INTRODUCTION
Ontogenetic changes in osmoregulatory ability (Charmantier et al., Reference Charmantier, Giménez, Charmantier-Daures and Anger2002), air exposure resistance, diet choices, predator avoidance (Hunt & Scheibling, Reference Hunt and Scheibling1997) and reproductive behaviour (Carr et al., Reference Carr, Tankersley, Hench, Forward and Luettich2004) may cause variations in habitat use during the life cycle of organisms (Etherington et al., Reference Etherington, Eggleston and Stockhausen2003; Lipcius et al., Reference Lipcius, Rochelle, Seitz, Seebo and Colon-Carrion2005). In addition, structured habitats may affect these habitat choices influencing possible interactions with other species or ages (Hines et al., Reference Hines, Lipcius and Haddon1987). Generally, vegetated intertidal areas, such as mangroves, seagrass beds or marshes, are important refuges or nursery habitats for many species (Beck et al., Reference Beck, Heck, Able, Childers, Eggleston, Gillanders, Halpern, Hays, Hoshino, Minello, Orth, Sheridan and Weinstein2001). Several species with complex life cycles depend upon these areas which enhance survival by diminishing predation and increasing food availability in comparison with non-vegetated areas (Beck et al., Reference Beck, Heck, Able, Childers, Eggleston, Gillanders, Halpern, Hays, Hoshino, Minello, Orth, Sheridan and Weinstein2001; Heck et al., Reference Heck, Hays and Orth2003; Minello et al., Reference Minello, Able, Weinstein and Hays2003).
Many crustaceans with spatial segregation of different size-classes may present differential body coloration depending on the habitat they inhabit. Either diet changes or predator avoidance, can promote phenotypic effects upon individuals. Some shrimps, for example, change their colour with different diets (Arredondo-Figueroa et al., Reference Arredondo-Figueroa, Pedroza-Islas, Ponce-Palafox and Vernon-Carter2003). Because there is an ontogenetic diet shift, juveniles and adults are easily recognized (Sagardes et al., Reference Sagardes, Castillo and Segonzac2000). The colour of the green crab Carcinus maenas is associated with intermoult duration because of pigment denaturalization (Reid et al., Reference Reid, Abelló, Kaiser and Warman1997). There is also spatial segregation in this case, because crabs moulting less often are more tolerant to dissecation, inhabiting in higher zones of the intertidal (McGaw & Naylor, Reference McGaw and Naylor1992; Reid et al., Reference Reid, Abelló, Kaiser and Warman1997; Styrishave et al., Reference Styrishave, Rewitz and Andersen2004). Colour also plays a key role in social behaviour. In several species, colour is related to reproductive success, directly by female choice, or indirectly as a visual signal of territoriality (Reid et al., Reference Reid, Abelló, Kaiser and Warman1997; Detto et al., Reference Detto, Zeil, Magrath and Hunt2004; Styrishave et al., Reference Styrishave, Rewitz and Andersen2004).
Colour in decapod crustaceans is determined by the number, types and distribution of chromatophores and by the carotenoid astaxanthin incorporated into the exoskeleton (Thurman, Reference Thurman and Wicksten1990). Since crustaceans are unable to synthesize carotenoids, they must acquire them from their diet (Sagardes et al., Reference Sagardes, Castillo and Segonzac2000) and thus, a diet change can modify individual coloration (Rao, Reference Rao, Bliss and Mantel1985). Carotenoids in crustaceans are assumed to have various functions being the most common cryptic coloration to avoid predation (Rao, Reference Rao, Bliss and Mantel1985; Thurman, Reference Thurman1988). Several crustaceans incorporate the appropriate colour by changing their diets throughout successive moults (Rao, Reference Rao, Bliss and Mantel1985). This also leads to differential coloration depending on the habitat. Among the functions proposed for carotenoids are thermoregulation (Silbiger & Munguia, Reference Silbiger and Munguia2008) and photoprotection to UV radiation (Moeller et al., Reference Moeller, Gilroy, Williamson and Grad2005), blocking light and the chemical damage to DNA (Edge et al., Reference Edge, McGarvey and Truscott1997). For this reason, we hypothesize that crustaceans that inhabit intertidal estuaries and use vegetated zones such as marshes, mangroves or seagrass beds during part of their life cycle may change their coloration either by changes in diet, UV intensity or any physical change that could modify moult rate.
