Introduction
Elasmobranchs are often the top predators in marine ecosystems (Ellis et al., Reference Ellis, Pawson and Sharckley1996); it is thus important to know the composition of their diets in order to understand trophic relationships and energy flows in the ecosystems they inhabit. Dietary information is also useful for understanding the life history of these fish and their role in the marine ecosystem, as well as the impact they have on a particular prey species' population. Knowing what a species eats can also provide information on its distribution and position in the food chain (Cortés, Reference Cortés1999). The analysis of stomach contents is one of the oldest and most frequently used methods of obtaining dietary information. It is particularly used for organisms that occupy intermediate to high trophic levels in food chains, fish probably being the best studied taxonomic group (Kling et al., Reference Kling, Fry and O'Brien1992).
Alopias pelagicus is a tropical shark that only occurs in the Pacific and Indian Oceans (Trejo, Reference Trejo2005). The species is essentially oceanic and epipelagic, but can also be found near the coast over the continental shelf, from the surface to a depth of 152 m. Pelagic thresher sharks feed on fish and squids which they gather by swimming around them, creating waves with their tails and then striking them, consuming those that are stunned or dead (Compagno, Reference Compagno1984, Reference Compagno2001; Compagno et al., Reference Compagno, Krupp, Schneider, Fischer, Krupp, Schneider, Sommer, Carpenter and Niem1995). The IUCN has classified the species as vulnerable, with a downward population trend (Reardon et al., Reference Reardon, Márquez, Trejo and Clarke2009).
Alopias pelagicus is a species of high commercial importance in Ecuador: from September 2007 to December 2011, 37,747,872 kg were caught, accounting for 67.4% of total landings of chondrichthyans recorded on the mainland coast according to data from Ecuador's Vice Ministry of Aquaculture and Fisheries (Ministerio de Agricultura, Ganadería, Acuicultura y Pesca [MAGAP], 2012). Martínez-Ortiz & García-Domínguez (Reference Martínez-Ortiz and García-Domínguez2013) confirm that the pelagic thresher is the main species caught by artisanal fisheries, representing 63–72% of the country's annual shark landings (by weight). All shark parts are used (meat, fins, jaws, teeth, cartilages, skin and viscera) and it can be found on both the domestic and export markets, sold as fresh or frozen meat.
Previous studies on the diet and feeding habits of A. pelagicus in Ecuador include Páez-Rosas et al. (Reference Páez-Rosas, Insuasti-Zárate, Riofrío-Lazo and Galván-Magaña2018), carried out in Galapagos Islands (province of Galápagos) by means of stable isotopes analysis (SIA) of carbon and nitrogen (N = 39). Rosas-Luis et al. (Reference Rosas-Luis, Navarro, Loor-Andrade and Forero2017) did a study in Manta (province of Manabí) and Santa Rosa de Salinas (province of Santa Elena), based on SIA and stomach contents (N = 19). Other studies include Estupiñán (Reference Estupiñán2016) in Galapagos Islands using SIA (N = 353); Polo-Silva et al. (Reference Polo-Silva, Newsome, Galván-Magaña, Grijalba-Bendeck and Sanjuan-Muñoz2013) in Manta using SIA and stomach contents (N = 111) and Polo-Silva et al. (Reference Polo-Silva, Rendón and Galván-Magaña2009) by means of stomach contents (N = 103).
In these studies A. pelagicus showed preference for consuming squids such as jumbo flying squid Dosidicus gigas, purpleback flying squid Sthenoteuthis oualaniensis and fishes such as Panama lanternfish Benthosema panamense. This species had a specialist predator behaviour, and presented a high overlap between sex and sexual maturity stage, and had a trophic level (3.8) consistent with a tertiary predator. In Perú, González-Pestana et al. (Reference González-Pestana, Acuña-Perales, Córdova, Coasaca, Alfaro, Alfaro-Shigueto and Mangel2019) studied A. pelagicus based on stomach contents (N = 38). Its diet comprised 10 prey taxa: nine cephalopods and one teleost, and the most important prey species were D. gigas and sharpear enope squid Ancistrocheirus lesueuri. It thus had a very high degree of specialization and its diet was dominated by a small number of prey species. Its average trophic position was high (4.4).
Although there are numerous studies about the diet and feeding behaviour of A. pelagicus based on stomach contents and SIA in Manta and Galápagos Islands, in Santa Rosa de Salinas studies are scarce and the only research carried out in this area was made with 19 individuals, so it is important to have updated information from this zone.
