INTRODUCTION
Day and night succession has a strong influence on the relative abundance of several species and changes the composition and richness of fish assemblages in sandy beaches (Clark et al., Reference Clark, Bennett and Lamberth1996). Very often, there is a seasonal change in the species composition, which is also reflected in the diel variations in assemblage structure (Horn, Reference Horn1980; Nash, Reference Nash1986, Reference Nash1988; Wright, Reference Wright1989). The diel and seasonal compartmentalization of space in the fish using open sandy beach habitat may be a key factor in understanding how so many species can be supported in a physically simple habitat.
In this study, two scales have been considered: diel and seasonal. Firstly, diel variation in species abundance is an indicator of species separation and habitat partition within a short time frame (i.e. niche differentiation). Such variation can present an adventitious reduction of competition for food and space and a reduction in predatory pressure (Ross, Reference Ross1986; Ross et al., Reference Ross, Mcmichael and Ruple1987). Additionally, some species appear to undergo a change in capture rate dependent on the prevailing photoperiod (Eriksson, Reference Eriksson and Thorpe1978; Muller, Reference Muller and Thorpe1978a, Reference Muller and Thorpeb; Nash, Reference Nash1986). Secondly, seasonal variation in the composition of the diel component could be the consequence of changes in behaviour patterns and assemblage structure. These changes can be caused by migrations or movements inshore/offshore that are dictated by ontogenetic changes (Gibson et al., Reference Gibson, Ansell and Robb1993).
In most environments, fish are: (i) diurnal and tend to feed primarily during the day; (ii) nocturnal and feed by night; (iii) feed primarily during crepuscular periods of twilight; or, (iv) less commonly, show no periodicity. Activity patterns in fish generally represent a direct response to changing light levels but are also affected by the activity patterns of their predators and prey. Predators and prey can mutually influence each other’s activities (Helfman, Reference Helfman and Pitcher1993). These behaviours allow the species to be characterized as diurnal or nocturnal through observation of periods of higher activity or rest, the latter including searching for shelter (Hobson, Reference Hobson1965).
Behaviour differs between species and there is often a difference in catchability or vulnerability of species relative to the point in the day–night light cycle (Parsley et al., Reference Parsley, Palmer and Burkhardt1989). It has been argued that individuals do not see nets at a great distance during the night, therefore increasing capture efficiencies (Wright, Reference Wright1989; Walsh, Reference Walsh1991; Nash et al., Reference Nash, Santos and Hawkins1994). However, some species show higher catches during the day than at night (Nash & Santos, Reference Nash and Santos1998). According to these authors the major disruptions occur when the assemblage undergoes restructuring through periods of recruitment of new year-classes and as the assemblage switches from an overwintering to a spring/summer structure. Successful feeding by piscivore fish occurs primarily during the transitional periods of evening and morning twilight, when diurnal or nocturnal groups essentially replace one another ecologically (Hobson, Reference Hobson and Sale1974; Parrish, Reference Parrish1992). Although their activities are concentrated at this time, in general, predatory fish are highly opportunistic and will take prey at any time of the day or night (Helfman et al., Reference Helfman, Collette and Facey1997). Pomadasys stridens, a carnivorous species, showed a marked diel change in catches with large numbers captured in the intertidal region at night (Wright, Reference Wright1989). This may reflect some net avoidance by these large active fish as well as a movement into the intertidal region at night. Plotosus lineatus also showed a marked diel difference in behaviour, since this species was captured exclusively at night (Unsworth et al., Reference Unsworth, Wylie, Smith and Bell2007). This probably reflects a movement into the intertidal region at night since the young-of-the-year would be unlikely to avoid the trawl net during the day.
This study examines the importance of diel fluctuations in nearshore fish communities, including: (i) the study of day/night catches of shallow-water fish over a 1-year period; and (ii) a description of the major assemblage types in this area and their seasonal variation.
MATERIALS AND METHODS
Study site
Guanabara Bay (Figure 1) is located on the coast of Rio de Janeiro, Brazil (22°50′S 43°10′W). The bay is 36 km long, with a mean depth of 7.6 m, reaching 30 m in the entrance channel (Amador, Reference Amador1997). Most of the circulation is accounted for by tidal currents within Guanabara Bay. The climate is humid-tropical (Kjerfve et al., Reference Kjerfve, Ribeiro, Dias, Filippo and Quaresma1997) with a warm rainy season (December through to March) and a cool dry season (July through to August) (Mayr et al., Reference Mayr, Tenenbaum, Villac, Paranhos, Nogueira, Bonecker, Bonecker, Magoon and Neves1989; Paranhos & Mayr, Reference Paranhos and Mayr1993). The bay is surrounded by one of the largest metropolitan areas in Brazil, with more than 11 million inhabitants. The sampling site is a large (approximately 1800 m) beach facing east in the entrance bay. The beach has low amplitude semi-diurnal tides with 0.7 m of mean variation and the maximum tidal range is 1.5 m with a maximum horizontal area of the beach at low water of approximately 30 m (i.e. 54,000 m2). The mean water temperature ranges from 20.5°C (winter) to 26.0°C (summer) and the mean salinity ranges from 30 to 35.
Fig. 1. Study area, Flamengo beach, Guanabara Bay, Brazil.
Data collection
Samples were collected between spring 2005 and winter 2006 (i.e. October 2005, January 2006, April 2006 and July 2006), to assure that samples were obtained during each season, using a beach seine net (10.0 × 2.0-m; 7 mm mesh size). The net was fitted with 30 m hauling ropes and set perpendicular to the shore at a depth of approximately 1.5 m. Two people performed seine hauls, one on each end of the rope, covering an extension of approximately 30 m. The total sampled area was considered to be the distance the net was laid offshore multiplied by the mean width of the haul, resulting in an effective fishing area of approximately 300 m2. This procedure was replicated three times during each 3-hour interval throughout the 48-hour period (samples collected between sunrise and sunset were classified as day samples, while samples collected after sunset and before sunrise were classified as night). Seine operations were conducted so coverage of the same area was not repeated. This design resulted in 192 samples: two days, 8 samples, and three replicates for four seasons (spring, summer, autumn and winter). The total number of individuals and total weight for each species were recorded. Individual weights (to the nearest 1 g) were obtained for each sample. The three samples at each sampling time were summed to give one total. Capture per unit effort (CPUE) was standardized by the number and weight of fish per 100 m2. All samples were taken during spring tide, near to the full moon. The average catch was then calculated for day and night in each season.
Diel variation in number of individuals and biomass was calculated as the proportion of the catch during the day relative to the night (day/(day + night)). Values above 0.5 indicate a greater catch during the day, and catches below 0.5 indicate night predominance. The degree of diel stability in assemblage structure was compared for each season using ANOSIM. Similarities in the percentage of numbers of individuals for the whole assemblage were compared between day and night for each season, and between season for the pooled day and night catches, using the SIMPER procedures in the PRIMER software package (Field et al., Reference Field, Clarke and Warwick1982; Clarke, Reference Clarke1993). The numerical abundance data were root–root transformed and converted into a triangular matrix of similarities between day and night for each season, and between each pair of seasons for both day and night.
Two-way analysis of variance (ANOVA) was used to test diel versus season interactions of number of individuals for the most abundant species. Logarithmic transformations [log10(x + 1)] of fish abundance (number) data were performed to meet assumptions of normality and homoscedasticity and to reduce the bias of abundant species. The consistency of diurnal or nocturnal variation in catches was examined using paired t-tests on the catch data for each species. These paired t-tests checked for differences in the number of individuals or biomass for either day or night catches in each season. ANOVA and t-tests were performed using STATISTICA 7.1.
RESULTS
A total of 25,788 individuals and 63 fish species were caught during the investigation. Over the entire year, spring 2005 to winter 2006, a slightly higher total number of individuals were measured during the day (13,001) than at night (12,787) (Table 1).
Table 1. Total catch during the day or night for each species at Flamengo beach, 2005/2006. D, species that occurred exclusively during the day; N, species that occurred exclusively during the night. +, value less than 0.01%.

