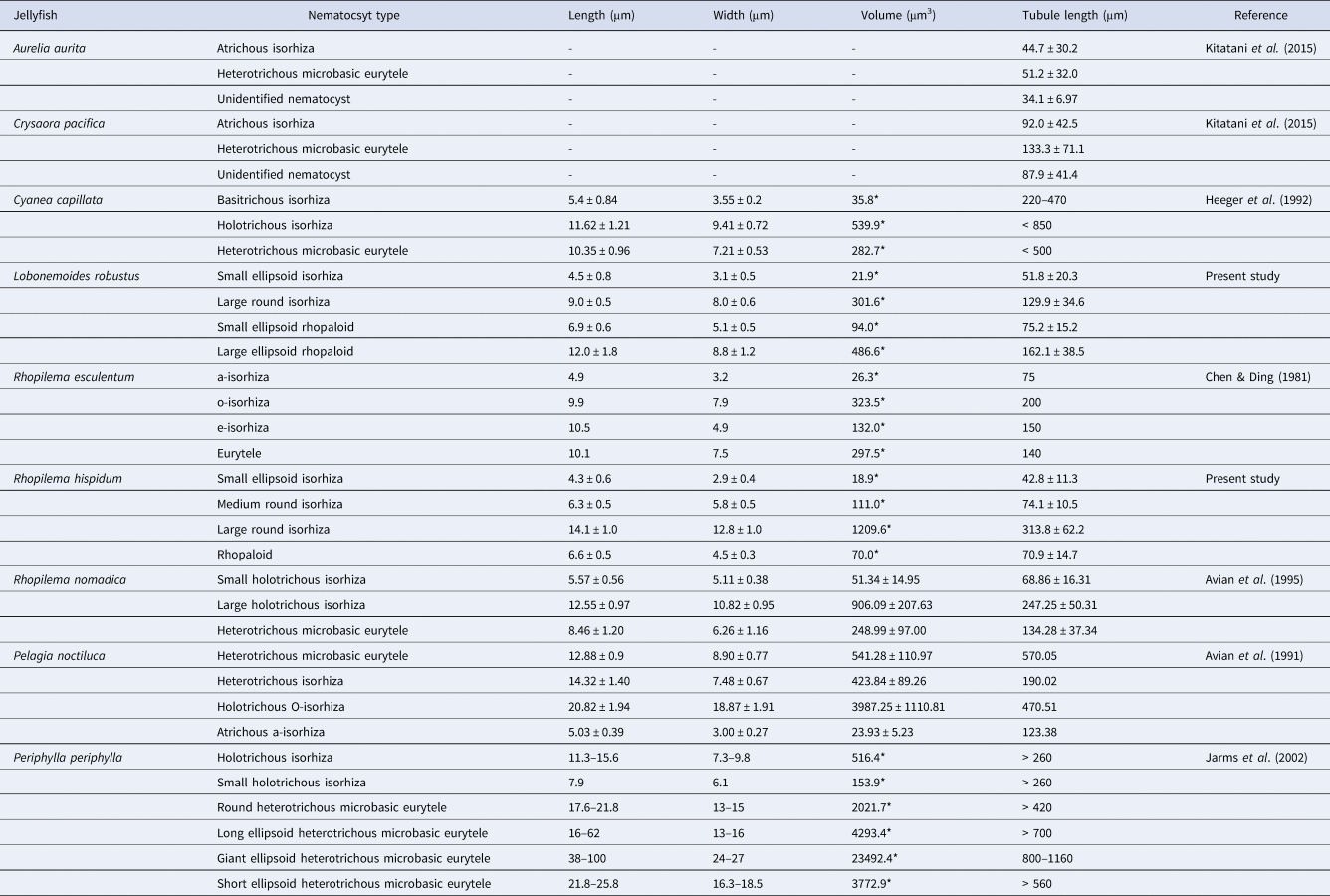
Introduction
Jellyfish are characterized by their possession of nematocysts (Mariscal, Reference Mariscal, Muscatine and Lenhoff1974; Hessinger & Lenhoff, Reference Hessinger and Lenhoff1988; Schuchert, Reference Schuchert1993; Kass-Simon & Scappaticci, Reference Kass-Simon and Scappaticci2002; Marques & Collins, Reference Marques and Collins2004; Technau et al., Reference Technau, Genikhovich, Kraus and Wanninger2015; Morandini et al., Reference Morandini, Custódio, Marques, Gopalakrishnakone, Haddad, Kem, Tubaro and Kim2016). Nematocysts consist of a capsule and an eversible tubule, and are classified into more than 30 morphological types (Östman, Reference Östman2000). Nematocysts, which contain various proteinaceous toxins, are used to capture prey, and for defence against enemies, such as medusivorous fish (Ates, Reference Ates1988; Arai, Reference Arai1997). When the jellyfish's tentacles contact a prey organism, the nematocysts discharge their tubules to sting the prey, thus injecting the toxin into the target's tissues (Burke, Reference Burke2002). Nematocysts are harmful not only to marine organisms, but also to humans; those of some species are highly dangerous and cause serious health problems to beachgoers and fishers worldwide (Purcell et al., Reference Purcell, Uye and Lo2007). In particular, many fishers suffer stings by jellyfish (Ghosh et al., Reference Ghosh, Gomes and Nag Chaudhuri1990; Al-Rubiay et al., Reference Al-Rubiay, Al-Musaoi, Alrubaiy and Al-Freje2009; Palmieri et al., Reference Palmieri, Barausse, Luisetti and Turner2014). They are stung when removing jellyfish from the fishing net during operations (Dong et al., Reference Dong, Liu and Keesing2010; Lucas et al., Reference Lucas, Gelcich, Uye, Pitt and Lucas2014; Palmieri et al., Reference Palmieri, Barausse, Luisetti and Turner2014). Victims can die in the worst cases (Li et al., Reference Li, Yu, Xue, Yue, Liu, Xing and Li2014).