Alopias pelagicus remains relatively unstudied in many aspects of its life history despite the fact that it supports an important target fishery; it too is vulnerable to overfishing, like all chondrichthyans. Research is thus needed in this zone to study the biological and ecological aspects of the species, to establish any similarities or differences between sizes and time of the year, as well as the ecology of prey species and their relation with apex predators. The objective of this study was to determine the diet composition of A. pelagicus and describe its feeding habits in Santa Rosa de Salinas, considering size, sex, sexual maturity stage of the specimens, as well as the season of the year (rainy or dry). This research is intended as a comparative study for analysing any similarities or differences in the feeding composition and the dynamics of species relationships in its environment.
Materials and methods
Sampling
Shark specimens were obtained weekly from the artisanal fishery landings at the port of Santa Rosa de Salinas, Santa Elena, Ecuador (02°12′56″S 80°57′26″W), between February 2008 and January 2009 (Figure 1).

Fig. 1. Location of Santa Rosa de Salinas, one of the main fishing ports of the Ecuadorian coast.
The sharks were identified, measured (total length (TL), in cm) and separated by size, sex, season and sexual maturity stage, based on external and internal sexual characteristics. The study sample comprised immature and mature sharks (including pregnant females), allowing to obtain valuable information on the dietary spectrum of sharks of all sexual maturity stages.
Stomachs were then removed and their degree of fullness was determined according to the categories proposed by Stillwell & Kohler (Reference Stillwell and Kohler1982): 0 (empty); 1 (25% full); 2 (50% full); 3 (75% full); and 4 (full). Stomach contents were placed in plastic bags and transported to the laboratory to be frozen and analysed later.
Analysis of samples and data
Prey items were classified by group, counted, measured (in cm) and weighed (in g). They were also classified by the degree of prey digestion according to Olson & Galván-Magaña (Reference Olson and Galván-Magaña2002): Degree 1 (individuals that have all the morphological characteristics of the species, making them easily identifiable); Degree 2 (individuals without skin, without eyes and with bare muscle); Degree 3 (individuals without head, with some body parts and with axial skeleton); and Degree 4 (presence of otoliths, skeletons and squid beaks only).
The fish prey digested to a minor degree (Degree 1) were identified based on fish identification keys (Chirichigno, Reference Chirichigno1980; Fischer et al., Reference Fischer, Krupp, Schneider, Sommer, Carpenter and Niem1995a, Reference Fischer, Krupp, Schneider, Sommer, Carpenter and Niem1995b; Jiménez & Beárez, Reference Jiménez and Béarez2004). Those digested to a higher degree (Degrees 3 and 4) were identified using their axial skeleton and otoliths; vertebrae counting based on Clothier (Reference Clothier1950) and Clothier & Baxter (Reference Clothier and Baxter1969), and the identification of otoliths, on García-Godos (Reference García-Godos2001), Mier (Reference Mier2011), Muñoz (Reference Muñoz2012) and Vinueza (Reference Vinueza2015). For cephalopods, the identification keys by Clarke (Reference Clarke1962, Reference Clarke1986), Wolff (Reference Wolff1982, Reference Wolff1984) and Ingrid et al. (Reference Ingrid, Iverson and Pinkas1971) were used. Lower and/or upper rostral length (i.e. the length of the lower and/or upper mandibles of cephalopod beaks) were measured in order to back-calculate weight, according to the equations proposed by Wolff (Reference Wolff1982) and Clarke (Reference Clarke1986).
The minimum number of stomachs required to validate this dietary study was determined as proposed by Hoffman (Reference Hoffman, Lipovsky and Simenstad1979). This method consists of plotting the cumulative number of prey species consumed against the number of stomachs sampled. The resulting cumulative curve shows the number of stomachs at which an asymptote is approached, i.e. the minimum sample size. For this purpose, the criteria proposed by Jiménez-Valverde & Hortal (Reference Jimenez-Valverde and Hortal2003) was used, with which a slope value less than 0.1 indicates the number of stomachs is reliable enough to characterize the diet. The asymptote curve value was also estimated. EstimateS V.8.0 software (Colwell, Reference Colwell2019) was used, on which the number of reviewed stomachs was subjected to 1000 permutations to eliminate bias with an α = 0.05. Statistica V.8.0 software (StatSoft, 2008) was used for estimating a and b parameters of Clench's equation.
