INTRODUCTION
Marine gastropods are one of the more diverse groups, displaying a wide range of feeding mechanisms including algae grazing, suspensivory, sessile animal and detritivory feeding, parasitism and predation. All these sorts of feeding mechanisms can be found throughout various trophic levels in marine communities (Taylor et al., Reference Taylor, Morris and Taylor1980). The group of the predators is extremely diverse, comprising 5000 to 6000 species (Boss, Reference Boss1971). In the majority of the surveyed marine areas, at least half of the gastropods can be classified as predators (Taylor et al., Reference Taylor, Morris and Taylor1980). Predator gastropods are numerically important in several communities of shallow waters, most of the species belonging to families of the order Caenogastropoda, especially suborder Neogastropoda (Taylor et al., Reference Taylor, Morris and Taylor1980). The act of predation implies a complex behaviour when compared to algae feeders; it includes searching, capture, immobilization, penetration of prey and subsequent ingestion. Predators differ from other gastropods in their anatomic and behavioural features. Ponder (Reference Ponder1973) has registered various characteristics which differentiate neogastropods from the remaining gastropods. There is a modification and elaboration of the anterior digestive system and the radule, including the formation of eversible proboscides, a well developed siphon and the elaboration of an extended superficial area through repeated folding in the chemoreceptor osfradium. Most of the anatomic differences between the families of Neogastropoda are centred in feeding structures. Similar modifications were seen in superfamilies Natacacea and Tonnacea (Ponder, Reference Ponder1973). Little is known about the diet and capture mechanisms in the Volutidae family and in Argentinean neogastropods in general.
Odontocymbiola magellanica (Gmelin, 1791) (Volutidae: Odontocymbiolinae) is very common and easy reachable in north Patagonian shallow waters. The anatomy of the anterior digestive system of O. magellanica was studied by Bigatti (Reference Bigatti2005). The anterior portion of the alimentary system contains a pleurombolic proboscis and paired white accessory salivary glands (AG) that are embedded in the salivary glands (SG). Both AG and SG are situated anterior to the valve of Leiblein which prevents the secretions of the mid-oesophagus from entering the anterior oesophagus. Ducts of AG and SG are very thin and run parallel to the anterior oesophagus (Bigatti & Pastorino, unpublished). The AG ducts join at the tip of the proboscis, while the SG ducts become embedded in the anterior oesophagus at mid-length, and enter the buccal mass. Odontocymbiola magellanica is a good bioindicator of tributyltin (TBT) contamination near harbours (Bigatti & Penchaszadeh, Reference Bigatti and Penchaszadeh2005; Bigatti & Carranza, Reference Bigatti and Carranza2007; Bigatti et al., Reference Bigatti, Primost, Cledón, Averbuj, Theobald, Gerwinski, Arntz, Morriconi and Penchaszadeh2009a). Studies about O. magellanica also include age and growth (Bigatti et al., Reference Bigatti, Penchaszadeh and Cledón2007) and reproduction (Bigatti et al., Reference Bigatti, Marzinelli and Penchaszadeh2008). The species was identified by Lasta et al. (Reference Lasta, Roux and Bremec2000) as a potential fishery resource of the artisanal fleet in Patagonian coasts, whereas Bigatti & Ciocco (Reference Bigatti and Ciocco2008) reported densities and suggested fishing policies for O. magellanica and other north Patagonian volutids.
In this work we studied the diet and feeding mechanism of O. magellanica in the field and aquarium as well as the biochemical composition of the secretions from AG and the aqueous extracts of salivary and accessory salivary glands in order to determine their function in the feeding behaviour.
MATERIALS AND METHODS
Sampling site
Adults of Odontocymbiola magellanica were sampled in Golfo Nuevo, Patagonia, Argentina (42°46′S 64°59′W) during September 2004 to July 2005. Bottoms consisted of mixed gravel and sand or sandy substrates. Dispersed patches of the bivalves Aulacomya atra, Protothaca antiqua and Eurhomalea exalbida are present in sandy bottoms, where O. magellanica inhabits (Bigatti, Reference Bigatti2005). The scallop Aequipecten tehuelchus was also dispersedly present. The algal assemblage was dominated by Codium vermilara and Dictyota dichotoma in addition to other small algal species (Casas et al., Reference Casas, Scrosati and Piriz2004), mainly co-inhabited by the gastropods Buccinanops globulosus, Natica isabeleana and Tegula patagonica.
Prey sampling
The diet components of the species were identified by capturing snails with their prey and placing each snail in a labelled plastic bag. The total length of O. magellanica and their prey were measured after identification and a correlation test between predator–prey lengths was performed. The stomachs of 20 individuals captured in different times of the year were dissected to perform a stomach content study under stereoscopic microscope.
Feeding mechanism
Feeding behaviour was observed by SCUBA diving, and animals capturing their prey with the foot were observed and sampled in the field. Sequential photographs were taken underwater to illustrate the feeding mechanism.