Jellyfish fisheries are intensively exploited in the world's oceans (Kingsford et al., Reference Kingsford, Pitt and Gillanders2000; Omori & Nakano, Reference Omori and Nakano2001; Omori & Kitamura, Reference Omori and Kitamura2004; Nishikawa et al., Reference Nishikawa, Thu, Ha and Thu2008, Reference Nishikawa, Ohtsuka, Mulyadi, Mujiono, Lindsay, Miyamoto and Nishida2015, Reference Nishikawa, Srinui, Ohtsuka, Kondo, Miyake, Lindsay and Iida2019; Richardson et al., Reference Richardson, Bakun, Hays and Gibbons2009; Nishida & Nishikawa, Reference Nishida, Nishikawa, Nishida, Fortes and Miyazaki2011; López-Martínez & Álvarez-Tello, Reference López-Martínez and Álvarez-Tello2013; Fujii et al., Reference Fujii, Kondo, Okada, Ohtsuka, Urata, Adati, Kato, Yamaguchi, Nakaguchi, Muranaka, Yoshino and Tsutsumi2014; Gul et al., Reference Gul, Jahangir and Schiariti2015; Brotz et al., Reference Brotz, Schiariti, López-Martínez, Álvarez-Tello, Peggy Hsieh, Jones, Quiñones, Dong, Morandini, Preciado, Laaz and Mianzanet2017; Behera et al., Reference Behera, Raju, Jishnudev, Ghosh and Saravanan2020). The main target of catch is rhizostome jellyfish, which are used in Chinese cuisine (Kingsford et al., Reference Kingsford, Pitt and Gillanders2000; Omori & Nakano, Reference Omori and Nakano2001). According to the fisheries statistics of the Food and Agriculture Organization (FAO, 2018), recent annual world jellyfish catches have been more than 500,000 metric tons. China has the highest annual jellyfish catch rate worldwide, followed by the South-east Asian countries, including Thailand, Indonesia, Malaysia and Vietnam (Kingsford et al., Reference Kingsford, Pitt and Gillanders2000; Omori & Nakano, Reference Omori and Nakano2001; Nishikawa et al., Reference Nishikawa, Thu, Ha and Thu2008, Reference Nishikawa, Ohtsuka, Mulyadi, Mujiono, Lindsay, Miyamoto and Nishida2015, Reference Nishikawa, Srinui, Ohtsuka, Kondo, Miyake, Lindsay and Iida2019; Nishida & Nishikawa, Reference Nishida, Nishikawa, Nishida, Fortes and Miyazaki2011; FAO, 2018). Worldwide, at least 17 species of rhizostomes have so far been known as main target species: Acromitus hardenbergi Stiasny, 1934; Cassiopea ndrosia Agassiz & Mayer, 1899; Catostylus mosaicus (Quoy & Gaimard, 1824); Catostylus perezi Ranson, 1945; Cephea cephea (Forskål, 1775); Crambione mastigophora Maas, 1903; Crambionella annadalei Rao, 1931; Crambionella helmbiru Nishikawa, Mulyadi & Ohtsuka, 2014; Crambionella orsini (Vanhöffen, 1888); Crambionella stuhlmanni (Chun, 1896); Lobonema smithi Mayer, 1910; Lobonemoides robustus Stiasny, 1920; Nemopilema nomurai Kishinouye, 1922; Rhizostoma pulmo (Macri, 1778); Rhopilema esculentum Kishinouye, 1891; Rhopilema hispidum (Vanhöffen, 1888); Stomolophus meleagris Agassiz, 1860 (Brotz, Reference Brotz, Pauly and Zeller2016). Of these, N. nomurai and R. pulmo have been reported to cause health problems from stings to fishers (Purcell et al., Reference Purcell, Uye and Lo2007; Mariottini & Pane, Reference Mariottini and Pane2010). Rhizostome stings are generally reported as mild skin inflammation (Fenner, Reference Fenner1993); however, they can also cause severe health hazards such as erythematous eruption, oedema and burn-like injuries (Figure 1), which have been reported in Acromitus rabanchatu Annandale, 1915, Rhopilema nomadica Galil, Spanier & Ferguson, Reference Galil, Spanier and Ferguson1990, R. pulmo, R. hispidum, N. nomurai and S. meleagris (Burnett & Calton, Reference Burnett and Calton1985; Galil et al., Reference Galil, Spanier and Ferguson1990; Ghosh et al., Reference Ghosh, Gomes and Nag Chaudhuri1990; Othman et al., Reference Othman, Fathilah, Mohd Saad, Mohd Yusof, Mustaffa and Azila1996; Williamson et al., Reference Williamson, Fenner, Burnett and Rifkin1996; Kokelj & Plozzer, Reference Kokelj and Plozzer2002; Fenner, Reference Fenner2005; Kawahara et al., Reference Kawahara, Uye, Burnett and Mianzan2006; Remigante et al., Reference Remigante, Costa, Morabito, Spada, Marino and Dossena2018; present study).

Fig. 1. Dermatitis caused by Rhopilema hispidum on 1 July 2013: (A) dermatitis on the backs of a woman's hands; (B) oedema on a man's wrist. Scale bars: A, 3 cm; B, 1 cm.
In Thailand, two species are mainly targeted by fisheries: R. hispidum and L. robustus (Omori & Nakano, Reference Omori and Nakano2001; Ohtsuka et al., Reference Ohtsuka, Kondo, Sakai, Shimazu, Shimomura, Komai, Yanagi, Fujita, Nishikawa, Miyake, Venmathi-Maran, Go, Nagaguchi, Yamaguchi, Dechsakulwatana, Srinui, Putchakarn, Mulyadi, Mujiono, Md F and Yusoff2010; Nishida & Nishikawa, Reference Nishida, Nishikawa, Nishida, Fortes and Miyazaki2011; Nishikawa et al., Reference Nishikawa, Srinui, Ohtsuka, Kondo, Miyake, Lindsay and Iida2019). Both species are distributed in the Gulf of Thailand, while only L. robustus appears in the Andaman Sea (Nishikawa et al., Reference Nishikawa, Srinui, Ohtsuka, Kondo, Miyake, Lindsay and Iida2019). Othman et al. (Reference Othman, Fathilah, Mohd Saad, Mohd Yusof, Mustaffa and Azila1996) reported that R. hispidum nematocysts exhibited toxicity, haemolytic activity and a relaxant effect on phenylephrine-induced smooth muscle contractions in rat aortas. In contrast, L. robustus nematocysts have never been analysed toxicologically. This study examined the cnidomes and toxicities of these two species of commercially harvested rhizostome jellyfish in Thailand. The current information on the nematocysts and toxicities of rhizostome jellyfish is insufficient compared with that on other jellyfish (Calder, Reference Calder1972; Kawahara et al., Reference Kawahara, Uye, Burnett and Mianzan2006). The purpose of this study is to accumulate basic data to prevent sting injuries caused by jellyfish.