The indices used to describe the diet of A. pelagicus were based on the numerical method (Hyslop, Reference Hyslop1980), the frequency-of-occurrence method and the gravimetric method (Peláez, Reference Peláez1997). The Index of Relative Importance (IRI) was computed following Pinkas et al. (Reference Pinkas, Oliphant and Iverson1971) and interpreted as proposed by Duarte & von Schiller (Reference Duarte and Von Schiller1997): 0–20 = incidental prey; 21–200 = secondary prey; and 201–20,000 = main prey.
The trophic niche width of predatory pelagic threshers was estimated using Levin's index (Bi; Krebs, Reference Krebs1985). Values for this index range from 0 to 1; a Bi lower than 0.6 indicates that the diet is dominated by few prey, making these sharks specialist predators, while a Bi higher than 0.6 indicates that they are generalist predators (Labropoulou & Eleftheriou, Reference Labropoulou and Eleftheriou1997).
The analysis of dietary overlap between the sexes, sexual maturity stages, size classes and season of the year was done using the Morisita–Horn index (C λ; Morisita, Reference Morisita1959; Horn, Reference Horn1966; Smith & Zaret, Reference Smith and Zaret1982). According to Langton (Reference Langton1982), a C λ of 0–0.29 indicates low dietary overlap; a C λ of 0.30–0.59, moderate overlap; and a C λ of 0.60–1, high overlap. A canonical correspondence analysis (CCA) was used to evaluate possible differences between all the categories– this test was carried out in Statistica V.8.0 software (StatSoft, 2008).
Trophic level was calculated using the following equation (Cortés, Reference Cortés1999):
 $$TL_{k} = 1 + \left({\sum_{n = 1}^{n = 19} Pj_{x} \times TL_{j}} \right)$$
$$TL_{k} = 1 + \left({\sum_{n = 1}^{n = 19} Pj_{x} \times TL_{j}} \right)$$where TLk is the trophic level of the species of interest, n is the number of prey species, Pjx is the relative proportion of each prey in the predator's diet and TLj is the trophic level of each prey.
One-way analysis of variance (ANOVA; F-test) was used to test for significant differences between sexes, sexual maturity stages, size classes and seasons of the year.
Results
Of the 104 individuals of Alopias pelagicus caught, 84 (81%) were females and 20 (19%) were males. The recorded range of sizes was 155–321 cm TL in females and 154–318 cm TL in males. From these 104 stomachs, 59 had food and 25 were empty for females and 14 had food and 6 were empty for males. The number of sharks caught varied each month (Table 1).
Table 1. Monthly variation of the number of sharks analysed in this research

The diet of A. pelagicus consisted of 19 prey items, of which 10 were cephalopods and 9 were bony fishes.
Determination of the minimum sample size
The number of stomachs with content sampled (N = 73) was sufficient to describe the general species' dietary spectrum (b = 0.05), and it was possible to cover 83% of the species that composed the A. pelagicus diet (Figure 2).

Fig. 2. General cumulative curve of the prey species consumed by Alopias pelagicus (N = 73).
According to sex, the number of stomachs studied with content for females (N = 59) was enough to describe the trophic spectrum for this category (b = 0.07), and it was possible to cover 80% of the species that composed females' diet. However, for males the number of stomachs with content analysed (N = 14) was not sufficient to describe the diet for this category (b = 0.12), but it was possible to cover 83% of the species that composed males' feeding (Figure 3).