For observations of feeding behaviour in the aquarium, snails captured in Golfo Nuevo were kept in 400 l aquaria thermostatized at 13°C and subjected to a photoperiod of 12 hours light/12 hours darkness (spring conditions). Artificial seawater, pumps, filters and aeration system were used to achieve optimal controlled conditions in culture. Salinity was checked weekly, reaching approximately 1030 ppm. A sandy and shell bottom was built to mimic the natural environment, in order to allow the development of the characteristic burial behaviour of the Volutidae family. Live bivalves and gastropods were offered to the snails in order to document the feeding mechanism.
Prey narcotization experiments
When feeding, O. magellanica releases a white and dense secretion (saliva) in the tip of the proboscis, originating in the salivary and accessory salivary glands (see Results). Experiments were performed with the bivalve Aulacomya atra (sizes 38 and 41 mm) and the gastropod Tegula patagonica (sizes 12 and 9 mm), to determine the effect of the salivary liquid of O. magellanica. Specimens were placed in plastic vials with 100 ml of seawater and kept acclimated for 1 hour in the same vial (water temperature: 18°C). Then, 4 ml of a solution containing the freshly ‘whole accessory salivary glands suspension’ (WAGS) of two O. magellanica mature individuals (between 10 and 16 cm length) was added. For the controls, only seawater was added. Fresh WAGS were obtained by cutting together the fresh AG and SG glands with a scissor and shaking it with distilled water, then the liquid plus the rest of the glands were added to the experiments. The product was a white dense liquid resembling that released by the proboscides of O. magellanica when feeding or disturbed.
Biochemical studies
The pH of several glands and secretions involved in feeding and digestion were measured: liquid secretion from the proboscides of living animals (SP), accessory salivary gland secretion (S), salivary glands extracts (SGE), accessory salivary glands extracts (AGE), Leiblein gland extracts and stomach extracts. They were measured using a digital pH meter (MV-RS 232; 0.01 unit) or pH indicator paper (Merck, range 0–14) for measurements done in the field. The secretion called ‘S’, was obtained by dissecting fresh accessory salivary glands from living individuals and placing only the pure liquid secretion contained inside (a white and dense liquid as observed in the mouth of the snails) in an Eppendorf tube (without rests of glandular tissues).
Another procedure was attempted in the laboratory to obtain the extracts of salivary (SGE) and accessory salivary glands (AGE). Namely, the gland contents were extracted in 30 ml distilled water by vigorous shaking of entire cut glands (organs) for 30 minutes. Cellular debris from glandular tissues was discarded by centrifugation at high speed. Supernatants were dialysed against distilled water during 24 hours and then freeze-dried. If precipitates appeared after dialysis, they were lyophilized separately (AGEp and SGEp).
The following analyses were performed for AGE and SGE, obtained as previously explained. Total protein content (Lowry et al., Reference Lowry, Farr and Randall1951), carbohydrate content (Dubois et al., Reference Dubois, Gilles, Hamilton, Rebers and Smith1956), hexosamines (Lane Smith & Gilkerson, Reference Lane Smith and Gilkerson1979) and uronic acids (Fillisetti-Cozzi & Carpita, Reference Fillisetti-Cozzi and Carpita1991) were determined colorimetrically. Sulphate content was estimated turbidimetrically (Dodgson & Price, Reference Dodgson and Price1962) as barium sulphate after hydrolysis with 1 M HCl (105°C, 5 hours). Identification of component monosaccharides was carried out by gas–liquid chromatography (GLC), after hydrolysis (2M trifluoroacetic acid, 120°C, 4 hours) and derivatization of samples to alditol acetates (Albersheim et al., Reference Albersheim, Nevins, English and Karr1967). GLC analysis was made using a HP Ultra 2 column (50 m × 0.32 mm, thickness of liquid phase, 0.17 µm) on a HP-5890 Gas Chromatograph equipped with a flame ionization detector. Nitrogen was used as a carrier gas (1 ml/minute). Runs were programmed starting at 160°C (held for 5 minutes), 1°C/minute to 220°C and then 2°C/minute to 250°C (held for 25 minutes) in the split mode (split ratio 1:80), while injector and detector were set at 270°C. For quantification, the peak areas were considered to be proportional to the mass of the corresponding derivative.
Identification of monosaccharides and hexosamines was aided by the use of standards and by GLC coupled to mass spectrometry. The mass spectra were performed in a Shimadzu QP 5050 coupled to a gas chromatograph GC-17A. Mass spectra were recorded over a mass range of 30–600 amu, using an ionization potential of 70 eV.
For polyacrylamide gel electrophoresis (10 and 16% polyacrylamide gels) (SDS-PAGE) (Laemmli, Reference Laemmli1970), S, SGE and AGE were loaded using a Mini-Protean II equipment (Bio-Rad). Separation was obtained by gradual voltage increment between 40 to 100V. The gels were stained with Coomasie blue R250 (Hames, Reference Hames1990), silver nitrate (Jay et al., Reference Jay, Culp and Jahnke1990) and periodic acid-Schiff (PAS) (Fairbanks et al., Reference Fairbanks, Steck and Wallach1971).
