egg sac; E, rostral area, ventral; F, left antennule, posterior; G, left antenna, posterior; H, labrum; I, left mandible. Scale bars: A, 300 μm; B, D, 200 μm; C, F, G, H, 50 μm; E, 100 μm; I, 20 μm.

Fig. 3. Stygiopontius senckenbergi Ivanenko & Ferrari, Reference Ivanenko and Ferrari2013, adult female (NSMT-Cr 28363). A, left maxillule, posterior; B, left maxilla, posterior; C, left maxilliped, posterior; D, left leg 1, anterior; E, right leg 2, anterior. Scale bars: A, B, 20 μm; C, 40 μm; D, E, 50 μm.

Fig. 4. Stygiopontius senckenbergi Ivanenko & Ferrari, Reference Ivanenko and Ferrari2013, adult female (NSMT-Cr 28363). A, right leg 3, anterior; B, left leg 4, anterior; C, partial view of fifth pedigerous somite and genital double somite, dorsal; D, right leg 5, dorsal. Scale bars: A, B, 100 μm; C, 50 μm; D, 40 μm.

Fig. 5. Stygiopontius senckenbergi Ivanenko & Ferrari, Reference Ivanenko and Ferrari2013, adult male (NSMT-Cr 28364). A, habitus, dorsal; B, anterior part of urosome, ventral; C, right antennule, posterior; D, second to fifth segments of right antennule, anterior; E, right maxilliped, posterior; F, right third endopodal segment of leg 2, anterior. Scale bars: A, 100 μm; B, D, 20 μm; C, E, 30 μm; F, 50 μm.

Fig. 6. Stygiopontius senckenbergi Ivanenko & Ferrari, Reference Ivanenko and Ferrari2013, nauplius I (NSMT-Cr 28374). A, habitus, ventral; B, left antennule, anterior; C, left antenna, anterior; D, left mandible, anterior. Scale bars: A, 100 μm; B–D, 40 μm.
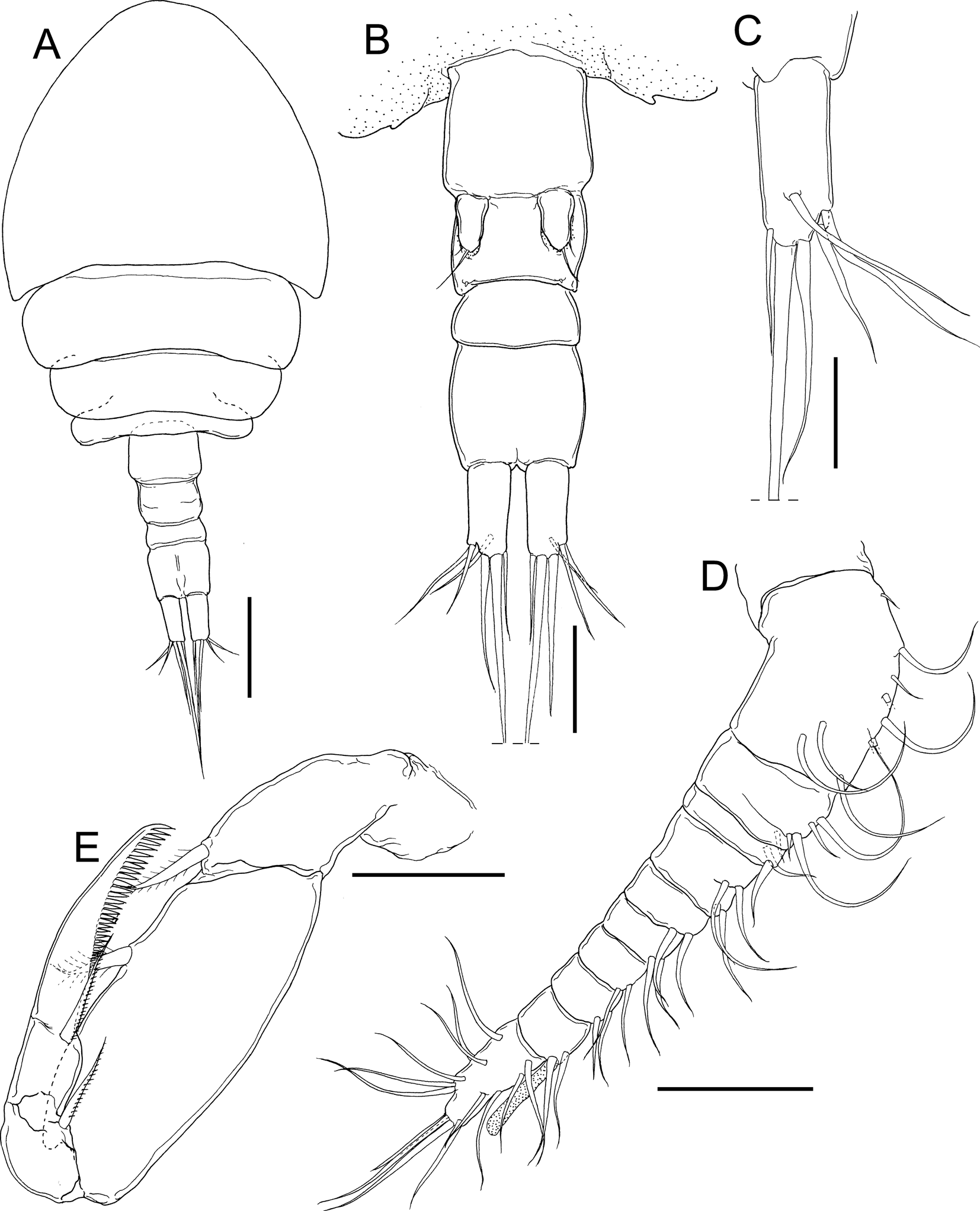
Fig. 7. Stygiopontius senckenbergi Ivanenko & Ferrari, Reference Ivanenko and Ferrari2013, copepodid IV (NSMT-Cr 28375). A, habitus, dorsal; B, urosome, ventral; C, right caudal ramus, dorsal; D, right antennule, anterior; E, left maxilliped, posterior. Scale bars: A, 100 μm; B, 50 μm; C, 30 μm; D, E, 40 μm.