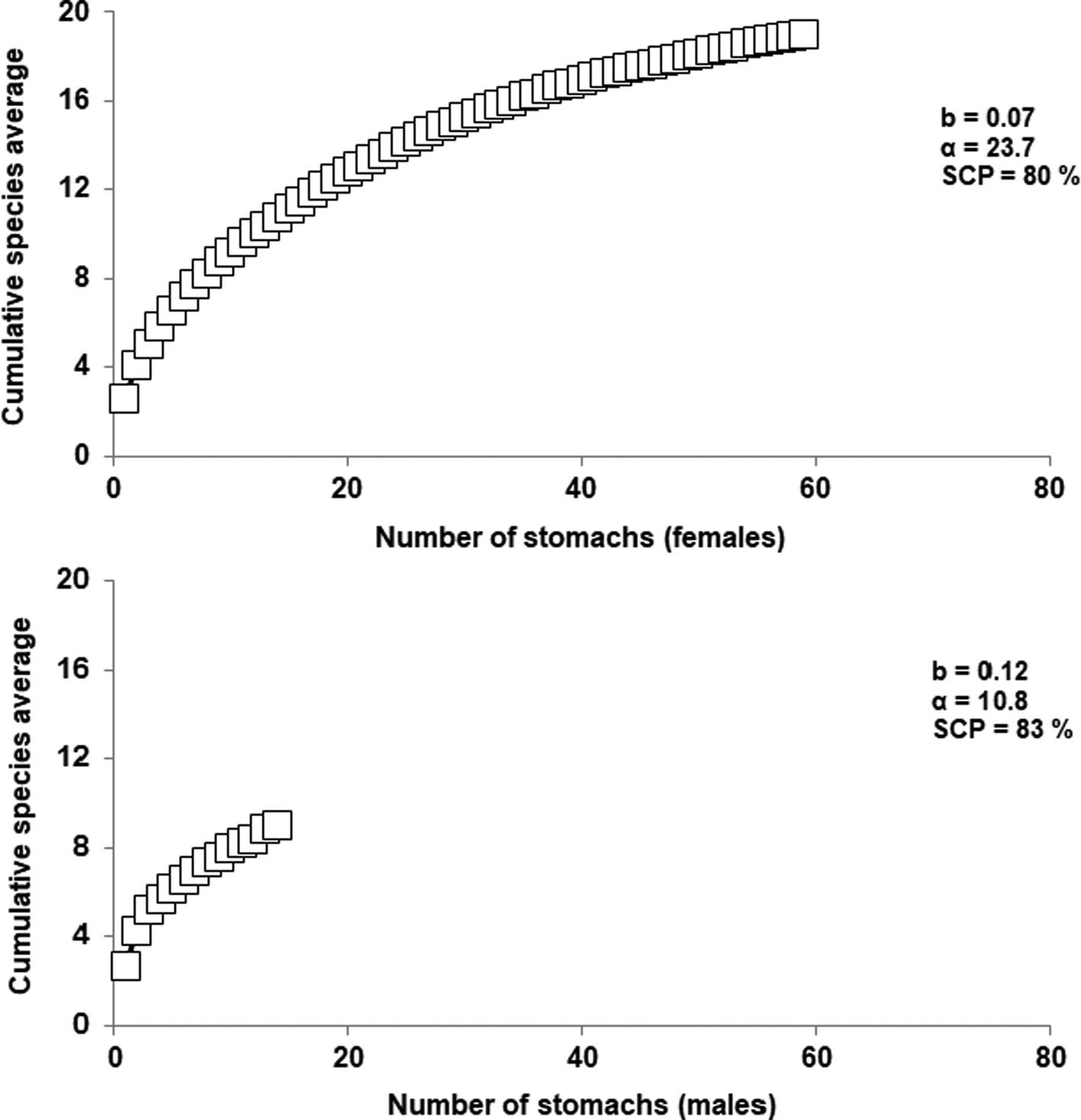
Fig. 3. Cumulative curve of the prey species consumed by Alopias pelagicus according to sex (N = 59 for females and N = 14 for males).
Trophic indices
All A. pelagicus analysed consumed 567 prey items, for a total weight of 126 kg, as follows: 364 cephalopods (125 kg) and 203 fishes (1 kg). According to the IRI, there were four main prey: Ommastrephes bartramii (53%), Dosidicus gigas (27%), Sthenoteuthis oualaniensis (12%) and Merluccius gayi (8%) (Table 2 and Figure 4).

Fig. 4. Diet of Alopias pelagicus as described by the numerical, gravimetric and frequency of occurrence methods.
Table 2. Dietary spectrum of Alopias pelagicus in Santa Rosa de Salinas, Ecuador, expressed in both absolute and percentage terms based on the numerical (N and %N), frequency-of-occurrence (FO and %FO), gravimetric (W and %W) and Index of Relative Importance (IRI and %IRI) methods

The females consumed 429 individuals (a total of 96 kg) belonging to 19 species, comprising 271 cephalopods and 158 fishes. The IRI showed four main prey species: O. bartramii, D. gigas, S. oualaniensis and M. gayi. The males fed on 139 prey individuals (a total of 30 kg) belonging to 9 species, comprising 93 cephalopods and 46 fish. According to the IRI, the four main prey in their diet were O. bartramii, D. gigas, S. oualaniensis and M. gayi (Figure 5). No significant differences in diet could be detected between females and males (F = 0.01; P = 0.94).

Fig. 5. Index of Relative Importance of the main prey consumed by each sex in Alopias pelagicus.