For gel permeation chromatography, a column (1.5 times 29 cm) packed with Bio-Gel P-100 equilibrated with 0.2 M acetic/acetate buffer pH 5 was employed. The sample ‘S’ was resuspended in buffer (0.5 ml) and added on top of the column. For the estimation of initial (15 ml) and final (40 ml) volumes, blue dextran and glucose were used. The elution profile was obtained reading absorbance at 280 nm in each of the fractions (1.1 ml). Fractions corresponding to the peaks were pooled, dialysed and lyophilized.
Trace cation microanalyses were performed for calcium, magnesium, potassium and sodium by atomic flame absorption in samples from freshly SG and AG separately.
RESULTS
Dietary composition
The analysis of 131 prey obtained in the field indicated that O. magellanica is a predator of the benthic community, its diet consisting of gastropods (54%) and bivalves (46%).
Gastrops ingested were mainly Tegula patagonica and Natica isabeleana, while Trophon geversianus and Buccinanops globulosus were consumed in lower percentages. Only in one case, predation on Crepidula sp. was registered (Figure 1). The main bivalve dietary item was the venerid Protothaca antiqua, followed by Ensis macha and Eurhomalea exalbida; the pectinid Aequipecten tehuelchus and the mytilid Aulacomya atra showed less relevance in diet composition (Figure 1). In sandy bottoms, where aggregation of O. magellanica