Materials and methods
Cnidomes
Rhopilema hispidum and L. robustus were collected from the coastal areas of Khampuan, Suksamran District, Ranong Province (9°21′43″−9°23′27″N 98°22′49″−98°23′33″E) and Nathung, Muang District, Chumphon Province (10°29′30″−10°29′52″N 99°14′42″−99°15′32″E), Thailand, on 3 and 4 December 2014, respectively (Figure 2). Each jellyfish was scooped with a 2-mm mesh scoop net with a long handle of ~1.5 m. The bell diameters and wet weights of the captured jellyfish were measured in situ immediately after collection. The marginal parts of the oral arms from each jellyfish were excised from fresh individuals, using clean scissors for cnidome analysis and toxicity bioassays.

Fig. 2. Sampling sites of jellyfish in Thailand. Open circle and closed circle indicate sampling sites of Rhopilema hispidum and Lobonemoides robustus, respectively.
To examine the cnidomes from each jellyfish species, a small piece of the oral arm was cut off with scissors and immersed in vinegar to discharge the nematocysts (Birsa et al., Reference Birsa, Verity and Lee2010), then subsequently fixed in 5% neutralized formalin/seawater. Approximately 1000 nematocysts per individual were counted and classified by type, capsule size and shape following Östman (Reference Östman2000) under an optical microscope BX53 (Olympus Corporation, Hachioji, Tokyo, Japan). The sizes and tubule lengths of the discharged nematocysts were measured using a microscope digital camera DP21 (Olympus Corporation, Hachioji, Tokyo, Japan) and image-processing software ImageJ, version 1.48 (Wayne Rasband National Institutes of Health, USA). In two species, 10 nematocysts of each type per individual were examined to measure the capsule lengths, widths and tubule lengths. The capsule size of each nematocyst type was compared using a Tukey test and Welch's t-test in R, version 3.0.1 (R Core Team, 2016). The differences in tubule lengths between R. hispidum and L. robustus were also analysed via Welch's t-test in R, version 3.0.1. Capsule volumes were estimated following Purcell (Reference Purcell1984) based on capsule length and width from previous and present studies. The correlation between capsule volume and tubule length was determined using Spearman's rank correlation coefficient test in R, version 3.0.1.
Toxicity
Marginal parts from the oral arms of the two jellyfish species were used in the toxicity bioassays. Oral arm parts were frozen on dry ice in the field, the wet weights were measured, and the arm parts were then lyophilized using a freeze-dryer FreeZone 6 (Labconco Corporation, Kansas City, USA) in the laboratory. The dry weights of the freeze-dried oral arms were recorded using an electronic scale PB602-S (Mettler Toledo International, Inc., Taito City, Tokyo, Japan), then homogenized with a spatula. The oral arm homogenate (mass ~0.10–0.25 g) was then placed in a 2-ml vial together with 1 ml of glass beads (diameter: 0.5 mm). The bottle was then filled with a 0.15 M NaCl 0.01 M phosphate buffer solution at pH 7.0. The bottle was placed in a homogenizer Mini-Beadbeater-1 (Bio Spec Products, Inc., Bartesville, USA) and run through 20 vibration cycles at 4800 rpm for 30 s, then cooled on crushed ice for 60 s. The samples were then moved to microtubes and run through two centrifugation cycles at 62,000 rpm for 30 s, then cooled for 30 s. The supernatant was recovered and diluted 1, 5, 10 and 15 times for R. hispidum and 1, 3, 5 and 7 times for L. robustus with a 0.15 M NaCl 0.01 M phosphate buffer solution at pH 7.0. For the lethality assay, the diluted solution was injected into the abdomens of three individual freshwater shrimp Palaemon paucidens De Haan, 1844 per each diluted concentration extracted from one individual jellyfish. The injection volume was calculated as 0.2 μl per 0.5 g of shrimp wet weight. Lethality (one unit) was defined as the minimum amount of venom that killed the tested shrimp within 4 h. The dilution ratio at which the shrimp died within 4 h was 5, 5, 10 times for R. hispidum and 3, 3, 3, 5 times for L. robustus, respectively. The shrimps injected with saline were used as a negative control to confirm that the shrimp did not die within 4 h. The lethality per gram of wet weight of the oral arm was formulated as follows:
Lethality per oral arm wet weight (unit g−1 wet weight) = lethality (unit)/homogenized oral arm (g) × [wet weight of fresh oral arm (g)/dry weight of freeze-dried oral arm (g)].
Differences in the toxicities between R. hispidum and L. robustus were tested via Welch's t-test in R, version 3.0.1.
Result
Cnidomes
Rhopilema hispidum cnidomes were composed of four nematocyst types: small ellipsoid isorhizas (Figure 3A, E), medium round isorhizas (Figure 3B, F), large round isorhizas (Figure 3C, G) and rhopaloids (Figure 3D, H) (Table 1). The average capsule dimensions were 4.3 ± 0.6 (length) × 2.9 ± 0.4 (width) μm (N = 30) for small ellipsoid isorhizas, 6.3 ± 0.5 × 5.8 ± 0.5 μm (N = 30) for medium round isorhizas, 14.1 ± 1.0 × 12.8 ± 1.0 μm (N = 30) for large round isorhizas, and 6.6 ± 0.5 × 4.5 ± 0.3 μm (N = 30) for rhopaloids (Figure 3A–H, Table 1). All three isorhiza capsule dimensions are significantly different (Tukey's test, P < 0.05). Tubule lengths were 42.8 ± 11.3 μm (N = 30) for small ellipsoid isorhizas, 74.1 ± 10.5 μm (N = 30) for medium round isorhizas, 313.8 ± 62.2 μm (N = 30) for large round isorhizas, and 70.9 ± 14.7 μm (N = 30) for rhopaloids (Table 1). The rhopaloids and medium round isorhizas were the major components of R. hispidum nematocysts, constituting 55.2–60.5% and 28.6–30.9%, respectively, irrespective of bell diameter (Figure 4). The small ellipsoid and large round isorhizas were less prevalent and comprised only 8.0–13.4% and 0.8–0.9% of the cnidomes, respectively (Figure 4).