Fig. 8. Stygiopontius senckenbergi Ivanenko & Ferrari, Reference Ivanenko and Ferrari2013, copepodid IV (NSMT-Cr 28375). A, left leg 1, anterior; B, right leg 2, anterior; C, left leg 3, anterior; D, left leg 4, anterior; E, anterior part of urosomites. Scale bars: A–D, 50 μm; E, 20 μm.

Fig. 9. Stygiopontius senckenbergi Ivanenko & Ferrari, Reference Ivanenko and Ferrari2013, mating pair, showing adult male grasping urosomite of copepodid IV. A, lateral view of mating pair; B, same, ventral view; C, urosomite of copepodid IV grasped by antennules of male. Scale bars: A, B, 100 μm; C, 30 μm.
Materials Examined
Adult female (NSMT-Cr 28363), ex Shinkaia crosnieri (Decapoda: Munidopsidae), the Iheya North Knoll (27°47.371′N 126°53.843′E), off Okinawa Island, East China Sea, 980 m depth, 16 November 2018. Adult male (NSMT-Cr 28364), collection data same as that of NSMT-Cr 28363. 25 adult females and 26 adult males (NSMT-Cr 28365), collection data same as that of NSMT-Cr 28363. Five females and three males (NSMT-Cr 28366), ex Sh. crosnieri (Onboard ID: HD1859 BC1-12, collected at Aki site in Iheya North hydrothermal vent field, 27°46.007′N 126°54.188′E), off Okinawa Island, East China Sea, 1095 m depth, 1 August 2015; one female and one male (NSMT-Cr 28367), ex Sh. crosnieri (Onboard ID: KIV673 BC1-02, Ana site 26°13.3777′N 126°28.3017′E), off Okinawa Island, East China Sea, 1085 m depth, 5 November 2015; nine females (NSMT-Cr 28368), ex Sh. crosnieri (Onboard ID: KIV717 BC1-3-1, Waka site in Futagoyama hydrothermal field, 24°52.0468′N 123°18.4832′E), off Okinawa Island, East China Sea, 1288 m depth, 21 November 2016; eight females (NSMT-Cr 28369), ex Sh. crosnieri (Onboard ID: KIV722 BC1-1, Gondo site, 26°18.4871′N 126°24.9294′E), off Okinawa Island, East China Sea, 1414 m depth, 1 December 2016; two females (NSMT-Cr 28370), ex Sh. crosnieri, the Izeha Hole (27°16.304′N 127°04.885′E), off Okinawa Island, East China Sea, 1308 m depth, 22 April 2005; five females (NSMT-Cr 28371), ex Sh. crosnieri, the Hatoma Knoll (24°51.486′N 123°50.512′E), off Yaeyama Islands, East China Sea, 1480 m depth, 24 April 2005; five females (NSMT-Cr 28372), ex Sh. crosnieri, the North of Iheya Ridge (27°47.430′N 126°53.850′E), off Okinawa Island, East China Sea, 1023 m depth, 3 September 2010; six females (NSMT-Cr 28373), ex Sh. crosnieri, the Iheya North Field (27°47.220′N 126°53.922′E), off Okinawa Island, East China Sea, 976 m depth, 26 September 1997; four nauplius I hatched from eggs (NSMT-Cr 28374), collection data same as that of NSMT-Cr 28363; one copepodid IV (NSMT-Cr 28375), collection data same as that of NSMT-Cr 28363.
Description
Adult female: body cyclopiform (Figure 2A) 970–1229 (1082 ± 66) long, composed of cephalothorax, second to fifth pedigerous somites, genital double somite, and 3-segmented abdomen (N = 11). Cephalothorax (Figure 2A) wider than long, 441–498 (466 ± 16) × 486–537 (509 ± 14), bearing pair of posterolateral pointed processes. Second to fourth pedigerous somites gradually narrower posteriorly. Prosome 647–767 (691 ± 33) long. Genital double somite (Figure 2A, B) slightly wider than long, 132–177 (149 ± 13) × 153–174 (160 ± 6). Abdomen (Figure 2A, B) composed of three free somites, 57–87 (69 ± 9) × 75–85 (80 ± 3), 48–67 (58 ± 9) × 71–77 (74 ± 2) and 45–66 (55 ± 8) × 60–73 (67 ± 4), respectively. Caudal rami (Figure 2A–C) 3.58–4.31 (3.97 ± 0.24) times longer than wide, 88–120 (102 ± 8) × 24–29 (26 ± 2), with six setae on distal tip. Egg sac (Figure 2D) containing two eggs.
Rostrum (Figure 2E) bearing round margin. Antennule (Figure 2F) 10-segmented; armature formula 14, 9, 2, 4, 2, 2, 2, 2, 2 + 1 aesthetasc, and 14; all setae naked. Antenna (Figure 2G) biramous, composed of coxa, basis, endopod, and exopod; coxa unarmed; basis ornamented with rows of marginal hairs; endopod 2-segmented, bearing one subterminal and three terminal setae on distal segment; exopod unsegmented, bearing one subterminal and one distal setae. Labrum (Figure 2H) shield shaped with median process. Mandible (Figure 2I) slender, bearing 10 distal teeth. Maxillule (Figure 3A) bilobed: large inner lobe (endite) with five setae and small outer lobe (palp) with three setae, respectively. Maxilla (Figure 3B) composed of syncoxa and claw; syncoxa robust bearing long inner seta near articulation with claw; claw elongate bearing pointed subterminal element and sharp tip. Maxilliped (Figure 3C) subchelate, comprising syncoxa, basis and 3-segmented endopod; syncoxa and basis bearing single inner seta, respectively; proximal endopodal segment bearing two tiny and single normal setae; middle segment of endopod bearing single seta; distal endopodal segment bearing single seta and pectinate claw with row of well-developed sharp processes on inner margin.