There were 19 immature and 85 mature individuals. The immature females had a dietary spectrum composed of 11 species and consumed a total of 112 prey items (11 kg), i.e. 50 cephalopods and 62 fishes. According to the IRI, five prey species dominated their diet: O. bartramii, M. gayi, D. gigas, S. oualaniensis and Benthosema panamense. The mature females consumed a total of 316 prey items (85 kg) belonging to 16 species, comprising 221 cephalopods and 95 fish. The IRI identified four main prey in their diet: O. bartramii, D. gigas, S. oualaniensis and M. gayi. No significant differences could be found between the diets of immature and mature females (F = 0.03; P = 0.88).
The immature males fed on 14 prey individuals (a total of 1 kg) belonging to 4 species, comprising 6 cephalopods and 8 fishes. Among these, there were three main prey: O. bartramii, M. gayi and D. gigas. The mature males fed on 163 prey items (a total of 29 kg), belonging to 9 species, comprising 125 cephalopods and 38 fish. There were four main prey: O. bartramii, D. gigas, S. oualaniensis and M. gayi. No significant differences in diet was observed between males of different sexual maturity stages (F = 0.10; P = 0.76).
The sharks of 141–230 cm TL consumed 31 prey items belonging to 7 species (a total of 4.6 kg), which included 22 cephalopods and 9 fish. The IRI suggested that four main elements were present: O. bartramii, D. gigas, M. gayi and S. oualaniensis. The sharks of 231–321 cm TL consumed 536 prey individuals belonging to 18 species (a total of 122 kg), including 342 cephalopods and 194 fishes. According to the IRI, there were four main prey items: O. bartramii, D. gigas, M. gayi and S. oualaniensis. No dietary difference could be observed between the two size classes (F = 0.05; P = 0.83).
The sharks of the rainy season consumed 480 prey items belonging to 18 species (a total of 92 kg) and included 281 cephalopods and 199 fish. The IRI suggested that four main elements were present: O. bartramii, D. gigas, M. gayi and S. oualaniensis. The sharks of the dry season consumed 87 prey individuals belonging to 10 species (a total of 32.7 kg), including 83 cephalopods and 4 fishes. According to the IRI, there were three main prey items: O. bartramii, S. oualaniensis and D. gigas. No dietary difference could be found between the two seasons of the year (F = 3.49; P = 0.07).
Ecological indices
The Levin's index had a general value of Bi = 0.20, confirming that the diet of pelagic threshers was dominated by four of the 19 species composing it, making them specialist predators. When calculated for males, Levin's index was twice that of females. The immature females had a higher Levin's index than the mature ones, and this was true also for males. Similarly, the sharks of 141–230 cm TL had a higher Levin's index than those of 231–321 cm TL and, the sharks in the rainy season had a higher index value than those of the dry season. The results indicate a preference for consuming certain prey of the dietary spectrum, as shown by the low Bi values obtained for all categories of sharks. Regardless of the number of prey items in their diet, these predators tended to prefer four particular prey species (Table 3).
Table 3. Trophic niche width according to Levin's index (Bi) for the categories of pelagic thresher sharks analysed

TL, total length.
Dietary overlap was high between all categories of sharks, with values close to 1, confirming the similarity in their diets described above. The diets of females and males were practically identical (C λ = 0.99), i.e. there was an almost total overlap between them. The same happened between the two size classes (141–230 and 231–321 cm TL). Following this trend, the immature and mature females had highly similar diets, and this was the same case between immature and mature males and, for rainy season and dry season (Table 4).
Table 4. Dietary overlap between the categories of Alopias pelagicus thresher sharks analysed according to the Morisita–Horn index (C λ)

TL, total length.
Similarities were found in the diet for all categories recorded above and confirmed by the CCA analysis, where no significant differences were observed (sex: canonical r = 0.38, P = 0.97; sexual maturity stage: canonical r = 0.54, P = 0.31; sizes: canonical r = 0.55, P = 0.26; seasons of the year: canonical r = 0.61, P = 0.75). The CCA showed that relationships between the trophic spectrum of females and mature females, males and mature males, rainy season and sizes were low, and during dry season and sizes were high; while immature females and immature males didn't show any relationship in their prey (Figure 6).

Fig. 6. Canonical correspondence analysis for categories related to sex, sexual maturity stages, sizes and seasons of year in Alopias pelagicus (N = 73).