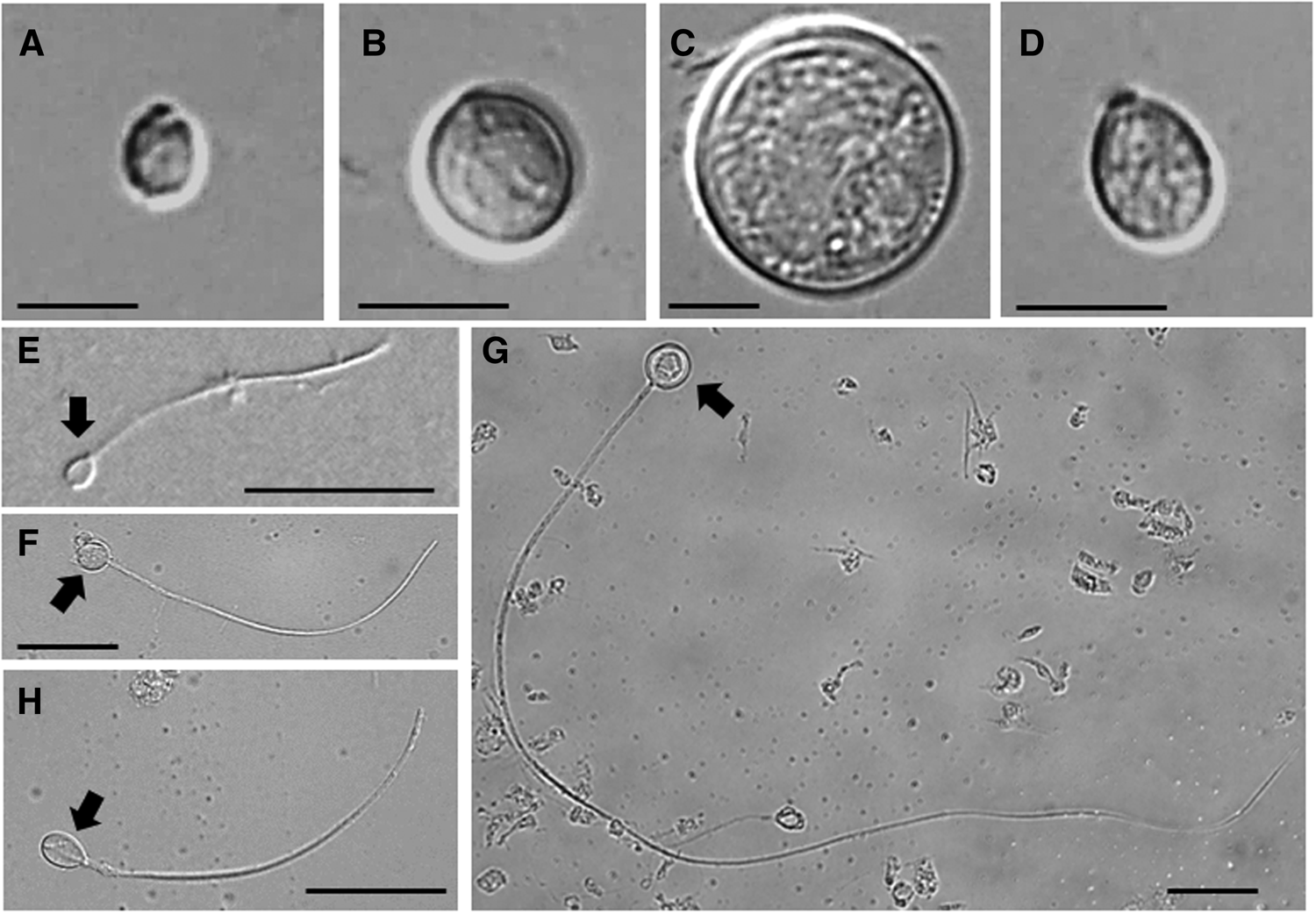
Fig. 3. Nematocysts of the oral arm of Rhopilema hispidum collected from the coastal area of Nathung, Thailand on 4 December 2014: (A) undischarged small ellipsoid isorhiza; (B) undischarged medium round isorhiza; (C) undischarged large round isorhiza; (D) undischarged rhopaloid; (E) discharged small ellipsoid isorhiza; (F) discharged medium round isorhiza; (G) discharged large round isorhiza; (H) discharged rhopaloid. Scale bars: A–D, 5 μm; E–H, 20 μm. Capsule indicated by arrow.

Fig. 4. Nematocyst compositions and proportions from Rhopilema hispidum collected from the coastal area of Nathung, Thailand on 4 December 2014.
Table 1. Size of nematocysts in the oral arms of Rhopilema hispidum.

BD, bell diameter; WW, wet weight.
Lobonemoides robustus cnidomes were also composed of four nematocyst types, but their compositions differed from those of R. hispidum: small ellipsoid isorhizas (Figure 5A, E), large round isorhizas (Figure 5B, F), small ellipsoid rhopaloids (Figure 5C, G) and large ellipsoid rhopaloids (Figure 5D, H) (Table 2). The average capsule dimensions were 4.5 ± 0.8 (length) × 3.1 ± 0.5 (width) μm (N = 40) for small ellipsoid isorhizas, 9.0 ± 0.5 × 8.0 ± 0.6 μm (N = 40) for large round isorhizas, 6.9 ± 0.6 × 5.1 ± 0.5 μm (N = 40) for small ellipsoid rhopaloids and 12.0 ± 1.8 × 8.8 ± 1.2 μm (N = 40) for large ellipsoid rhopaloids (Figure 5A–H, Table 2). There was a significant difference between the capsule dimensions of the two isorhizas (Welch's t-test, P > 0.05). The two rhopaloids are also significantly different (Welch's t-test, P > 0.05). The tubule lengths were 51.8 ± 20.3 μm (N = 40) for small ellipsoid isorhizas, 129.9 ± 34.6 μm (N = 40) for large round isorhizas, 75.2 ± 15.2 μm (N = 40) for small ellipsoid rhopaloids and 162.1 ± 38.5 μm (N = 40) for large ellipsoid rhopaloids (Table 2). The small ellipsoid rhopaloids were the most dominant nematocysts in L. robustus, constituting over 70% of the cnidome, irrespective of bell diameter (Figure 6). The small ellipsoid isorhizas and large round isorhizas comprised 6.3–12.8% and 5.9–17.0% of the total cnidome, respectively (Figure 6). The large ellipsoid rhopaloids comprised <1% of the nematocysts in the cnidome (Figure 6).