Legs 1 to 4 (Figures 3D, E & 4A, B) biramous; both rami 3-segmented, except 2-segmented endopod of leg 4. Leg armature formula shown in Table 1. Intercoxal sclerites of legs 1 to 4 (Figures 3D, E & 4A, B) unornamented. All spines serrated. All setae on rami plumose, except one seta with short marginal hairs on proximal endopodal segment of leg 4. Rami of legs 1 to 4 bearing pointed processes on outer, inner and distal margins. Leg 5 (Figure 4C, D) 2-segmented; proximal segment bearing 1 basal naked element; distal segment bearing three distal plumose setae. Leg 6 (Figure 4C) represented by single small element at genital opening.
Table 1. Armature formula of legs 1 to 4 of Stygiopontius senckenbergi Ivanenko & Ferrari, Reference Ivanenko and Ferrari2013, adult female. Arabic numbers = number of setae, Roman numbers = number of spines

Adult male: body (Figure 5A) cyclopiform, 590–652 (627 ± 22) long, composed of cephalothorax, second to fifth pedigerous somites, genital somite and 4-segmented abdomen (N = 10). Cephalothorax wider than long, 248–263 (256 ± 5) × 290–310 (302 ± 7), bearing pair of posterolateral pointed processes. Prosome 386–422 (406 ± 12) long. Genital somite (Figure 5A, B) wider than long, 63–78 (71 ± 6) × 85–90 (88 ± 2). Abdomen (Figure 5A) composed of four free somites, 35–50 (41 ± 5) × 49–57 (53 ± 2), 29–41 (36 ± 4) × 46–52 (49 ± 2), 28–41 (32 ± 6) × 41–46 (43 ± 2) and 28–43 (32 ± 6) × 39–48 (42 ± 2), respectively. Caudal rami (Figure 5A) 2.16–2.79 (2.50 ± 0.21) times longer than wide, 32–44 (38 ± 4) × 14–19 (15 ± 2), with six setae on distal tip.
Antennule (Figure 5C, D) 11-segmented, geniculated between ninth and tenth segments; armature formula 1, 14, 7, 2, 2, 4, 2, 2, 4, 4 + 1 aesthetasc, and 12; all setae naked except one stout serrated seta on fourth segment (Figure 5D); third segment bearing digitiform process with accessory element; fourth and sixth segments each bearing three conical processes. Antenna, mandible, maxillule and maxilla as in female. Maxilliped (Figure 5E) as in female except terminal pectinate claw with row of processes less well developed than in female.
Armature formula of legs 1 to 4 as in female except third endopodal segment of leg 2: I, II, 1, 2 (Figure 5F). Leg 5 (Figure 5B) composed of protopod with outer seta incorporated in fifth pedigerous somite and exopod bearing three slender outer and two stout distal setae. Leg 6 (Figure 5B) represented by genital flap bearing serrated spine and seta on outer margin.
Nauplius I: body ovoid (Figure 6A), longer than wide, 198–206 (203 ± 4) × 138–158 (148 ± 8) (N = 4), filled with yolk. Caudal rami represented by pair of slender setae (Figure 6A). Naupliar eye not observed. Mouth and anal opening absent. Antennule (Figure 6B) unsegmented, two setae on ventral margin and three setae on distal tip. Antenna (Figure 6C) biramous: coxa without masticatory process incompletely fused to unarmed basis; exopod 5-segmented bearing 1, 1, 1, 1 and 2 setae per segment; endopod 2-segmented bearing proximal segment unarmed and distal segment with one middle and two distal setae. Mandible (Figure 6D) biramous: unarmed coxa incompletely fused to unarmed basis; exopod 4-segmented bearing 1, 1, 1 and 2 setae per segment; endopod unsegmented bearing two inner setae and two distal setae.
Copepodid IV: body cyclopiform (Figure 7A) 646 long (N = 1), composed of 4-segmented prosome (cephalothorax, second to fourth pedigerous somites) and 4-segmented urosome (one pedigerous, genital and two abdominal somites). Cephalothorax (Figure 7A) wider than long, 301 × 328. Prosome 445 long. Urosome 221 long. Caudal rami (Figure 7A–C) 2.31 times longer than wide, 46 × 20, with one subdistal and five distal setae.
Antennule (Figure 7D) 10-segmented; armature formula 9, 5, 2, 4, 2, 2, 2, 2, 2 + 1 aesthetasc, and 14; all setae naked. Antenna, mandible, maxillule, and maxilla as in adult female. Maxilliped (Figure 7E) subchelate, comprising syncoxa, basis, and 2-segmented endopod; syncoxa and basis each bearing single inner seta; proximal endopodal segment bearing normal seta; distal endopodal segment bearing single seta and pectinate claw with row of well-developed sharp processes on inner margin.
Legs 1 to 4 (Figure 8A–D) biramous; both rami 2-segmented. Leg armature formula shown in Table 2. Leg 5 (Figures 7B & 8E) unsegmented bearing one basal naked element and three distal elements. Leg 6 (Figure 8E) represented by single element on lateral lobe.
Table 2. Armature formula of legs 1 to 4 of Stygiopontius senckenbergi Ivanenko & Ferrari, Reference Ivanenko and Ferrari2013, copepodid IV. Arabic numbers = number of setae, Roman numbers = number of spines

Remarks
Based on both sexes, Stygiopontius senckenbergi belongs to a group characterized by leg 1 with an inner coxal seta and leg 4 with four spines on the distal exopodal segment which comprises the following eight congeners: S. brevispina, S. flexus Humes, Reference Humes1987, S. hispidulus Humes, Reference Humes1987, S. lauensis, S. mirus Humes, Reference Humes1996, S. pectinatus, S. senckenbergi and S. sentifer Humes, Reference Humes1987 (Ivanenko & Ferrari, Reference Ivanenko and Ferrari2013). Among those congeners, four species (S. brevispina, S. hispidulus, S. lauensis and S. pectinatus) share the same armature formula on legs 1 to 4 with S. senckenbergi but clearly differ by lack of a pointed conical distal process on the leg 4 endopod.