The trophic level of A. pelagicus was estimated at 5.00, which is typical of top predators (quaternary consumers or tertiary carnivores). This agrees with the prey data from the stomach contents (squids and fish) having trophic levels between 2.61 and 4.48, confirming that pelagic threshers belong to a long food chain composed of five links, typical of areas that are close to the coast. The highest estimated trophic level was for immature males (5.16) and the lowest for immature females (4.93; Table 5).
Table 5. Trophic levels estimated according to Cortés (Reference Cortés1999) for the categories of pelagic thresher sharks analysed

TL, total length.
Discussion
This study showed that 62 stomachs were sufficient to describe the general dietary spectrum of Alopias pelagicus in the area. The smaller minimum number of stomachs estimated here may be due to the smaller number of prey recorded in the dietary spectrum (19) and the large predominance of a few prey (N = 4). Despite the elapsed time from the first studies on the diet composition and feeding behaviour of A. pelagicus, the availability of prey species (squids and teleostean fishes) has probably remained constant due to the characteristics of the tropical and epipelagic marine ecosystems (primary and secondary productivity). The results of this study confirm the importance of this site for A. pelagicus as a feeding habitat.
Most stomachs (70%) were found with food and 74% of the prey found in the stomachs were completely digested, which is consistent with previous studies by Polo-Silva et al. (Reference Polo-Silva, Newsome, Galván-Magaña, Grijalba-Bendeck and Sanjuan-Muñoz2013; 77% of stomachs were with food), Polo-Silva et al. (Reference Polo-Silva, Rendón and Galván-Magaña2009; 91%) and González-Pestana et al. (Reference González-Pestana, Acuña-Perales, Córdova, Coasaca, Alfaro, Alfaro-Shigueto and Mangel2019; 85%). This is most probably due to the time elapsed between the time sharks were caught and their arrival at the landing location, i.e. about 14 h. Indeed, 12 h is enough time for gastric juices to break down and digest the food consumed by these predators, even after their death (Bowen, Reference Bowen, Murphy and Willis1996).
Because of this elapsed time, the weight of fish prey was underestimated, no information being available about the back calculation of weight from the anatomical structures found in stomach contents. This was not the case with cephalopods, for which mantle length and total weight could be estimated from beak structures.
A higher number of food items (19 species: 10 cephalopods and 9 fish) was found in this study compared with those recorded by Rosas-Luis et al. (Reference Rosas-Luis, Navarro, Loor-Andrade and Forero2017; 8 species: 4 cephalopods and 4 fish) and González-Pestana et al. (Reference González-Pestana, Acuña-Perales, Córdova, Coasaca, Alfaro, Alfaro-Shigueto and Mangel2019; 10 species: 9 cephalopods and 1 fish). However, in this study the number of prey was smaller than found by Polo-Silva et al. (Reference Polo-Silva, Rendón and Galván-Magaña2009; 20 species: 9 cephalopods and 11 fish) and Polo-Silva et al. (Reference Polo-Silva, Newsome, Galván-Magaña, Grijalba-Bendeck and Sanjuan-Muñoz2013; 24 species: 1 crustacean, 7 cephalopods and 16 fish). The importance of these two groups of prey in the diet of A. pelagicus (known to be mainly teuthophagous and piscivorous) was confirmed. Also, the species maintained its feeding grounds in both the coastal and oceanic areas.
The IRI allowed identification of four main components of the A. pelagicus diet: Ommastrephes bartramii, Dosidicus gigas, Sthenoteuthis oualaniensis and Merluccius gayi, which agrees with what was reported by Polo-Silva et al. (Reference Polo-Silva, Rendón and Galván-Magaña2009, Reference Polo-Silva, Newsome, Galván-Magaña, Grijalba-Bendeck and Sanjuan-Muñoz2013), Rosas-Luis et al. (Reference Rosas-Luis, Navarro, Loor-Andrade and Forero2017) and González-Pestana et al. (Reference González-Pestana, Acuña-Perales, Córdova, Coasaca, Alfaro, Alfaro-Shigueto and Mangel2019). The importance of these prey is probably related to their abundance in the study area. In the case of squids, two aspects should be emphasized: (1) the year-round availability of all three species; and (2) the continuous presence of O. bartramii, which was found to be the most important component of the A. pelagicus current diet. Thus, a change seems to have occurred towards a higher preference for this squid. In a study done in the same area for other top predators, Rosas-Luis et al. (Reference Rosas-Luis, Navarro, Loor-Andrade and Forero2017) found five main components: D. gigas, A. lessueuri, skipjack tuna Katsuwonus pelamis, M. gayi and yellowfin tuna Thunnus albacares for shortfin mako Isurus oxyrinchus, blue shark Prionace glauca, silky shark Carcharhinus falciformis and swordfish Xiphias gladius, while Auxis sp. was a predominant prey for blue marlin Makaira nigricans and Indo-Pacific sailfish Istiophorus platypterus.