occurred in the absence of other mollusc species, intraspecific cannibalism (alive individuals) was registered in 4.7% of the studied cases, i.e. between bigger and smaller specimens. Other field observations included intraspecific cannibalism with up to 3 individuals of O. magellanica feeding on the same prey (a live smaller individual). In some isolated cases, animals were registered feeding on carrion: dead molluscs with degraded tissues. No remnants of animal tissues were found in the stomach of the 20 individuals analysed; we only found a mucous mass.

Fig. 1. Percentage of prey consumed by Odontocymbiola magellanica in Golfo Nuevo shallow waters.
No correlation between prey and predator sizes was found (P of correlation: 0.385). Cases of small O. magellanica individuals feeding on big prey and vice versa were registered (Figure 2).
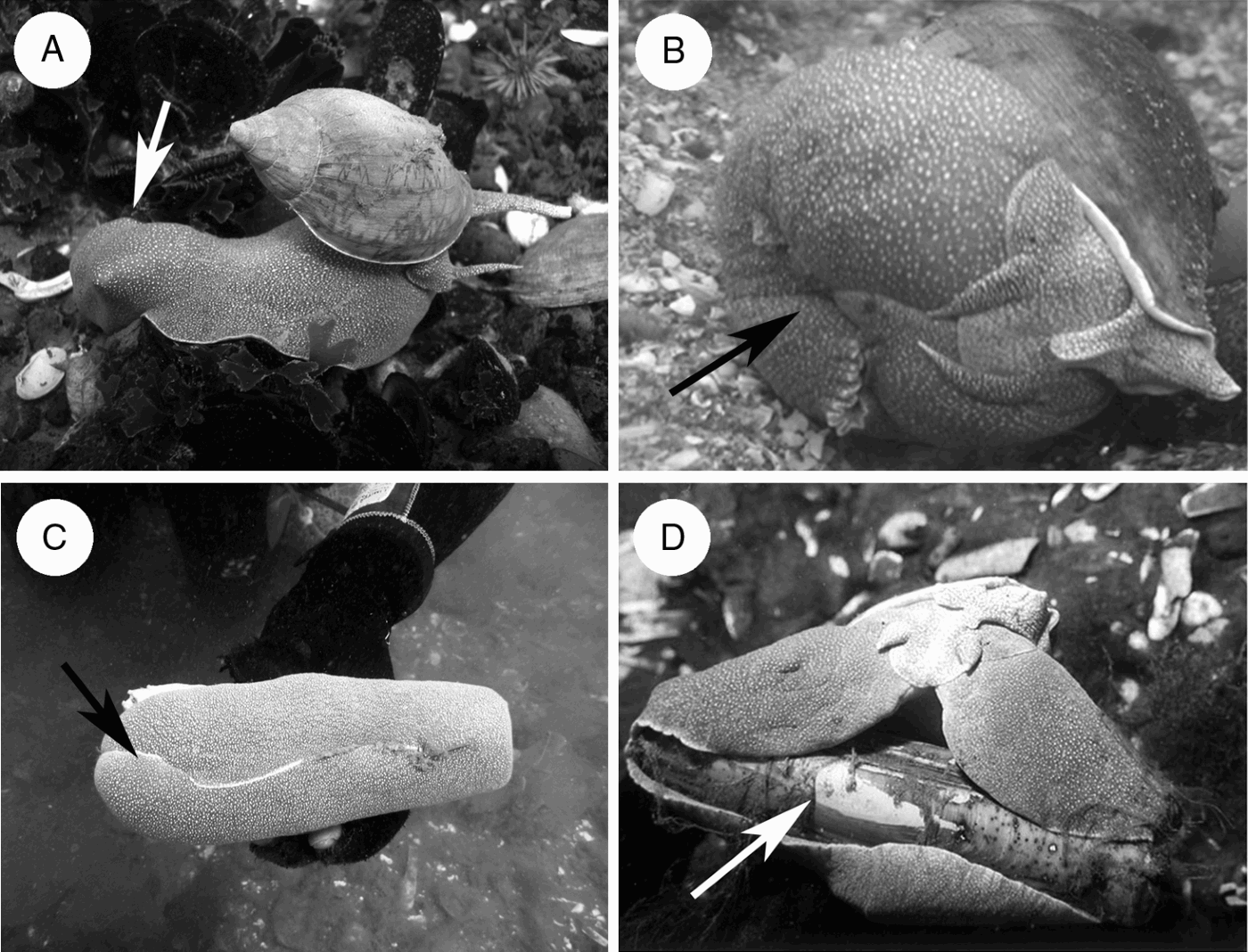
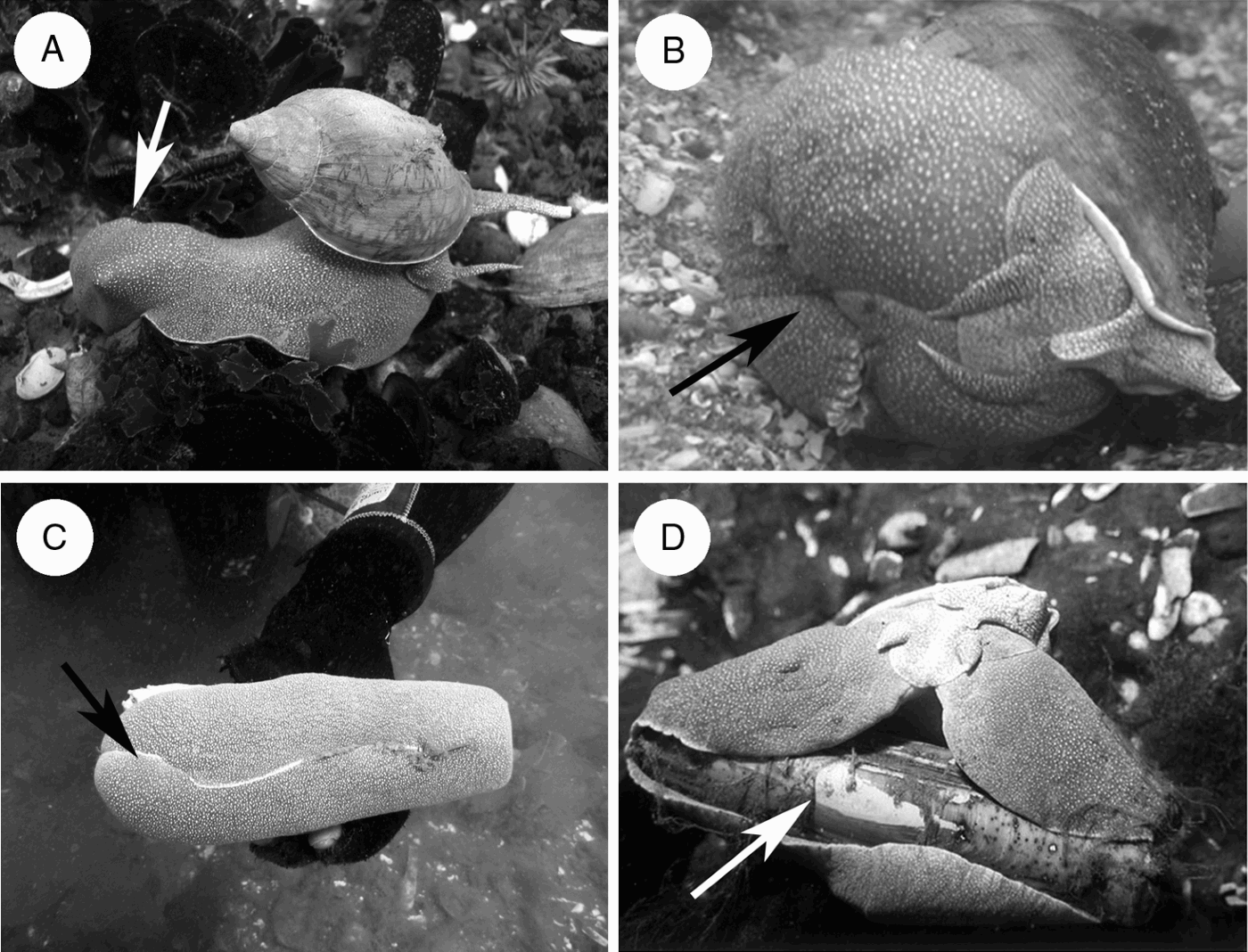
Fig. 2. Diet mechanism of Odontocymbiola magellanica (arrows indicate prey in the foot). (A) The prey is captured, immobilized in the metapodial zone and carried to soft bottoms before the narcotization; (B) during narcotization prey is strongly engulfed by the foot while saliva is continuously introduced, creating a semi sealed cavity with a pH around 10 (individual in aquarium); (C) the ‘razor clam’ Ensis macha engulfed by the foot and consumed alive (D).