One of the examined males was found in mate guarding, with its antennules grasping onto the second urosomite of a copepodid IV female (Figure 9).
Newly established Japanese name: ‘Ohmu-kudakuchi-mijinko’ for the species. ‘Ohmu’ is the symbolic creature in Japan's leading fantasy animation movie. The multiple blue dots distributed on the copepod's body are reminiscent of the eyes in the normal condition of ‘Ohmu’.
Genus Dirivultus Humes & Dojiri, Reference Humes and Dojiri1981
Dirivultus kaiko sp. nov.
(Figures 1I, 10–13)
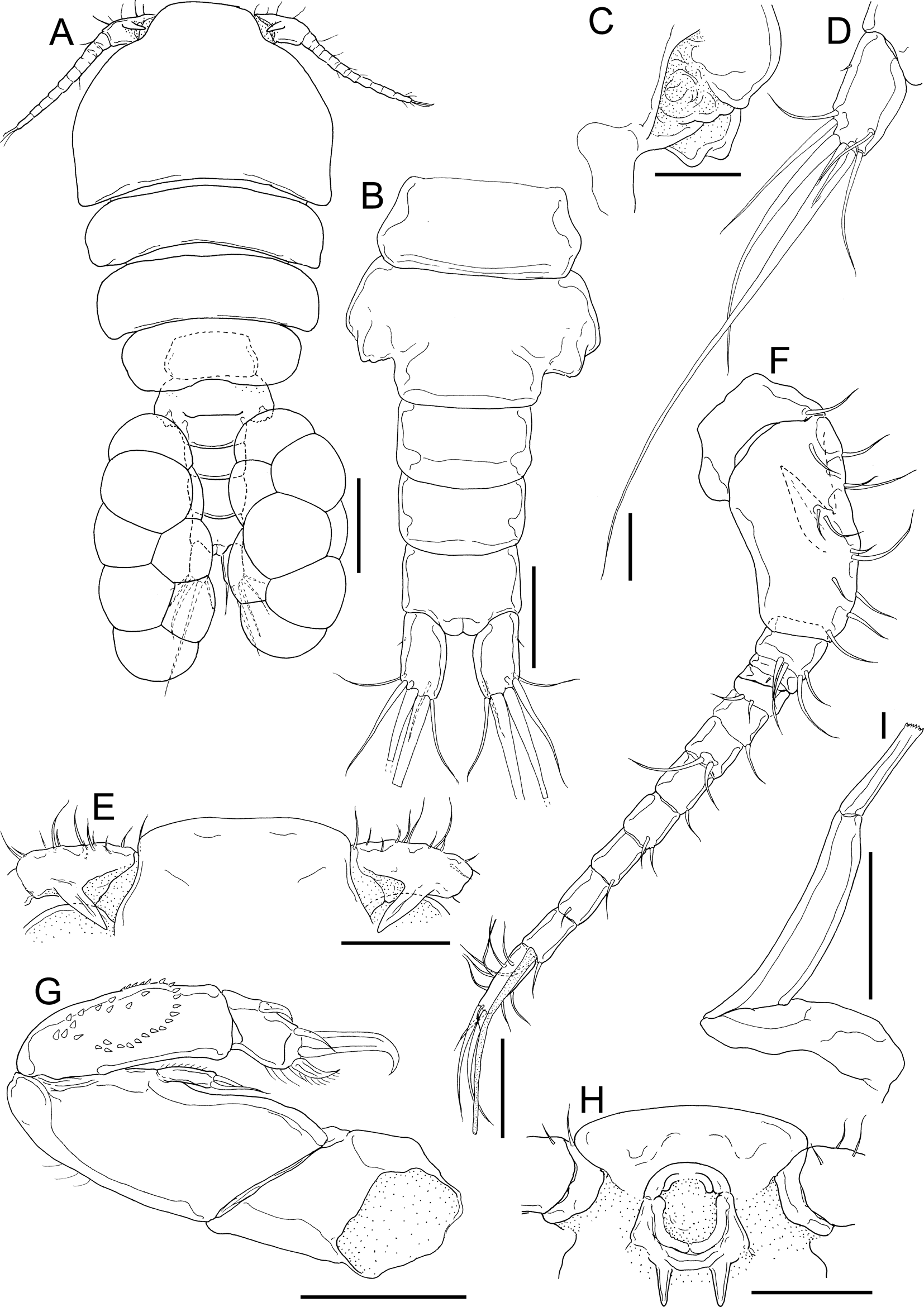
Fig. 10. Dirivultus kaiko sp. nov., adult female, holotype (NSMT-Cr 28376). A, habitus, dorsal; B, urosome, ventral; C, right genital aperture; D, left caudal ramus, dorsal; E, rostral area and basal part of antennules, dorsal; F, left antennule, posterior; G, left antenna; H, oral cone; I, right mandible, anterior. Scale bars: A, 200 μm; B, E, H, 100 μm; C, I, 40 μm; D, F, G, 50 μm.