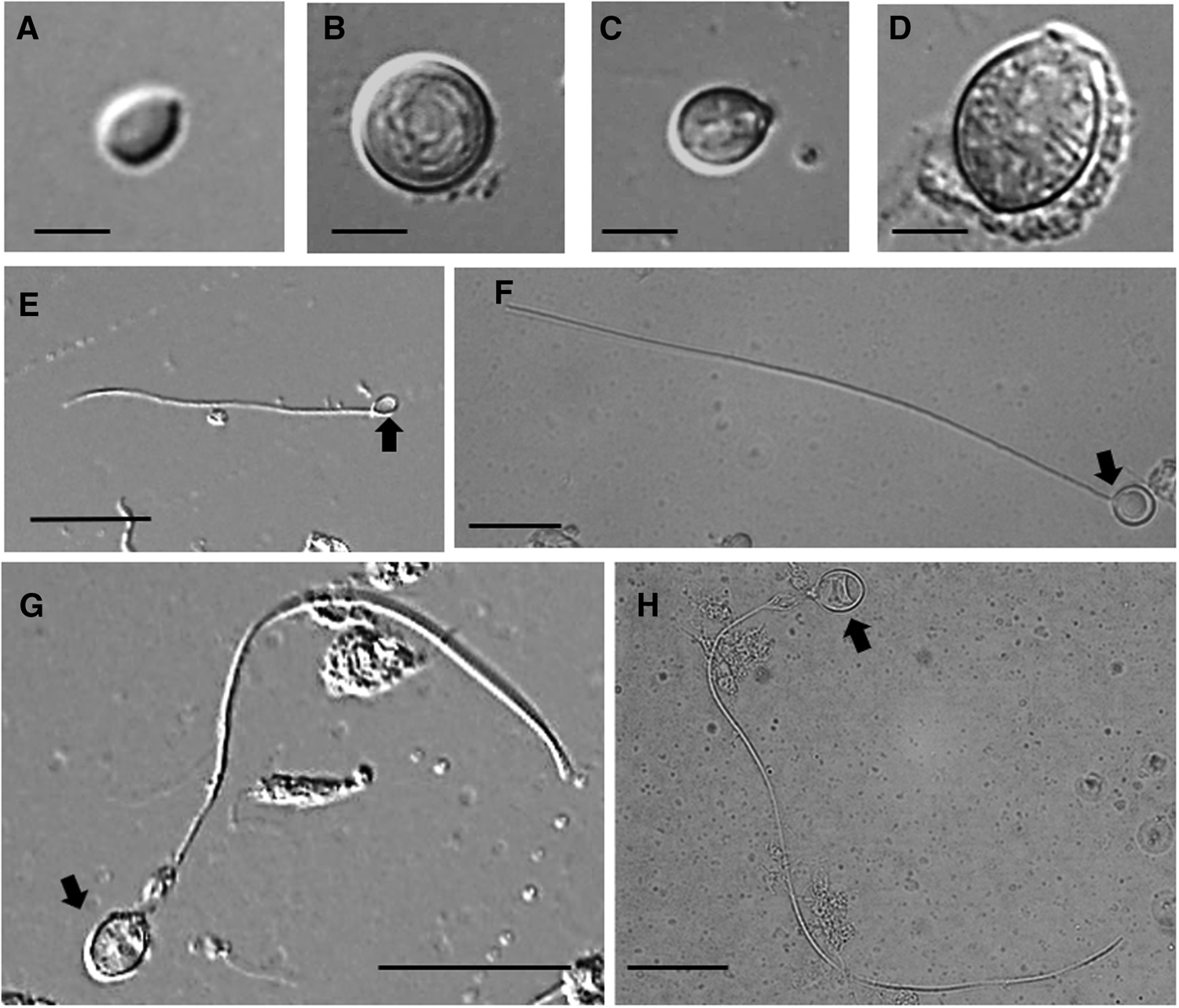
Fig. 5. Nematocysts from the oral arms of Lobonemoides robustus collected from the coastal area of Khampuan, Thailand on 3 December 2014: (A) undischarged small ellipsoid isorhiza; (B) undischarged large round isorhiza; (C) undischarged small ellipsoid rhopaloid; (D) undischarged large ellipsoid rhopaloid; (E) discharged small ellipsoid isorhiza; (F) discharged large round isorhiza; (G) discharged small ellipsoid rhopaloid; (H) discharged large ellipsoid rhopaloid. Scale bars: A–D, 5 μm; E–H, 20 μm. Capsule indicated by arrow.

Fig. 6. Nematocyst compositions and proportions of Lobonemoides robustus collected from the coastal area of Khampuan, Thailand on 3 December 2014.
Table 2. Size of nematocysts in the oral arm of Lobonemoides robustus.

BD, bell diameter; WW, wet weight.
The tubule lengths of the most dominant nematocyst types did not differ significantly between R. hispidum (rhopaloids) and L. robustus (small ellipsoid rhopaloids) (Welch's t-test, P > 0.05) (Figure 7, Tables 1 and 2). However, tubule lengths of the large round isorhizas in R. hispidum were significantly longer than those of the large ellipsoid rhopaloids in L. robustus (Welch's t-test, P < 0.05) (Figure 7, Tables 1 and 2).