Feeding mechanism
FIELD OBSERVATIONS
The observations done in the natural environment revealed that in a first step, O. magellanica captures their prey and strongly engulfed it with the metapodial zone of the foot (Figure 2A), immobilizes and transports it to sandy bottoms. The foot creates a closed cavity which is not completely isolated from its environment (Figure 2B, C). After some time prey are narcotized by saliva introduced inside this cavity by the proboscis. As a result, the bivalves opened their adductor muscles or in the case of gastropods, they lost reaction in the retraction of the foot muscles. Prey narcotization lead to lower muscular speed reaction, enabling O. magellanica to feed on living prey tissues (Figure 2D) by means of its radula. Field observations suggested that narcotization was produced due to the constant release of salivary liquid into the foot-generated cavity, where the prey was immobilized. In this cavity, water is probably not abundant, consequently enabling a proportionally higher concentration of salivary liquid.
PREY NARCOTIZATION EXPERIMENTS
We made experiments immediately after sampling the animals, using WAGS, freshly extracts of both AG and SG glands as explained before. The solution was applied to the bivalve Aulacomya atra and to the gastropod Tegula patagonica. The initial pH of the treatment was 8.63 and the final pH was 8.32. The controls maintained a constant pH of 7.82, the same as seawater. After 4 hours of WAGS solution application we observed a reduction in the muscle reactions of molluscs. The narcotization (no contraction) of the adductor muscles of the bivalve and the foot of the gastropods was produced after 15 hours of exposure to the extract. In the controls, the organisms continued alive and showed fast muscle reactions (contraction) when stimulated.
FEEDING BEHAVIOUR IN AQUARIUM
After maintaining animals in captivity during 4 years without noticeable results, we could finally observe O. magellanica feeding on one live bivalve offered. One male 100 mm long captured a scallop (Aequipecten tehuelchus) 39 mm long. When the snail was stimulated, it carried the prey by capturing it from the methapodial zone and moved. The same behaviour was observed in the field several times (Figure 2A), suggesting that the individuals capture their prey in the areas of mollusc patches, and then feed on sandy areas with lower population density. The predator was sealing its prey as shown in Figure 2B during a total of 10 hours. After that the prey were alive, their muscles were narcotized and valves could be opened easily with the hands.
Biochemical studies
pH OF THE DIGESTIVE SYSTEM
The pH of the different organs of the digestive system of 20 freshly collected animals was measured after dissection. The pH of the salivary liquid extracted from the proboscis (SP) and from the accessory salivary gland secretion (S) showed identical values than the aqueous SGE plus the AGE ranging between around 8.5–10.5 (mean = 9.76; SD = 0.56). The pH of the aqueous extract of the Leiblein gland was 6.8–7.6 (mean = 7.23; SD = 0.40); in the stomach pH varied between 7.5 and 7.6 (mean = 7.57 SD = 0.07). Following Leiblein gland, the medium and posterior oesophagus and the stomach are found. The pH of these organs was similar (around 7.5) and lower than that recorded in the area of the anterior oesophagus (around 10).
BIOCHEMICAL COMPOSITION OF SALIVARY SECRETIONS
The composition obtained by colorimetric analysis of proteins, carbohydrates, hexosamines, and uronic acids of the accessory salivary gland secretion (S), AGE and SGE is shown in Table 1. The composition of sugars obtained after hydrolysis with TFA 2M (120°C, 4 hours), and derivatization of the resulting monosaccharides to the corresponding acetylated alditols is shown in Table 2. When the entire glands were subjected to aqueous extractions, the resulting products (SGE and AGE) showed large proportions of protein (Table 1). The proportion of neutral carbohydrates was low but similar to that of amino sugars. This is also true for S, though protein proportion is much lower. The fraction AGEp did not add exactly 100%; this could be due to salt content which might have not been completely dialysed. The samples indicated presence of sulphate in trace amounts.
Table 1. Yield and composition of AG secretion (S) and products extracted from salivary (SGE) and accessory glands (AGE). 1Number between brackets indicates product batch; 2suffix s, soluble, non-dialysable product; 3suffix p, insoluble, non-dialysable product.

n.d., not determined.
Table 2. Monosaccharide composition (in moles%) of AG secretion (S) and the soluble, products extracted from salivary (SGE) and accessory glands (AGE). 3This product contains small proportions of inositol; 4SGEp only contained traces of fucose, manose, galactose and galactosamine. For nomenclature, see Table 1.

The composition of sugars obtained after hydrolysis is depicted in Table 2. In the precipitated fraction (SGEp (2) and AGEp (2)) sugar peaks could only be identified for the accessory salivary gland extract (AGEp). Except for AGEs (2), where the hexoses to hexosamine relationship was approximately 1:1, the rest of the products showed a slight predominance of hexoses (1.7–1.9:1). The detection of amino sugars suggested the presence of glycoproteins in all the products. Galactosamine content was higher in all the aqueous extracts of both glands (AGE and SGE); only accessory salivary gland secretion (S) showed a slightly higher value for glucosamine. Galactose and glucose were the main hexoses. The detection of an unusual sugar like 3-O-methylfucose in all of the analysed fractions is noteworthy. To the best of our knowledge, this sugar has been previously identified only in lipid fractions of the bivalves Corbicula sp. (Itasaka, Reference Itasaka1966) and in Hyriopsis schlegelii (Hori et al., Reference Hori, Sugita, Ando, Kuwahara, Kumauchi, Sugie and Itasaka1981).