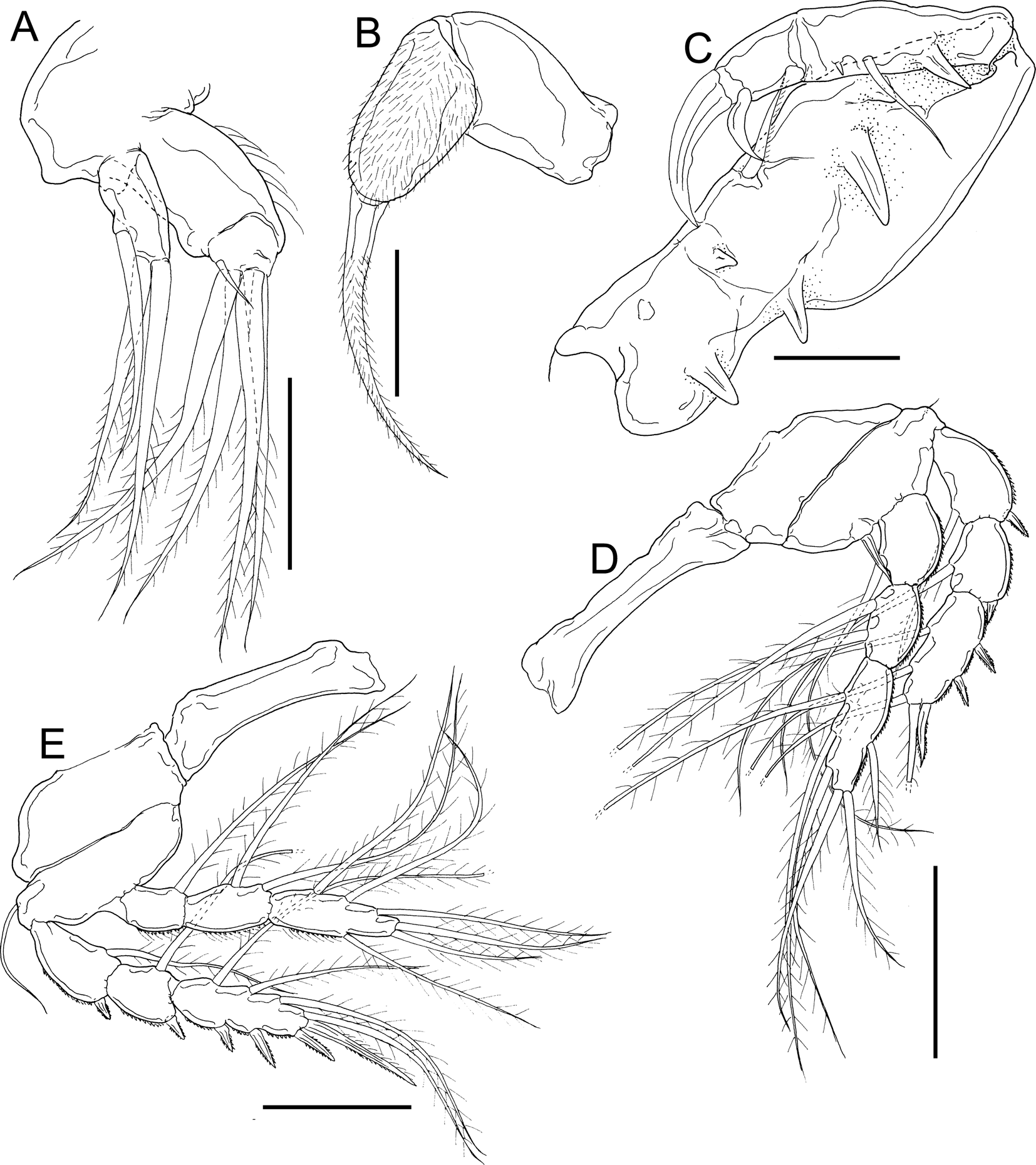
Fig. 11. Dirivultus kaiko sp. nov., adult female, holotype (NSMT-Cr 28376). A, right maxillule, anterior; B, left maxilla, anterior; C, left maxilliped, posterior; D, left leg 1, anterior; E, right leg 2, anterior. Scale bars: A, B, 40 μm; C, 50 μm; E, F, 100 μm.

Fig. 12. Dirivultus kaiko sp. nov., A–C, adult female, holotype (NSMT-Cr 28376), and D, E, adult male, allotype (NSMT-Cr 28377). A, right leg 3, anterior; B, right leg 4, anterior; C, partial fifth pedigerous somite with left leg 5; D, habitus, dorsal; E, urosome, ventral. Scale bars: A, B, E, 100 μm; C, 40 μm; D, 200 μm.
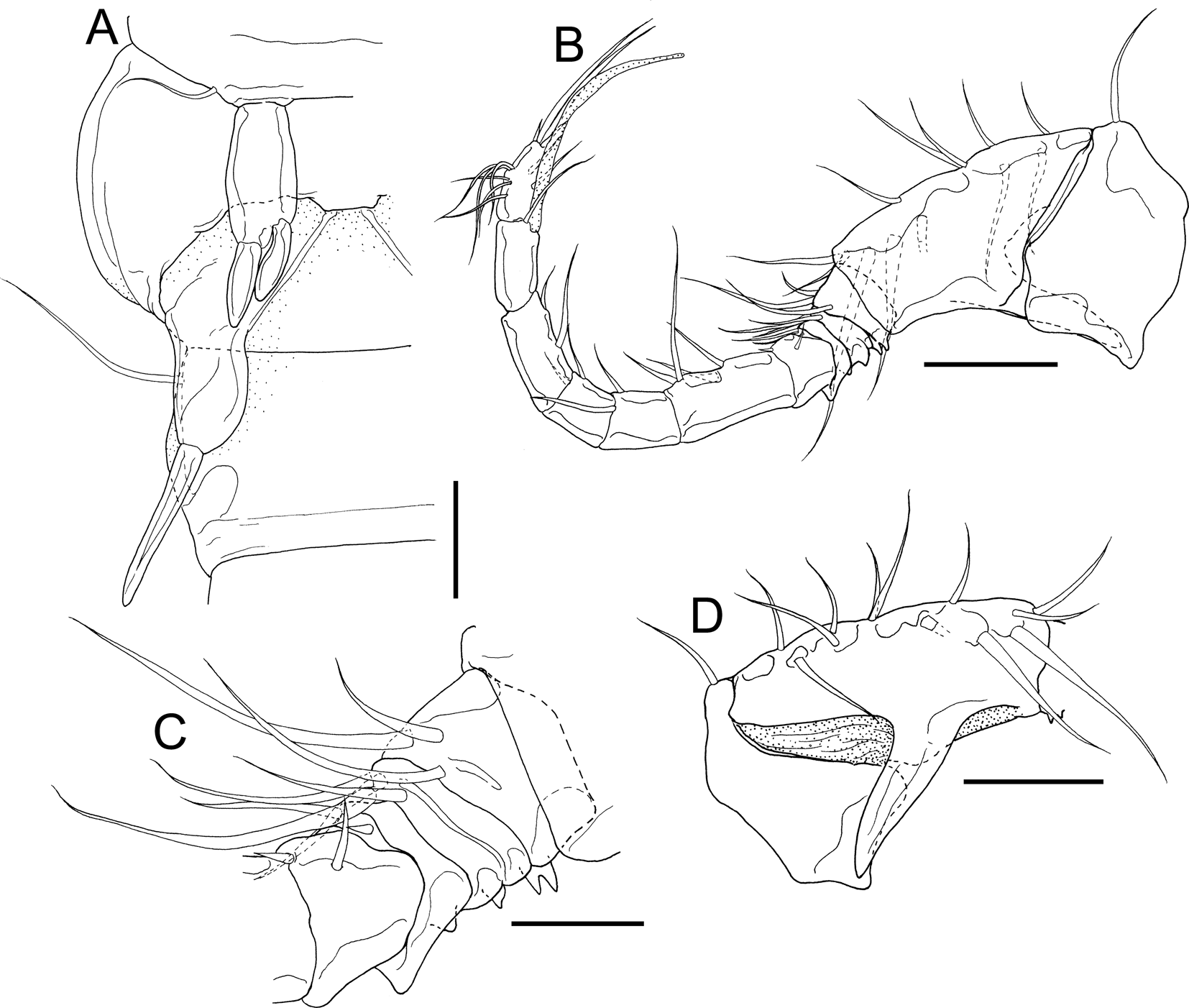
Fig. 13. Dirivultus kaiko sp. nov., adult male, allotype (NSMT-Cr 28377). A, right portion of anterior part of urosome; B, right antennule, posterior; C, third to sixth segments of right antennule, posterior; D, first and second segments of antennule, anterior. Scale bars: A, C, 20 μm; B, D, 50 μm.
Type Material
Holotype: adult female (NSMT-Cr 28376), ex Lamellibrachia columna Southward, 1991 (Annelida: Siboglinidae), the Iheya North Knoll (27°47.371′N 126°53.843′E), off Okinawa Island, East China Sea, 987 m depth, 16 November 2018.
Allotype: adult male (NSMT-Cr 28377), collection data same as that of holotype.
Paratypes: 3 adult females and 2 adult males (NSMT-Cr 28378), collection data same as that of holotype.
Attachment Sites
The copepods inhabited the inner lamellar pockets in the tentacular crown of L. columna (Figure 1H, I).
Etymology
The species name ‘kaiko’ refers to the ROV ‘Kaiko 7000II'.
Description
Holotype adult female: body cyclopiform (Figure 10A) 1240 long, composed of cephalothorax, second to fifth pedigerous somites, genital double somite and 3-segmented abdomen. Cephalothorax (Figure 10A) wider than long, 434 × 554. Second to fourth pedigerous somites gradually narrower posteriorly. Prosome 822 long. Genital double somite (Figure 10A, B) distinctly wider than long, 154 × 256; paired genital openings situated on dorsal surface (Figure 10C). Abdomen (Figure 10A, B) composed of three free somites, 75 × 127, 89 × 123 and 90 × 115, respectively. Caudal rami (Figure 10A, B, D) 1.95 times longer than wide, 91 × 47, with six setae on distal tip. Egg sac multiseriate (Figure 10A).