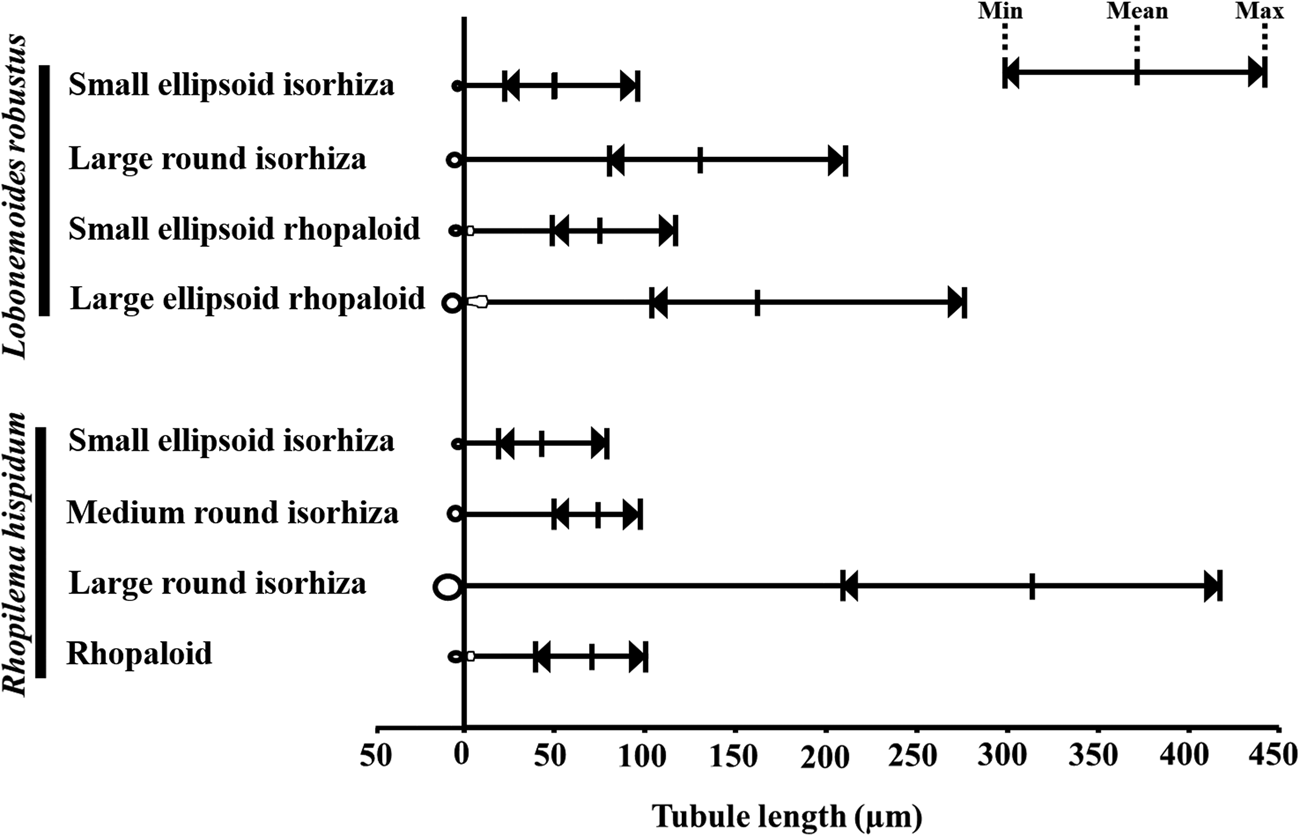
Fig. 7. Comparison of mean tubule length between Rhopilema hispidum and Lobonemoides robustus.
The capsule volume and tubule length of several scyphozoans were significantly positively correlated (Spearman's rank correlation coefficient test, P < 0.05, r = 0.82) (Figure 8, Table 3). The following equation was obtained from the relationship between capsule volume (x) and tubule length (y): y = 174.14×10−1E–04x.

Fig. 8. Correlation between capsule volume and tubule length of nematocysts.
Table 3. Types, capsule sizes, volumes and tubule lengths of scyphomedusae nematocysts.

*, Volumes estimated following Purcell (1984) based on capsule length and width.
Toxicity
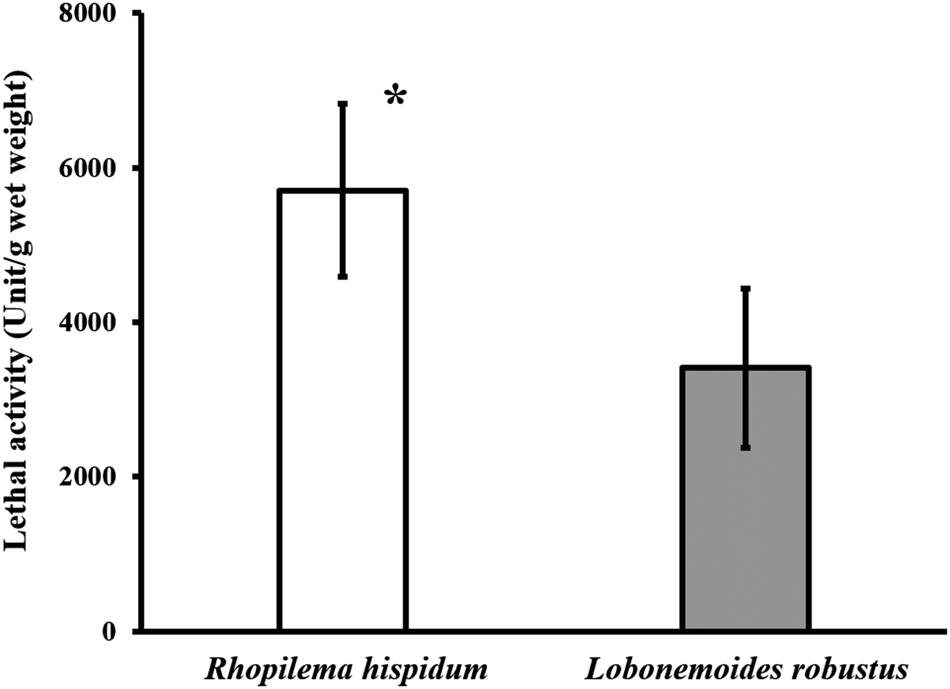
In R. hispidum, the lethality per wet weight (g) of the oral arms ranged from 5020.0–6995.6 unit g−1 wet weight (mean ± SD = 5705.3 ± 1118.1 unit g−1 wet weight, N = 3). In L. robustus, the lethal activity ranged from 2871.5–4956.6 unit g−1 wet weight (3408.3 ± 1032.9 unit g−1 wet weight, N = 4) (Figure 9). The lethal activity of R. hispidum was significantly greater than that of L. robustus (Welch's t-test, P < 0.05).