Intertidal zones of estuaries and embayments in the south-western Atlantic (southern Brazil to central Argentina) are dominated by the burrowing grapsoid crab Neohelice (Chasmagnathus) granulata (e.g. Boschi, Reference Boschi1964; Spivak et al., Reference Spivak, Anger, Luppi, Bas and Ismael1994; Iribarne et al., Reference Iribarne, Bortolus and Botto1997) which are characterized by extensive mudflats surrounded by salt marshes dominated by Spartina spp. (Isacch et al., Reference Isacch, Costa, Rodríguez-Gallego, Conde, Escapa, Gagliardini and Iribarne2006). Neohelice granulata reaches 40 mm carapace width (CW) and their physiological adaptations allow them to occupy the uppermost parts of the intertidal from salt marshes to the lowest mudflat zones in the intertidal (Spivak et al., Reference Spivak, Anger, Luppi, Bas and Ismael1994; Luquet et al., Reference Luquet, Cervino, Ansaldo, Carrera Pereyra, Kocmur and Dezi1998; Halperin et al., Reference Halperin, Ansaldo, Pellerano and Luquet2000). Nevertheless, the environmental conditions in the higher part of the marsh are generally more stressful for these organisms, so densities are usually higher in the lower zones (Spivak et al., Reference Spivak, Anger, Luppi, Bas and Ismael1994; Bortolus et al., Reference Bortolus, Schwindt and Iribarne2002). In the high marsh, vegetation ameliorates the physical stress caused by high temperatures allowing crabs to colonize this area during the summer season (Bortolus et al., Reference Bortolus, Schwindt and Iribarne2002). Crabs are primarily deposit feeders in mud flats and herbivorous–detritivorous in the salt marsh (Iribarne et al., Reference Iribarne, Bortolus and Botto1997; Botto et al., Reference Botto, Valiela, Iribarne, Martinetto and Alberti2005), with Spartina spp. their main food source (Botto et al., Reference Botto, Valiela, Iribarne, Martinetto and Alberti2005). In addition to these differences between habitats and diet crab changes, because of the size-dependent spatial segregation present in this species it is common to see crabs with coloration brighter in the marsh than in the mudflat. In this research we analyse the relationship between size, zone, and intermoult length and the colour of Neohelice granulata.
MATERIALS AND METHODS
Study area
Experiments and field samples were performed in Mar Chiquita coastal lagoon (37° 46′S 57°27′W, Argentina). This is a body of brackish water (46 km2) of low tidal amplitude (~1 m) permanently connected to the sea (Reta et al., Reference Reta, Martos, Perillo, Piccolo, Ferrante and Iribarne2001). The main habitats around the lagoon are intertidal mudflats and large tidal plains irregularly flooded (10 to 15 times per month) dominated by the cordgrass Spartina densiflora (Isacch et al., Reference Isacch, Costa, Rodríguez-Gallego, Conde, Escapa, Gagliardini and Iribarne2006). The crabs Neohelice granulata are distributed in both the S. densiflora salt marsh and the intertidal mudflats generating large burrowing beds (Spivak et al., Reference Spivak, Anger, Luppi, Bas and Ismael1994; Iribarne et al., Reference Iribarne, Bortolus and Botto1997; Botto et al., Reference Botto, Valiela, Iribarne, Martinetto and Alberti2005).
Differences in crab coloration
To investigate differences in N. granulata coloration between marsh and mudflat zones, crabs were collected randomly by hand at both zones and photographed with a digital camera (following Tlusty & Hyland, Reference Tlusty and Hyland2005), analysed with Adobe Photoshop program. Each individual was dorsally and ventrally photographed with a white reference square in order to standardize colour values of the photographs. Pictures were opened in red–green–blue (RGB) colour mode, and a part of the crab could be selected giving the average value for red, green and blue from a histogram plot provided by the program. Each colour value ranged from 0 to 255, where 0 is black. Values of 255 in the 3 bands represent white and 0 black, while a pure colour (red, green or blue) is 255 for that particular colour and 0 for the other two. A total of 6 crab regions were analysed, 3 ventral zones, abdomen (Ab), sternite (St), 3rd maxilliped (Mx) and 3 dorsal zones, chela (Q), carapace (Cap) and carpus (Car). Each region of the crab was compared between marsh and mudflat zones with a tc test (t-test corrected) for unequal variances (Welch approximation tc; Zar Reference Zar1999) separately by colour bands (RGB). The tc is equal to the t-value when sample sizes are the same, but degrees of freedom decrease as the difference between variances of the 2 groups increases (Zar, Reference Zar1999). To analyse if crab size is related to coloration, the 6 regions were analysed with correlation analysis (Zar, Reference Zar1999) between colour values and crab size, separately by colour band.