The elution of S in Bio-Gel P-100 (Figure 3) showed only one peak, unfortunately with a very low yield (900 µg/each individual accessory salivary gland secretion (S)). This result is in agreement with the low protein content of this product (Table 1). Note that analytical characterization of the sample is far from 100%, suggesting large proportions of salts.

Fig. 3. Gel permeation chromatography of ‘S’ on Bio-Gel P-100. Vi and Vf, indicate initial and final volumes, respectively.
In agreement with the high protein content of the aqueous extracts, a fractionation in SDS-PAGE was attempted. Protein fractionation of SGs and AGs by means of SDS-PAGE (Figure 4, lanes II and III) revealed several bands with molecular weights ranging from 13,800 and 51,900 Da with major bands at 23,200 and 13,800 Da. Minor products of molecular weight 34,700 and 51,900 could also be detected. Major bands also showed a light positive glycoprotein staining. An identical band pattern was observed for both batches (1 and 2) of the aqueous extracts (AGE and SGE). On the other hand, SDS-PAGE of S (Figure 4, lane I), showed a higher proportion of bands in the 32,000–60,000 Da range; namely an important one at 37,900 Da, and three less intense bands of 48,500, 43,400 and 32,200 Da, in addition to important low molecular bands of 23,200 and 13,800 Da. Product from fractionation in Bio-Gel showed a unique broad band of approximately 53,100 Da (Figure 4, lane IV).

Fig. 4. SDS-PAGE separation. Lanes I, S; II, AGE; III, SGE; IV, S-Bio-Gel. Bars indicate Dalton Mark Molecular Weight standards. Loading lanes: 10 µL.
Microanalysis
The microanalysis of cations showed a higher calcium and magnesium concentration in the AG than in SG (Figure 5), while sodium and potassium concentration were similar for both glands. These results could suggest that a low molecular weight compound could have been lost in the dialysis process. In fact during dialysis of AG we could observe the presence of small crystals in the external media, probably CaCO3. The calcium proportion was 12 times higher in AG than SG, whereas that for magnesium was almost 5 times higher.

Fig. 5. Percentage weight/weight (%w/w) for each cation analysed in the accessory salivary gland (AG) and salivary gland (SG). Concentration of calcium and magnesium is significantly higher in the AG.
DISCUSSION
Dietary composition
The preference of mollusc prey (gastropods and bivalves) registered in Odontocymbiola magellanica is in agreement with the observations registered for other species of the family Volutidae. From nine subfamilies described by Weaver & Dupont (Reference Weaver and Dupont1970), information was found only for members of subfamilies Cymbiinae and Zidoninae. For the latter, Weaver & Dupont (Reference Weaver and Dupont1970) explain that the volutid Adelomelon beckii ‘is captured by means of hooks with bait, raising the assumption that this species is carnivore’. Bigatti et al. (Reference Bigatti, Sánchez Antelo, Miloslavich and Penchaszadeh2009b) stated that Adelomelon ancilla a sympatric volutid (from the same zone to that studied in this work) consumes mainly bivalves (88.9%), gastropods (9.5%) and, rarely sea urchins (1.6%), probably denoting a food niche separation between both species. An isotopic analysis made on Zygochlamcys patagonica beds on the coasts of northern Argentina showed that the scallop contributes to the diet of O. magellanica and A. ancilla with similar isotopic signatures. However, they are not the main prey (Botto et al., Reference Botto, Bremec, Marecos, Shcejter, Lasta and Iribarne2006). Ponder (Reference Ponder1970) observed that the volutid Alcithoe arabica is an intertidal organism, preying on molluscs such as Chione stutchbury (Veneridae), Macoma liliana (Tellinidae) and the gastropods Lunilla smaragda (Turbinidae) and Cominella adspersa (Buccinidae). Weaver & Dupont (Reference Weaver and Dupont1970) claim that Cymbiola (Aulica) aulica (subfamily Cymbiinae) is carnivore, the fishermen usually capture them with ‘hooks with bait’. Morton (Reference Morton1986) says that Thorson in his Christmas card for 1954 illustrated a specimen of Cymbium neptuni eating a venerid bivalve, covering it with the foot. Marche-Marchad (Reference Marche-Marchad1977) comments: ‘C. cymbium (Linné), C. glans (Gmelin), C. peppo (Lightfoot), C. moratum (Link) and Cymbium sp. seem to eat gastropods and lamellibranchs almost exclusively’ and illustrated a C. glans specimen of 8.2 kg ingesting a smaller individual of C. cymbium. Wilson & Gillet (Reference Wilson and Gillet1971) showed a specimen of Melo amphora feeding on another volutid, Zebramoria zebra. This photograph was then illustrated by Taylor who pointed out that ‘the gastropods members of the Volutidae family are mainly predators of other mollusks bivalves and gastropods’ (Morton, Reference Morton1986), being able to eat sessile or mobile individuals (Taylor et al., Reference Taylor, Morris and Taylor1980).