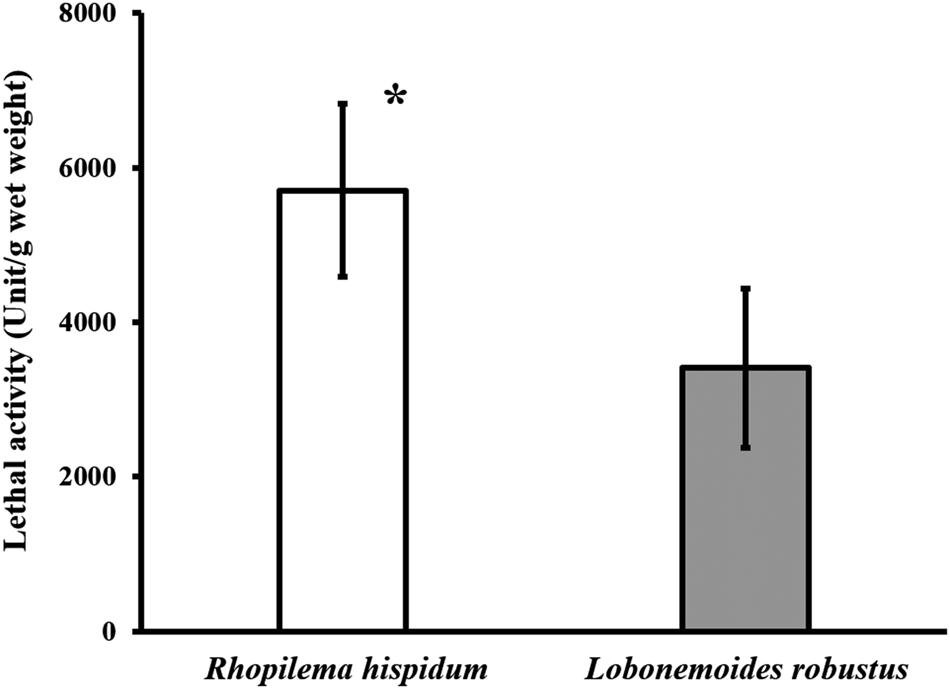
Fig. 9. Comparison of lethal activity between Rhopilema hispidum and Lobonemoides robustus. Asterisk indicates significant difference (Welch's t-test, P < 0.05).
Discussion
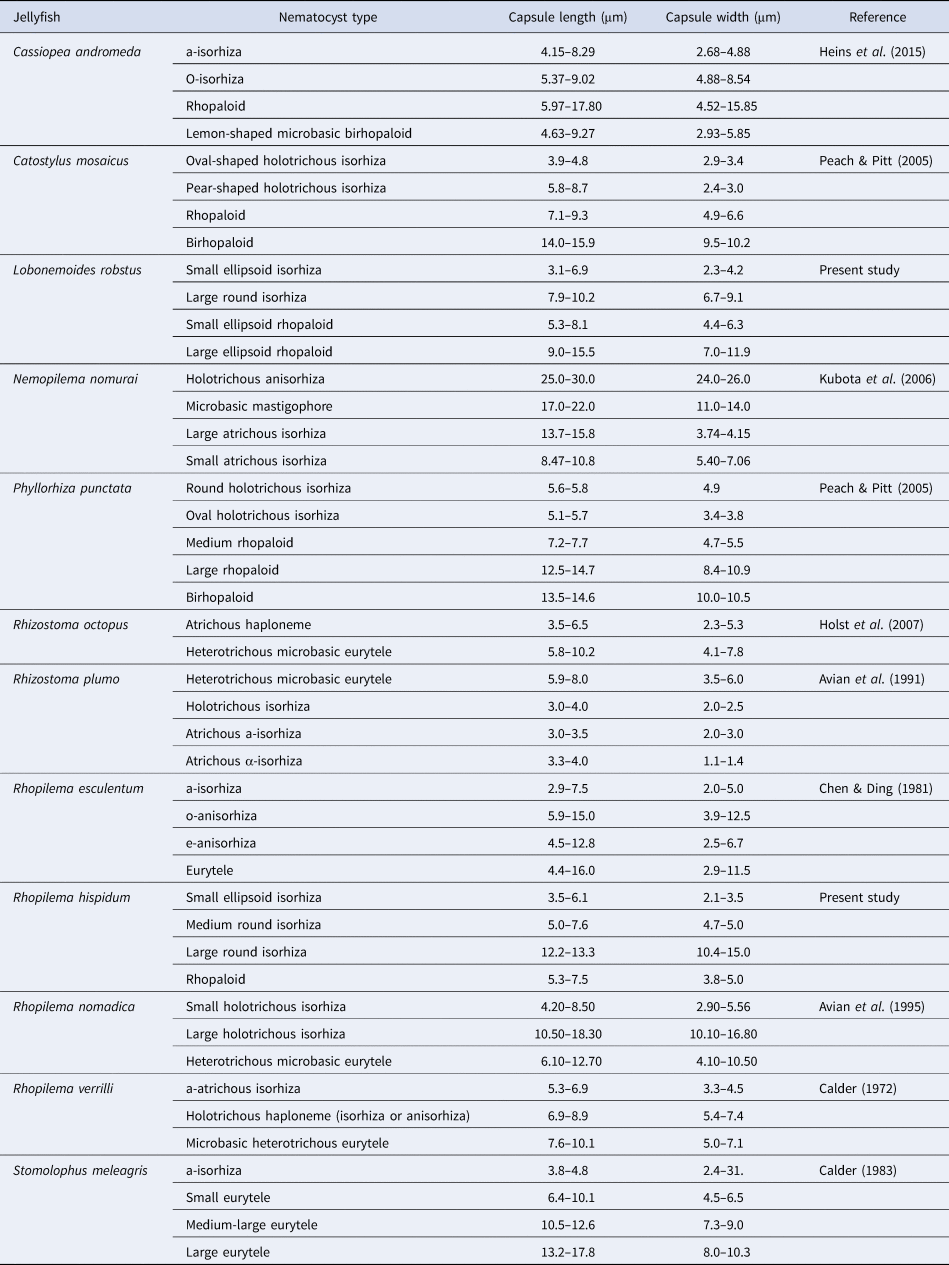
Scyphozoans, including rhizostomes, have fewer nematocyst types than do anthozoans and hydrozoans (Kubota, Reference Kubota1985; Purcell & Mills, Reference Purcell, Mills, Hessinger and Lenhoff1988). In many scyphozoans, isorhizas and rhopaloids are the main components (Arai, Reference Arai1997). Rhopilema hispidum and L. robustus cnidomes were also composed of isorhizas and rhopaloids. Rhopilema hispidum had three isorhiza types and one rhopaloid type, while L. robustus had two of each type. Othman et al. (Reference Othman, Fathilah, Mohd Saad, Mohd Yusof, Mustaffa and Azila1996) observed three nematocyst types (atrichous isorhizas, holotrichous isorhizas and heterotrichous microbasic euryteles) in R. hispidum tentacles. This differed from the nematocyst composition of the oral arm, suggesting that R. hispidum cnidomes vary among body parts. Previous studies have reported that rhizostome jellyfish have between two and five nematocyst types (Table 4). Mastigophores are rare (Table 4). Cnidomes from five species of the genus Rhopilema have been reported: Rhopilema verrilli (Fewkes, 1887) (Calder, Reference Calder1972); R. esculentum (Chen & Ding, Reference Chen and Ding1981); R. hispidum (Othman et al., Reference Othman, Fathilah, Mohd Saad, Mohd Yusof, Mustaffa and Azila1996; present study); R. nomadica (Avian et al., Reference Avian, Spanier and Galil1995). Previous and present studies have revealed that cnidomes of the genus Rhopilema mainly consist of isorhizas and rhopaloids (Table 4). The cnidome of L. robustus was first recorded in the rhizostome family, Lobonemidae. They have two nematocyst types, isorhizas and rhopaloids (present study). In both jellyfish species examined in this study, small nematocysts of >10 μm in capsule length dominated the oral arms cnidome, while relatively large nematocysts were rare. Similar cnidomes were found in other rhizostomes, such as S. meleagris (Calder, Reference Calder1983), R. nomadica (Avian et al., Reference Avian, Spanier and Galil1995), C. mosaicus and Phyllorhiza punctata von Lendenfeld, 1884 (Peach & Pitt, Reference Peach and Pitt2005).