Differences in crab colour between zones may be related to differences in crab size. To avoid this confounding factor, crabs from the same size-range (22–36 mm) were compared between zones. The 6 regions were compared for each colour band with tc tests (Zar, Reference Zar1999).
Intermoult length can also affect crab coloration patterns. To analyse changes in crab colour through intermoult an individual inclusion experiment was performed with cylindrical boxes (6 replicates, 40 cm diameter, 1 mm mesh size). In each cage, 1 recently moulted adult crab was included. The different crab regions were compared with tc test for dependent samples at the beginning and 15 days after the crabs moulted.
RESULTS
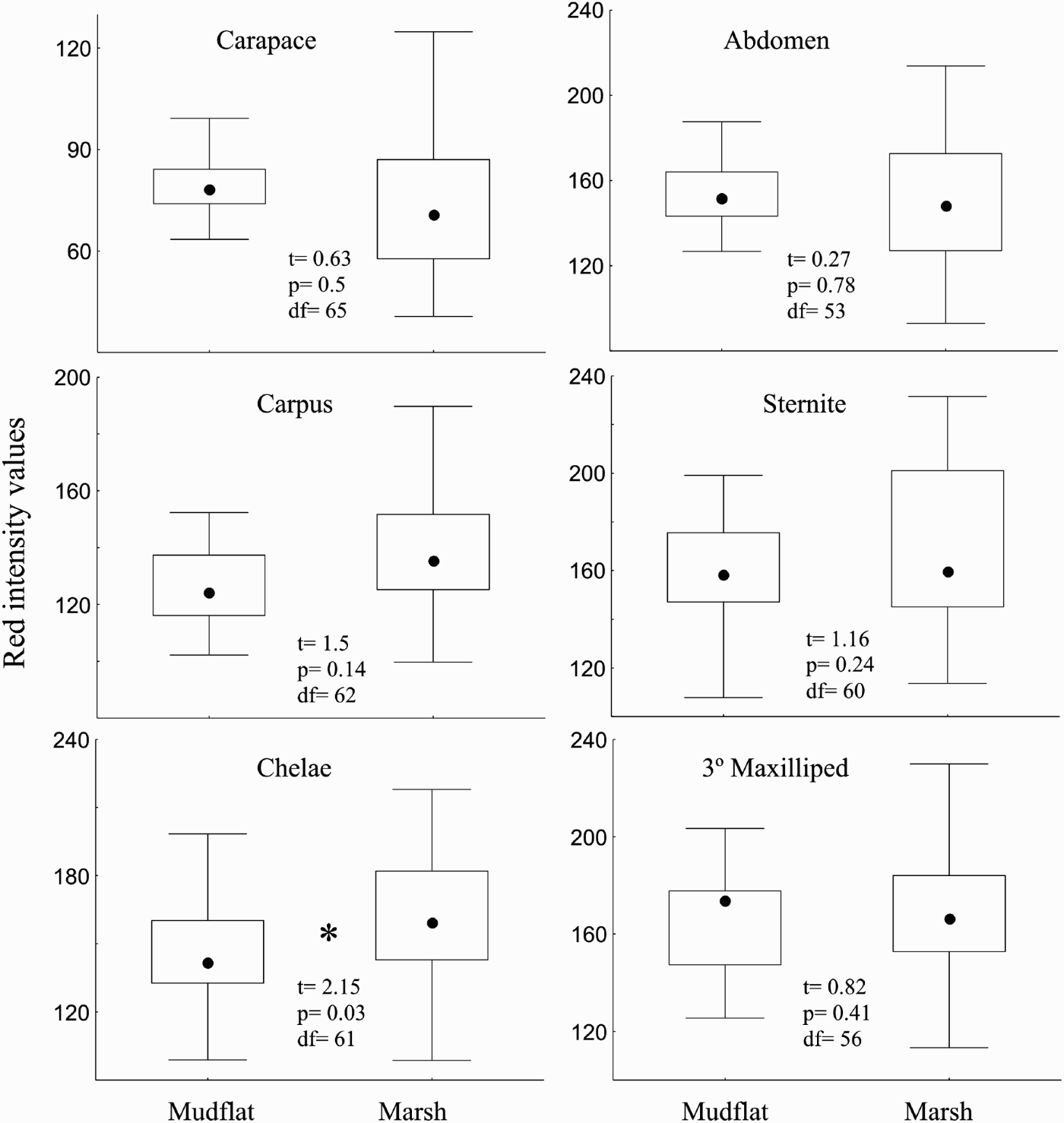
All crab regions analysed had differences in colour at least in one of the colour bands. The red value for the chela was highest in the marsh (Figure 1) while the carapace showed highest green values at the mudflat (Figure 2). The 3 dorsal regions had differences for the blue band with higher values in the mudflat than the crabs in the marsh (Figure 3) and the ventral regions had differences for green values being higher in the marsh than in the mudflat (Figure 2).