Feeding mechanism
Taylor et al. (Reference Taylor, Morris and Taylor1980) and Ponder (Reference Ponder1970) suggested that the prey captured by volutids is asphyxiated by sealing it with the posterior part of the foot. Morton (Reference Morton1986) found that the preferred prey of the volutid Melo melo were other gastropods inhabiting the same community, mainly Hemifusus tuba (Melongenidae) and Babylonia lutosa (Buccinidae). The same author indicated that the predator foot covers the prey and builds a sealed cavity, possibly secreting a toxin by means of the salivary glands to kill the prey. Novelli & Novelli (Reference Novelli and Novelli1982) observed that Adelomelon brasiliana (Volutidae from Brazilian coasts) also covers its prey with the foot, and suggested that they are killed by asphyxia, although they observed a white viscous fluid coming from the mouth, which they believed could be prey narcotizing. We registered a similar behaviour in O. magellanica; prey are engulfed by the foot and narcotized by the saliva and probably partially digested before ingestion (as we did not register the rest of tissues in the stomachs, just a mucous mass). This pre-digestion step suggests the presence of proteases in the salivary secretion*, although the Leiblein gland and posterior oesophagi also acts in the digestion process. While proteases in salivary glands of other volutids were not studied nor found yet, Ghose (Reference Ghose1961) observed that Acatina fulica saliva and rectum contain only traces of protease while Rao (Reference Rao1975) did not find indication of digestive enzymes (proteases) in the salivary glands of Cellana radiata. Graham (Reference Graham1932) in Patella vulgata and Ward (Reference Ward1966) in Fissurella barbadensis also did not observe any digestive enzymes in the salivary glands.
In the sympatric species Adelomelon ancilla an analogous feeding behaviour and pH of the digestive glands to O. magellanica were registered (Bigatti et al., Reference Bigatti, Primost, Cledón, Averbuj, Theobald, Gerwinski, Arntz, Morriconi and Penchaszadeh2009).
*Note added in proof: we have recently confirmed proteolytic activity of the saliva in zymograms using commercial gelatin as substrate (unpublished results).
The time taken in consuming or narcotizing a prey could not be determined in the field, since the process is relatively slow. The observations on feeding behaviour in aquaria enabled us to determine a time of at least 10 hours is required for prey narcotization at 13°C (mean temperature in the field). After narcotization, the prey is consumed alive by means of mechanical action of the radula. The change in the trophic habits to carrion or cannibalism in absence of other living molluscs indicates that this species is very voracious and adapts itself to food scarcity circumstances typical of the sandy bottoms community they inhabit. Fisheries studies made with volutids from north Patagonia revealed that no species were captured in baited traps (Bigatti & Ciocco, Reference Bigatti and Ciocco2008), suggesting also a preference for live prey. Galván et al. (in press) found that two perciform reef fish consume O. magellanica in the study area: the Argentine sandperch Pseudopercis semifasciata and the Argentinean sea bass Acanthistius patachonicus. These results and those obtained in the present work suggest that O. magellanica is not a ‘top predator’ but an active predator from the benthic communities it inhabits.
Biochemical studies and saliva action
The salivary and accessory salivary glands of O. magellanica are typical of the subfamily Odontocymbiolinae (Bigatti, Reference Bigatti2005); externally they are embedded in a similar disposition to all the members of this subfamily (Clench & Turner, Reference Clench and Turner1964; Leal & Bouchet, Reference Leal and Bouchet1989; Leal & Harasewych, Reference Leal and Harasewych2005). Experiences with WAGS indicate that the secretion products (saliva) cause narcotization of prey before ingestion. Salivary (SG) and accessory salivary gland (AG) ducts end separately; the former ends in the anterior oesophagus and the latter at the tip of the mouth (Bigatti & Pastorino, unpublished). The differentiated opening of the ducts from both glands could correspond to different functions. The ducts of the AG secrete the white viscous fluid that is observed in the predation events, probably with narcotizing effects on the muscles of molluscs. Ducts of the SG finish in the anterior oesophagus and pour their secretion in that area; this fact would avoid the contact of the narcotizing liquid from AG ingested together with the prey, by covering the oesophagus epithelium with secretions from SG (without the narcotizing compound). The pH of the salivary liquid (SP) is around 10, and the higher calcium concentration found in the AG could be binding proteins secreted by the salivary glands. After passing through the Leiblein valve, the pH of the digestive system decreases up to approximately 7.5. This shift in pH would allow the inactivation of the salivary fluid from AG (with narcotizing function), avoiding toxicity for the producer in its digestive system. The pH registered in the mid and posterior oesophagus zone (~7.5) would be given by digestive enzymes secreted by the Leiblein gland, the posterior oesophagus or the stomach. The Leiblein valve would then have the function of retaining the enzymatic secretions of the Leiblein gland, and the dorsal glandular folds (inside the medium and posterior oesophagus), as it has been observed for other volutids (Ponder, Reference Ponder1973) or other caenogastropods (Andrews & Thorogood Reference Andrews and Thorogood2005). The fact that no remnants of prey tissues were observed in the stomach contents analysed sustains the hypothesis that a pre-digestion is produced before ingestion. The secretions of the salivary glands in gastropods have been related to different physiological functions, including lubrication and food ingestion, initial phase of external digestion and prey capture (Andrews, Reference Andrews1991). Andrews et al. (Reference Andrews, Page and Taylor1999) registered 6 types of salivary secretions in Cymatium intermedius with actions including enzymatic, toxic, acid and protection of the digestive tract (as the one postulated in this work). In the case of O. magellanica the narcotization of the prey due to the relaxation of pedal muscles in gastropods or adductor muscles in bivalves seems to be given by the action of saliva on the prey, particularly the S secretion from AG. Analysis of the latter indicates that it contains proteins, glycoproteins and calcium. The lower viscosity of the extracted products AGE and SGE could be attributed to a decrease in molecular weight. High speed centrifugation might have precipitated the high molecular weight (less soluble) proteins. On the other hand, cations contributing to the stabilization of quaternary structure might have been lost during dialysis. In either case, the resultant points to a decrease in molecular weight and biological activity, as evidenced by comparing the SDS-PAGE patterns of S with those of AGE and SGE.