Table 4. Capsule size of Rhizostomeae species nematocysts.

The types and tubule lengths of scyphozoan nematocysts were compiled from previous studies (Table 3). The purple jellyfish, Pelagia noctiluca (Forsskål, 1775), is a highly venomous species, with nematocysts containing tubules longer than 400 μm (Avian et al., Reference Avian, Del Negro and Rottini Sandrini1991; Mariottini et al., Reference Mariottini, Giacco and Pane2008). The lion's mane jellyfish, Cyanea capillata (Linnaeus, 1758), which occasionally causes serious damage to humans, has nematocysts with tubule lengths that reach ~850 μm (Heeger et al., Reference Heeger, Möller and Mrowietz1992). Kitatani et al. (Reference Kitatani, Yamada, Kamio and Nagai2015) clarified that nematocyst tubule length is directly correlated with pain in humans; stings from longer tubules exert more severe pain. The average longest tubules in R. hispidum (313.8 μm) and L. robustus (162.1 μm) were longer than those in the harmful Japanese sea nettle, Chrysaora pacifica (Goette, 1886) (133.3 μm) (Yasuda et al., Reference Yasuda, Ueno and Adachi2003; Kitatani et al., Reference Kitatani, Yamada, Kamio and Nagai2015; present study). Therefore, these two species of edible jellyfish can potentially cause damage in humans. Our results showed that the tubule lengths of the large round isorhizas in R. hispidum were significantly longer than those of the large ellipsoid rhopaloids of L. robustus, suggesting that R. hispidum is more harmful than L. robustus. Our lethality bioassay also showed that the toxicity of the former was approximately twice that of the latter. Rhopilema hispidum has longer tubules than do other Rhopilema species (Chen & Ding, Reference Chen and Ding1981; Avian et al., Reference Avian, Spanier and Galil1995; present study) (see Table 3). Othman et al. (Reference Othman, Fathilah, Mohd Saad, Mohd Yusof, Mustaffa and Azila1996), Williamson et al. (Reference Williamson, Fenner, Burnett and Rifkin1996) and Kawahara et al. (Reference Kawahara, Uye, Burnett and Mianzan2006) reported that R. hispidum caused considerable damage to human skin. In fact, when one of the authors and one of the aquarium staff were stung by R. hispidum in Thailand on 1 July 2013, dermatitis and oedema occurred on their hands (Figure 1). In contrast, L. robustus has not been observed to cause such severe damage since we first studied it in 2009. The nematocyst volume and tubule length were significantly positively correlated (Figure 8, Table 3). The tubule is helically coiled in the capsule before discharge (Avian et al., Reference Avian, Del Negro and Rottini Sandrini1991, Reference Avian, Spanier and Galil1995; Östman, Reference Östman2000). Purcell (Reference Purcell1984) suggested that large-volume capsules could accommodate longer tubules and could more effectively penetrate and capture prey animals. Jellyfish with large capsules and long tubules are highly likely to be dangerous species.
This study revealed the cnidomes and toxicities of two commercially harvested rhizostome jellyfish in Thailand. Rhopilema hispidum causes more severe symptoms because its toxicity is stronger than that of L. robustus. Fortunately, no fatal stings by rhizostomes have been reported in Thai waters (Fenner et al., Reference Fenner, Lippmann and Gershwin2010). Stings from rhizostomes such as Catostylus, Lobonema and Phyllorhiza are usually relatively mild (Halstead, Reference Halstead1965; Williamson et al., Reference Williamson, Fenner, Burnett and Rifkin1996; Marsh & Slack-Smith, Reference Marsh and Slack-Smith2010); however, the sting of N. nomurai has been reported to be fatal in the worst cases (Williamson et al., Reference Williamson, Fenner, Burnett and Rifkin1996; Dong et al., Reference Dong, Liu and Keesing2010; Kim et al., Reference Kim, Han and Durey2018). Further research is needed, as toxicity varies by jellyfish species. This study is the first report to investigate the toxicity and cnidome of L. robustus, but the rhizostome toxicity information is insufficient. Understanding cnidome biology, toxins and jellyfish behaviour is important in preventing stings to fishers and beachgoers.
Acknowledgements
We thank the members of Enoshima Aquarium for providing us with the pictures. We would like to express our sincere thanks to Prof. Shuhei Nishida for encouragement throughout this study.
Financial support
This study was partially supported by grants from the Japan Society of Promotion of Science (JSPS), KAKEN (grant numbers 20380110, 25304031, 26304030 and 18K05688), and the Core to Core Program (B. Asia-Africa Science Platforms).