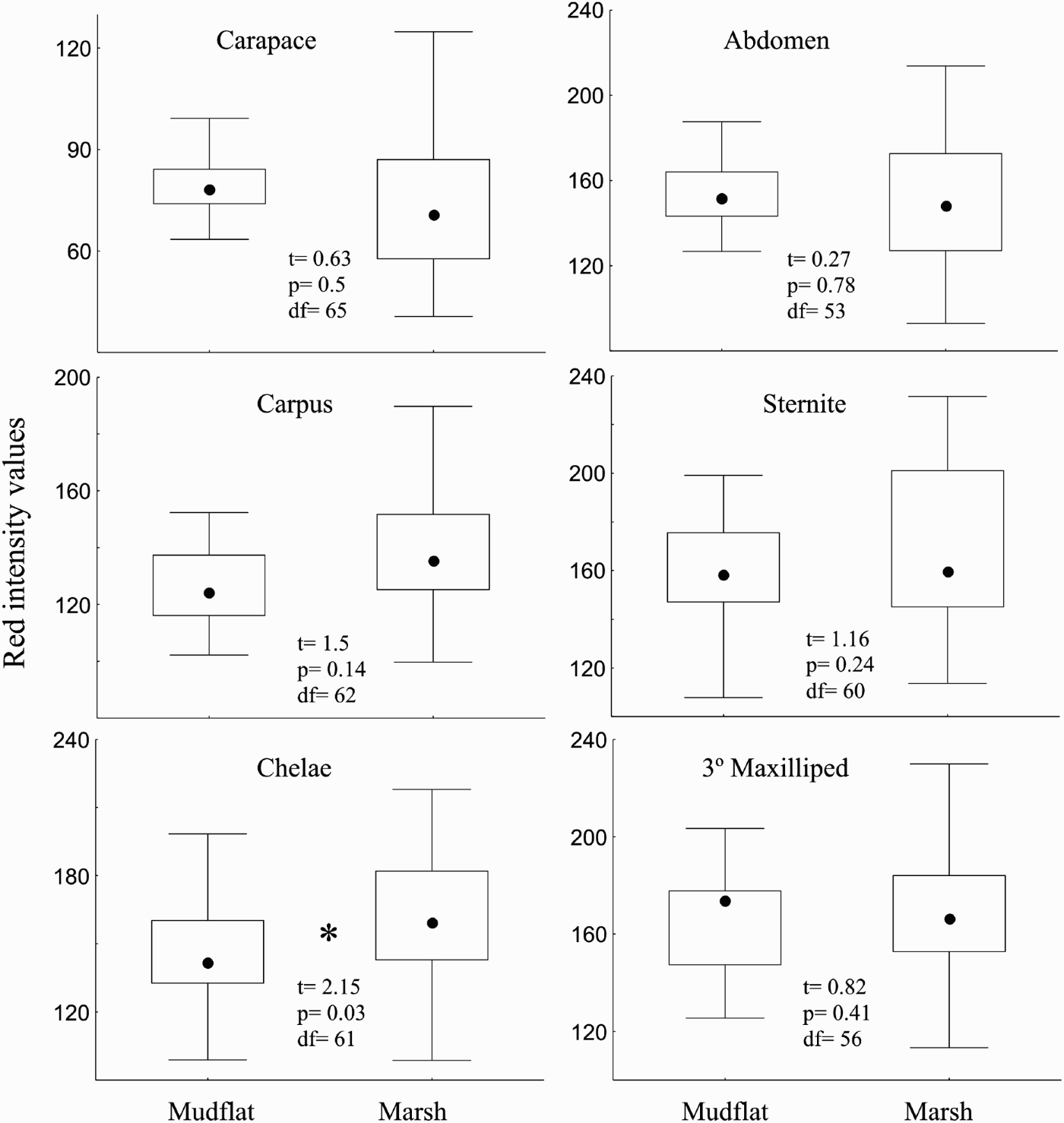
Fig. 1. Box-plots comparing intensity values for the red colour band between marsh and mudflat zones for the 6 body regions analysed. Here and thereafter box-plots are constructed with limits of boxes being the 75th and 25th percentile; lines represent 10th and 90th percentiles and squares inside boxes are medians. Inside: t-test results.