SDS-PAGE of the only peak of S in the Bio-Gel fractionation suggests homogeneity after the column purification step. Yet, it must be taken into account that a Bio-Gel elution profile was drawn on the basis of absorbance at 280 nm due to aromatic amino acids. Thus, other peptides (especially those with lower molecular weight) appearing in the electrophoresis pattern of SGE, AGE or S might have a proportion of aromatic amino acids below the limit of the detection.
Siegman et al. (Reference Siegman, Moers, Li, Narayan, Trinkle-Mulcahy, Watabe, Hartshorne and Butler1997) suggested that the phosphorylation state of a ~600 kDa protein regulates catch in the anterior byssus retractor muscle (ABRM) of Mytilus edulis. Experiments on both permeabilized and intact muscles indicate that when the muscle is stimulated with acetylcholine and the intracellular calcium concentration increases the phosphorylation of the high molecular weight protein decreases, probably through the action of a calcium-activated phosphatase. The dephosphorylation of the protein does not alter the force output as long as the calcium concentration is high, but when the calcium returns to low levels, continued force maintenance (catch) requires the protein to be dephosphorylated. Somehow, the interaction between contractile filaments resulting in force output is maintained at the low calcium concentration if the protein is dephosphorylated. When serotonin is added, there is an increase in cAMP, and the high molecular weight protein is phosphorylated by A kinase. This results in the loss of the catch state and a rapid relaxation of the muscle. The secretions form AG and SG of O. magellanica could be involved in the catch mechanism of bivalves or contraction of gastropod muscles. One hypothesis would be that one of the low molecular weight proteins observed in the electrophoresis experiments of this work depolarizes the membrane of the muscular cells, and the calcium in excess in the saliva enters the cytoplasm releasing neurotransmitters like acetylcholine which take part in the contraction of muscles (Katz & Miledi, Reference Katz and Miledi1967). This calcium excess, acts as an intracellular signal liberating the neurotransmitter (Llinas, Reference Llinas, Alberts, Bray, Lewis, Raff, Roberts and Watson1994). The effect of a high calcium concentration outside and inside cells would be an initial catch contraction and then a total relaxation of the muscle cells. The other hypothesis would be that the peptides secreted by the salivary and accessory glands aggregate in the presence of calcium ions. The resulting quaternary protein structure would be the active form which causes prey narcotization and/or activates proteolytic activity. Several mollusc (glyco)proteins with glycosidase activity were found recently, such as those in Spisula sachalinensis (Verigina et al., Reference Verigina, Burtseva, Ermakova, Sova, Pivkin and Zvyagintseva2005), Littorina kurila (Pesentseva et al., Reference Pesentseva, Kusaykin, Anastiuk, Sova and Zvyagintseva2006), and in the scallop Patinopecten yessoensis (Nakai et al., Reference Nakai, Iisuka, Okuyama, Mori, Chiba and Kimura2006). For the latter several ion-dependent enzymes were found with molecular weights between 30 and 61 kDa.
The results of this work demonstrate that the prey is not killed by asphyxia due to the fact that water enters the cavity made by the foot and consequently prey respiration is not inhibited. The continuous secretions from the salivary and accessory salivary glands generate a pH of approximately 10 into this cavity with narcotizing properties which relax the muscles of the captured individuals. Activity of the AG and SG glands must be tested separately to isolate and purify the compounds responsible for the prey narcotization.
ACKNOWLEDGEMENTS
Carlos Sanchez Antelo, Andrés Averbuj, Miguel Angel Diaz, Julio Rúa, Ricardo Vera, Nestor Ortiz, Norberto de Garín and Fabián Quiroga helped in field activities. This project was partially supported by PICTR 01869, PICT 2008-0323, PIP 5699 (CONICET) UBACyT 171 and X-214.