When calculated by sex, size, sexual maturity stage and season, the IRI showed no dietary differences between categories of sharks, all showing a preference for the same prey (Polo-Silva et al., Reference Polo-Silva, Newsome, Galván-Magaña, Grijalba-Bendeck and Sanjuan-Muñoz2013). Thus, there is no segregation of any type in A. pelagicus, and there is a common food source and feeding area that is used by the whole population, as reported by Rosas-Luis et al. (Reference Rosas-Luis, Navarro, Loor-Andrade and Forero2017) who determined similarity in the δ13C values recorded for seven top and pelagic predators indicating they exploit a marine area for their feeding.
The squid Ommastrephes bartramii is an oceanic species that is found from the surface to a depth of 1500 m and undergoes daily vertical migrations to access food between subsurface and deeper waters at night and during daytime, respectively (Roper et al., Reference Roper, Sweeney, Hochberg, Fischer, Krupp, Schneider, Sommer, Carpenter and Niem1995). Based on their biology, pelagic threshers most probably hunt at night, taking advantage of the abundance of the food resource when O. bartramii searches for food. A high consumption of juvenile squids was also observed. Because of its high availability in certain months and continuous presence over the year, O. bartramii is an essential part of the A. pelagicus diet and has increased in importance in recent years.
The squid Dosidicus gigas is abundant in oceanic, neritic as well as coastal areas and is found from the surface to a depth of 500 m. It has a broad distribution in the eastern Pacific, where large-sized individuals were recorded (Roper et al., Reference Roper, Sweeney, Hochberg, Fischer, Krupp, Schneider, Sommer, Carpenter and Niem1995). The species undertakes important migrations to Peru and Chile, where it searches for food in winter and summer (Cabrera, Reference Cabrera2003). Juveniles migrate to the surface at night to feed, while adults remain at a depth of 10–35 m when they migrate (Markaida & Sosa, Reference Markaida and Sosa2003). Due to its high abundance in the study area in recent years, it has very often been identified as a main prey in the diet of A. pelagicus and other marine predators. There is apparently a preference for juvenile squids, which are captured while migrating to the surface.
The squid Sthenoteuthis oualaniensis is an oceanic species that may be found from the surface to a depth of 1000 m (Roper et al., Reference Roper, Sweeney, Hochberg, Fischer, Krupp, Schneider, Sommer, Carpenter and Niem1995). These pelagic squids actively search for food at night and are often found in small groups near the continental shelf, but they may also be found in oxygen-depleted waters during the day (400–1100 m; Markaida & Sosa, Reference Markaida and Sosa2003). Pelagic threshers probably swim towards the continental shelf at night to capture this prey, which was the third most important component of their diet and present throughout the year of study.
The demersal fish Merluccius gayi, probably M. gayi peruanus (distributed from 5–14°S), is a mesopelagic species that is found in shallow waters of the continental shelf (50 m deep) as well as the upper part of the continental slope (500 m deep). The main spawning season was found to be from August to March in the area extending from 4–8°S (Cohen et al., Reference Cohen, Inada, Iwamoto and Scialabba1990), so A. pelagicus probably consumed this prey mainly in January. The period of maximum abundance of M. gayi in the shark diet is only one month, although this prey was found to be present throughout the year.
The fish Benthosema panamense is an oceanic and mesopelagic species that can be found on continental and island shelves from the surface to depths of over 2000 m. These lanternfish undertake vertical migrations to a depth of 200 m, where they remain during the day, and then continue to reach the upper water layer at night (Fischer et al., Reference Fischer, Krupp, Schneider, Sommer, Carpenter and Niem1995b). Studies on the feeding habits of D. gigas have shown that B. panamense is its main prey (Nesis, Reference Nesis1970; Bennet, Reference Bennet1978; Markaida & Sosa, Reference Markaida and Sosa2003); so A. pelagicus may thus have captured B. panamense and D. gigas. Benthosema panamense was found to be present only in January and was the fifth main prey in the diet of A. pelagicus.