Fig. 2. Box-plots comparing intensity values for the green colour band between marsh and mudflat zones for the 6 body regions analysed, with t-test results inside.

Fig. 3. Box-plots comparing intensity values for the blue colour band between marsh and mudflat zones for the 6 body regions analysed, with t-test results inside.
Correlation analysis between colour values and size showed relationships (Table 1) for the same bands that showed differences between zones. Ventral regions showed a decrease in green values with size (Figure 4), and dorsal regions showed an increase in blue values with crab size (Figure 5). Nevertheless, the r2 values are moderate or low, explaining between 20 to 40% of the variability in colour.

Fig. 4. Correlation analysis between green colour values and crab size for the crab parts with a significant relationship.

Fig. 5. Correlation analysis between blue colour values and crab size for the crab parts with a significant relationship.
Table 1. Correlation analysis between colour values and crab size for the six crab parts analysed. St, sternite; Ab, adomen; 3° Mx, 3° maxilliped; Cap, carapace; Car, carpus; Ch, chela; r2, coefficient of determination ***P < 0.0001; **P < 0.01; *P < 0.5.

When the same size-range was considered, some of the colour differences between zones were eliminated. Green values for the abdomen and carapace regions had no differences in their values (tc Ab = 0.79, df29, P = 0.4; tc Cap = 0.44, df = 39, P = 0.6). Carapace and carpus had no differences for the blue values (tc Cap = 1.66, df = 39 P0.1; tc Car = 1.02, df = 39, P = 0.3). Green values for the sternite and the 3rd maxilliped had differences between zones (tc St = 2.65, df = 37, P = 0.01; t Mx = 2.27, df = 35, P = 0.03) as well as differences in blue values for the chela (tc = 2.3, df = 40, P = 0.02).
Crabs recently moulted and those 15 days postmoult had differences in colour. Green (tc = 2.16, P = 0.05) and red (tc = 2.3, P = 0.05) values of the carapace diminished during intermoult stage, while the sternite showed an increase in green value (tc = 2.9, P = 0.05). The other regions showed no differences along this period (all P > 0.01).
DISCUSSION
Coloration of Neohelice granulata showed differences between the low unvegetated intertidal and the high vegetated intertidal areas. Some of these differences were caused by colour changes related to crab size. There was a moderate relationship between crab size and colour; because of the spatial size segregation present in this species, this affects the coloration patterns between zones. Nevertheless, ventral zones and chela had differences between zones unrelated to size. Intermoult duration also affected crab colour in field experiments.
Colour associated with social interactions is often related to claws. Many male crabs have bright chelae which are used in aggressive interactions with other males and waving displays to attract females (Crane, Reference Crane1975; Christy & Salmon, Reference Christy and Salmon1984). Neohelice granulata showed differences in chelae colour between marsh and mudflat zone, and chelae were the body region with the highest association with size. If chelae are used in social interactions such as courtship or agonistic encounters as reported for other crab species (Uca: Crane, Reference Crane1975), colour can be a useful sign to predict the outcome of competitive disputes (Detto et al., Reference Detto, Zeil, Magrath and Hunt2004). Body size generally is a good indicator of the outcome (Huntingford et al., Reference Huntingford, Taylor, Smith and Thorpe1995), but when size differences are small, claw size is a more reliable indicator of fight outcome than body size (Sneddon et al., Reference Sneddon, Huntingford and Taylor1997). In some species, claw colour is the predominant morphological feature in identifying conspecific mates from a distance (Uca mjoebergi: Detto et al., Reference Detto, Hemmi and Backwell2008). In Heloecius cordiformis colour is also an important signal during courtship or aggressive interactions (Detto et al., Reference Detto, Zeil, Magrath and Hunt2004). In consequence, colour claw could reduce the need to approach rivals or mates to determine the likely outcome of a fight for a male or a female.