Rostrum (Figure 10E, H) bearing round margin without distinct apex. Antennule (Figure 10F) 13-segmented; armature formula 1, 12, 4, 1, 2, 1, 2, 1, 2, 2, 1, 1 + 1 aesthetasc, and 12; all setae naked; second segment bearing long recurved process directed posteromedially (Figure 10E, F). Antenna (Figure 10G) biramous, composed of coxa, basis, endopod, and exopod; coxa unarmed; basis armed with row of marginal hairs; endopod 2-segmented, bearing spinules on proximal segment and one subterminal and three terminal setae on distal segment; exopod unsegmented, bearing two distal setae.
Labrum forming oral cone with labium (Figure 10H). Oral cone with two dentiform processes on posterior part of base.
Mandible (Figure 10I) slender, bearing fine distal teeth. Maxillule (Figure 11A) bilobed: large inner lobe (endite) with four long and one short distal setae and small outer lobe (palp) with one subterminal and two distal setae. Maxilla (Figure 11B) 2-segmented; syncoxa robust, unarmed; second segment covered with fine setules with elongate claw-like spine. Maxilliped (Figure 11C) subchelate, comprising syncoxa, basis and 2-segmented endopod; syncoxa incompletely incorporated to basis, bearing one seta and three pointed processes; basis bearing two processes; proximal endopodal segment bearing one seta, one small spiniform element and one process; distal endopodal segment bearing one spine-like seta and terminal claw with median protrusion on concave margin.
Legs 1 to 4 (Figures 11D, E & 12A, B) biramous; both rami 3-segmented, except endopod of leg 4. Leg armature formula shown in Table 3. Intercoxal sclerites of legs 1 to 4 (Figures 11D, E & 12A, B) unornamented. All spines serrated. All setae on rami plumose. Leg 5 (Figure 12C) represented by one minute element. Leg 6 not observed.
Table 3. Armature formula of legs 1 to 4 of Dirivultus kaiko sp. nov., adult female. Arabic numbers = number of setae, Roman numbers = number of spines

Allotype adult male: body (Figure 12D) cyclopiform, 1180 long, composed of cephalothorax, second to fifth pedigerous somites, genital somite, and 4-segmented abdomen. Cephalothorax wider than long, 400 × 574. Prosome 694 long. Genital somite (Figures 12D, E & 13A) wider than long, 144 × 217. Abdomen (Figure 12D, E) composed of four free somites, 109 × 146, 90 × 131, 81 × 113, and 77 × 109, respectively. Caudal rami (Figure 12D, E) 1.80 times longer than wide, 86 × 48, with six setae on distal tip.