The estimated values of trophic niche width showed that A. pelagicus is a specialist predator (Bi = 0.20), confirming the results of previous studies (Polo-Silva et al., Reference Polo-Silva, Rendón and Galván-Magaña2009, Bi = 0.33; Polo-Silva et al., Reference Polo-Silva, Newsome, Galván-Magaña, Grijalba-Bendeck and Sanjuan-Muñoz2013, Bi = 0.02; González-Pestana et al., Reference González-Pestana, Acuña-Perales, Córdova, Coasaca, Alfaro, Alfaro-Shigueto and Mangel2019, Bi = 0.16). However, immature sharks were found to have a generalist behaviour, with juveniles of 137–162 cm TL having a Bi = 0.94 (Polo-Silva et al., Reference Polo-Silva, Rendón and Galván-Magaña2009). This confirms the findings of Tytler & Calow (Reference Tytler and Calow1985), Castro (Reference Castro1993) and Gerking (Reference Gerking1994) that the feeding behaviour of some fishes can change over their lifetime in response to changing energy requirements linked to their hunting skills, growth and sexual maturation, as well as the season of the year, habitat and food availability.
The intraspecific similarity of the diets is a consistent pattern in A. pelagicus, with the four categories of sharks compared; this was also observed in previous studies (Polo-Silva et al., Reference Polo-Silva, Rendón and Galván-Magaña2009, Reference Polo-Silva, Newsome, Galván-Magaña, Grijalba-Bendeck and Sanjuan-Muñoz2013). Exceptions to this pattern were pointed out by Polo-Silva et al. (Reference Polo-Silva, Rendón and Galván-Magaña2009) for sharks of 137–162 cm TL (C λ = 0.13) and 189–249 cm TL (C λ = 0.10). Based on these studies, the study area is a feeding ground, and this zone is being exploited by the fishing activity.
The trophic level of A. pelagicus was estimated at 5.00, which is higher than the values previously estimated for the species (4.50 by Froese & Pauly, Reference Froese and Pauly2018; 4.20 by Polo-Silva et al., Reference Polo-Silva, Newsome, Galván-Magaña, Grijalba-Bendeck and Sanjuan-Muñoz2013; 4.40 by González-Pestana et al., Reference González-Pestana, Acuña-Perales, Córdova, Coasaca, Alfaro, Alfaro-Shigueto and Mangel2019) and other species of the same genus such as A. superciliosus (4.20 by Polo-Silva et al., Reference Polo-Silva, Newsome, Galván-Magaña, Grijalba-Bendeck and Sanjuan-Muñoz2013; Cortés, Reference Cortés1999) and A. vulpinus (4.20 by Cortés, Reference Cortés1999). This may be because these studies used standardized values of trophic levels for the prey species (e.g. 3.24 for teleost fishes and 3.20 for cephalopods).
Indeed, this tends to underestimate trophic levels because no single trophic level value can represent all these species, which, depending on their diet, can occupy very different trophic positions, e.g. 2.61 for Sardinops sagax and 4.48 for the dorado Coryphaena hippurus. Similarly, the standardized trophic level used for cephalopods in these studies is 3.20 when the trophic level of D. gigas, for example, is estimated at 4.14, and that of Octopus bimaculatus at 3.44, both above the average trophic level assigned to this taxon. The highest trophic level estimated was for immature males (5.16), and the lowest, for immature females (4.93). This may be due to the fact that immature males have only four diet components with trophic levels ranging from 3.44–4.30, resulting in an estimated trophic level higher than mature males, with nine diet components of various trophic levels. In contrast, the dietary spectrum of immature females included 8 of the 16 prey species present in mature females, with similar trophic levels, resulting in more similar estimated trophic levels.
Information about diet composition and feeding habits of A. pelagicus is given for this part of the South-east Pacific Ocean, in relation to sex, size, sexual maturity stages and season. Sample minimum size, feeding behaviour, intraspecific comparison of the A. pelagicus diet and trophic position were reviewed. All these features are necessary for understanding the relations of the top predator and its prey in the tropical marine ecosystem.
Acknowledgements
MDCM thanks Erika Carrera García for her help in editing the map of the study area. FGM thanks the Instituto Politécnico Nacional for fellowships (Comisión para el Fomento de Actividades Académicas [COFAA] and Estímulo al Desempeño de los Investigadores [EDI]). Thanks to Isabelle Gamache for translating the original manuscript to English.
Financial support
MDCM thanks Mexico's Consejo Nacional de Ciencia y Tecnología (CONACYT) for the graduate scholarship granted to study and gaining my Master degree. This paper is derived from my Master's thesis.