Ventral regions had colour differences between marsh and mudflat zones independently from the crab size. Since crustaceans are unable to synthesize carotenoids de novo, individuals must acquire them from their diet (Chien & Jeng, Reference Chien and Jeng1992). Because N. granulata change their diet from deposit feeder in the mudflat to herbivore in the marsh (Iribarne et al., Reference Iribarne, Bortolus and Botto1997), this diet change could be affecting pigment incorporation, resulting in a differential colour. Pigments can be extracted by crabs from both zones, since algae in the mudflat and plants in the marsh can synthesize precursors (Davenport et al., Reference Davenport, Healy, Casey and Jeffron2004) and stable isotopes were used to determine that their main source of food of crabs in mudflat areas is also Spartina (Botto et al., Reference Botto, Valiela, Iribarne, Martinetto and Alberti2005). Physical and biological conditions in each zone might affect crab colour. Plant cover in the marsh can modify colour by providing pigments through consumption and by modifying the UV intensity which alter pigments change of colour (Davenport et al., Reference Davenport, Healy, Casey and Jeffron2004). Plant canopy also reduces temperature by shading (Bortolus et al., Reference Bortolus, Schwindt and Iribarne2002). Higher temperatures generate shorter intermoult lengths by enhancing growth rates (Hartnoll, Reference Hartnoll2001). Mudflats are more frequently flooded by tides than the marsh zone, so burrow temperatures are more variable in the mudflat and more stable for longer periods in the marsh (Silva et al., Reference Silva, Luppi, Spivak and Anger2009).
There were also changes in coloration during moulting. In experiments, crabs changed coloration after moulting. The average intermoult period for the size of crab range selected for the experiment (22–24 mm) is about 150 days (ambient temperature; Luppi et al., Reference Luppi, Spivak, Bas and Anger2004). After this selection 15 differences occurred in crab colour suggesting that intermoult length could be an important factor determining crab coloration. In crustaceans, colour changes can be affected by intermoult length (Carcinus maenas: Reid et al., Reference Reid, Abelló, Kaiser and Warman1997; Styrishave et al., Reference Styrishave, Rewitz and Andersen2004), by individual age or size (Panulirus spp.: Melville-Smith et al., Reference Melville-Smith, Wing Cheng and Thomson2003; Wade et al., Reference Wade, Goulter, Wilson, Hall and Degnan2005). Smaller crabs moult more frequently than large crabs (Luppi et al., Reference Luppi, Spivak, Bas and Anger2004). Thus, in N. granulata the colour may be a combination of these factors. In other species (e.g. Uca capricornis: Detto et al., Reference Detto, Hemmi and Backwell2008) carapace coloration is determined by ontogenetic colour changes during moulting. In addition to individual identity, the ontogenetic colour changes in U. capricornis provide information about the sex, size, and reproductive status of the individual and also have the potential to signal competitive ability. Rapid colour changes in crustaceans have been emphasized in several publications (Hultgren & Stachowicz, Reference Hultgren and Stachowicz2008; for a review: Stuart-Fox & Moussalli, Reference Stuart-Fox and Moussalli2009) while slow changes have received relatively little attention. Even the effects of colour change were not evaluated in this work; the potential social significance of ontogenetic colour changes in N. granulata compared with other well-studied species such as Uca spp., highlights the importance of studying the effects of ontogenetic colour changes. This research demonstrates that colour in N. granulata is related to size, zone and intermoult duration, especially in the chela, suggesting that this body part could be an intraspecific signal.
ACKNOWLEDGEMENTS
We thank two anonymous referees and the Executive Editor, Dr Ann Pulsford for helpful comments on the manuscript. This project was supported by Universidad Nacional de Mar del Plata, ANPCyT (01272) and CONICET (PIP5669 all granted to O.I.). A.M.C. was supported by a Doctoral scholarship from CONICET.