Antennule (Figure 13B–D) 12-segmented; armature formula 1, 12, 3, 4, 2, 2, 4, 2, 2, 2, 1 + 1 aesthetasc, and 12; all setae naked; second segment bearing long recurved process directed posteromedially (Figure 13D); third segment bearing bifid process on posterior margin (Figure 13C); fourth and fifth segments bearing digitiform processes on posterior margins (Figure 13C); fifth segment bearing distal pointed corner. Antenna, mandible, maxillule, maxilla, and legs 1 to 4 as in female. Maxilliped as in female except syncoxa with two processes (i.e. lacking second small pointed process shown on female).
Leg 5 (Figure 13A) composed of protopod incorporated in fifth pedigerous somite with outer naked seta and unsegmented exopod bearing two spatulate terminal setae. Leg 6 (Figure 13A) represented by genital flap bearing blunt distal spine and outer naked seta.
Variability: morphology of the female paratypes as in the holotype. The measurements of the body parts (N = 3) are as follows: body length, 1071–1327 (1211 ± 130); cephalothorax wider than long, 417–457 (432 ± 22) × 492–535 (515 ± 22); prosome length, 772–898 (837 ± 63); genital double somite wider than long, 117–165 (148 ± 27) × 242–247 (244 ± 2); first and second urosomites and anal somite length and width as follows, 60–83 (71 ± 12) × 115–123 (117 ± 5), 51–80 (67 ± 15) × 108–109 (109 ± 0), and 79–103 (93 ± 12) × 92–105 (98 ± 6); caudal ramus 2.18–2.33 (2.27 ± 0.08) times longer than wide, 83–89 (86 ± 3) × 37–39 (38 ± 1). Morphology of the male paratypes as in the allotype. The measurements of the body parts (N = 2) are as follows: body length, 1309–1320 (1314 ± 8); cephalothorax wider than long, 409–466 (438 ± 40) × 586–597 (591 ± 8); prosome length, 757–802 (780 ± 32); genital somite wider than long, 148–159 (153 ± 8) × 215–220 (218 ± 3); first to third urosomites and anal somite length and width as follows, 98–120 (109 ± 15) × 150–154 (152 ± 3), 102–105 (103 ± 2) × 122–131 (126 ± 7), 77–92 (85 ± 11) × 113–114 (114 ± 0) and 83–91 (93 ± 12) × 106–108 (107 ± 1); caudal ramus 1.83–2.19 (2.01 ± 0.25) times longer than wide, 81–94 (88 ± 9) × 43–44 (44 ± 1).
Remarks
So far, two congeners, Dirivultus dentaneus Humes & Dojiri, Reference Humes and Dojiri1981 and D. spinigulatus Humes, Reference Humes1999, were described based on both sexes (Humes & Dojiri, Reference Humes and Dojiri1981; Humes, Reference Humes1999). Dirivultus kaiko sp. nov. is clearly separated from those congeners by many characters (Table 4). In particular, antennules of both sexes with large recurved process directed posteromedially on the second segment are useful, only the males of D. dentaneus share a similar process on the sixth segment. In addition, these three congeners can be distinguished even by egg sac: with uniseriate eggs on D. spinigulatus, single egg on D. dentaneus and multiseriate eggs on the new species.
Table 4. Major distinguishing characters of adult stages of the three species of Dirivultus Humes & Dojiri, 1981 constructed after Humes & Dojiri (Reference Humes and Dojiri1981) and Humes (Reference Humes1999)

Newly established Japanese name: ‘Haori-kudakuchi-mijinko-zoku’ and ‘Haori-kudakuchi-mijinko’ for the genus and species, respectively. Those names allude to the Japanese name of siboglinid tubeworms ‘Haori-mushi’.
Discussion
Stygiopontius senckenbergi was originally described based on two adult males from the New Ireland Fore-Arc system, Papua New Guinea (Ivanenko & Ferrari, Reference Ivanenko and Ferrari2013). The present study represents the second record of the species as well as the first discovery of its adult female, nauplius I and copepodid IV. Now, both sexes have been described on 11 species of Stygiopontius (S. brevispina, S. cladarus Humes, Reference Humes1996, S. geminus Lee, Kim & Kim, Reference Lee, Kim and Kim2020, S. horridus, S. lauensis, S. quadripaxillifer Lee, Kim & Kim, Reference Lee, Kim and Kim2020, S. quadrispinosus Humes, Reference Humes1987, S. regius Humes, Reference Humes1996, S. senckenbergi, S. senokuchiae and S. serratus Humes, Reference Humes1996) (Humes, Reference Humes1987, Reference Humes1991, Reference Humes1996; Ivanenko et al., Reference Ivanenko, Defaye, Segonzac, Khripounoff, Sarrazin and Ferrari2011; Uyeno et al., Reference Uyeno, Watanabe and Shimanaga2018; Lee et al., Reference Lee, Kim and Kim2020; present study). In almost all of these congeners, sexual dimorphism is distinctly shown on the antennule and legs 2 and 5. Three or four setae on the outer margin of the third endopodal segment of leg 2 are replaced into spines in males, except in S. horridus and S. regius. Stygiopontius horridus shows minor differences in leg 1. In the female, leg 5 is elongate and situated laterally, and its proximal segment is distinctly separated from the pedigerous somite. That of the male is short, situated ventrally, and its proximal segment is incorporated to the pedigerous somite.
The male antennule of Stygiopontius is geniculated between the penultimate and antepenultimate segments. During precopulatory mate guarding, the male of S. quadrispinosus uses the antennule to grasp the anterior portion of the urosome of both adult and copepodid V females (Huys & Boxshall, Reference Huys and Boxshall1991). In this study, the adult male of S. senckenbergi was attached on the second urosomite of the copepodid IV by the antennules (Figure 9A–C). A stout serrated seta on the fourth segment works to pinch the dorsal side of the abdomen of the copepodid.
The general body form and armature of the nauplius I of S. senckenbergi are similar to those of S. pectinatus (see Ivanenko et al., Reference Ivanenko, Martínez Arbizu and Stecher2007). As in S. pectinatus, the nauplius I of S. senckenbergi has characteristics typical of lethithotrophy, i.e. hairs are absent on the antennal coxa in the masticatory process and an oral opening with a labrum is not observed. The antenna, mandible, maxillule and maxilla of the copepodid IV are basically similar to those of adults. The antennule is also similar to that of the adult female, but there are fewer setae on the proximal to third segments. In the order Siphonostomatoida, sexual dimorphisms are generally observed from copepodid V (see Ivanenko et al., Reference Ivanenko, Ferrari and Smurov2001; Ivanenko & Ferrari, Reference Ivanenko and Ferrari2003b), except in several taxa e.g. Pennellidae which shows sexual dimorphism from copepodid II or III (chalimus I or II) on the maxilliped (Schram, Reference Schram1979; Perkins, Reference Perkins1983; Ismail et al., Reference Ismail, Ohtsuka, Venmathi Maran, Tasumi, Zaleha and Yamashita2013). Copepod IV is probably female, because adult males grasp individuals as is done in precopulatory mate guarding.
In this study, specimens of both sexes of S. senckenbergi were found on the ventral setae of the deep-sea squat lobster Shinkaia crosnieri which are covered with filamentous bacteria (Figure 1D, E). Although the instances of symbiosis by invertebrates are poorly known in hydrothermal vent fields, the members of the family Dirivultidae are associated with a variety of invertebrates (Boxshall & Halsey, Reference Boxshall and Halsey2004; Gollner et al., Reference Gollner, Ivanenko, Martínez Arbizu and Bright2010). Crustacean hosts including alvinocaridid shrimps, Rimicaris spp. and the bythograeid crab Bythograea thermydron Williams, 1980 have been reported (Humes, Reference Humes1987, Reference Humes1996; Humes & Lutz, Reference Humes and Lutz1994; Ivanenko et al., Reference Ivanenko, Martínez Arbizu and Stecher2006). Stygiopontius senckenbergi was originally described on the basis of specimens found without hosts in sediment samples collected by box-core from the New Ireland Fore-Arc system, Papua New Guinea (Ivanenko & Ferrari, Reference Ivanenko and Ferrari2013). Shinkaia crosnieri is distributed in hydrothermal vent fields off the Bismarck Archipelago, Papua New Guinea, close to the type locality of S. senckenbergi mentioned above (Baba & Williams, Reference Baba and Williams1998). The discovery of the host of S. senckenbergi in this study represents the first record of a dirivultid from Shinkaia crosnieri.
All three species of Dirivultus were found on the tentacular crowns of siboglinid tubeworms from the Pacific Ocean (D. dentaneus from Lamellibrachia barhami Webb, 1969 off southern California, eastern North Pacific; D. spinigulatus from an unidentified host close to Escarpia Jones, 1985 off Papua New Guinea, western South Pacific; and D. kaiko sp. nov. from L. columna in the Okinawa Trough, western North Pacific) (Humes & Dojiri, Reference Humes and Dojiri1981; Humes, Reference Humes1999; present study, Figure 1H, I). However, there are limited records of copepods associated with siboglinid tubeworms. Members of the family Dirivultididae have been found in wash residues from siboglinid tubeworms and associated invertebrates, except for species of Chasmatopontius Humes, 1980 and Rimipontius Humes, 1996 (e.g. Humes, Reference Humes1987, Reference Humes1989; Ivanenko & Ferrari, Reference Ivanenko and Ferrari2003a; Gollner et al., Reference Gollner, Ivanenko, Martínez Arbizu and Bright2010). Of those species found in association with siboglinid tubeworms, only three species of Dirivultus, as well as Ceuthoecetes aliger Humes & Dojiri, Reference Humes and Dojiri1980, have been recorded from the tentacular crowns (Humes & Dojiri, Reference Humes and Dojiri1980; Humes, Reference Humes1987, Reference Humes1999; present study). In other families, Tychidion guyanense Humes, Reference Humes1973 (Cyclopoida: Erebonasteridae) was found from the same site as Lamellibrachia luymesi van der Land & Nørrevang, 1975 (= Lamellibrachia sp.) off Guyana, North Atlantic (Humes, Reference Humes1973, Reference Humes1984).
As a result of the present study, three species of the family Dirivultidae have now been recorded from Japanese waters since S. senokuchiae was reported from Izu-Bonin Arc (Uyeno et al., Reference Uyeno, Watanabe and Shimanaga2018).
Acknowledgements
We are grateful to the captains, crews and operation teams of the R/V ‘Kairei’, the ROV ‘Kaiko’, the R/V ‘Natsushima’ and the ROV ‘Hyper-Dolphin’ (JAMSTEC); to Akihiro Okamoto for providing decapod and annelid samples collected for the project ‘Isolation of host nonspecific viruses targeting harmful bacteria toward environmental and medical technology’ carried out during KR18-15; and to Chong Chen, Katsunori Fujikura, Shinsuke Kawagucci, Junichi Miyazaki, Ken Takai and Shinji Tsuchida (JAMSTEC) for their help in collecting other materials; Jenna Moore (Dauphin Island Sea Lab) for her kindness in correcting the English of this manuscript. This study is an outcome of the project directed by the second author, ‘Universal adaptive traits for extreme environment’.
Financial support
This study was partly funded by the Japan Society for Promotion of Science (JSPS) [KAKENHI grant numbers JP17H01913 for the first author, and JP16K18597 for the second author]; ‘Establishment of Global Research and Education Network in the Amami Islands’ project of Kagoshima University adopted by the Ministry of Education, Culture, Sports, Science and Technology, Japan.



