The day catch, as a proportion of the total catch in both number of individuals and biomass for each season, was used to compare changes in diel catch rate. The relative number of individuals caught during the day and night varied between seasons (Figure 2). The spring (0.44) and winter (0.27) were characterized by a higher relative number of individuals at night, while summer (0.61) and autumn (0.55) had more individuals during the day. On the other hand, spring (0.46), summer (0.46) and winter (0.30) had more biomass at night. Mean biomass and number of species were significantly higher at night, while number of individuals did not differ between day and night (Table 2; Figure 3).

Fig. 2. Seasonal variation in the proportion, day relative to night, of number of individuals (closed circles) and biomass (open circles).

Fig. 3. Seasonal variation in the numbers of species during either the day (open triangles) or night (closed triangles).
Table 2. Day or night predominance of catches at Flamengo beach, Brazil. D, day; N, night.

Harengula clupeola, Atherinella brasiliensis, Pomatomus saltatrix, Trachinotus carolinus, Umbrina coroides, Sardinella janeiro and Anchoa lyoleps were the seven most abundant species, accounting for 96.1% of the total number and 90.5% of the total biomass (Table 1).
Seven species were caught exclusively during the day while seventeen species were caught exclusively at night (Table 1). However, this could be considered as ‘non-representative’ for all species due to their low number of individuals.
During both day and night, there were low similarities between spring and winter, autumn and winter, and summer and winter, both in the distribution of number of individuals and biomass (Table 3). It indicates that there were major differences in assemblage structures in the winter compared with the other seasons.
Table 3. Percentage similarity in assemblage structure between successive seasons.

At the assemblage level, diel changes were detected only during the summer according to ANOSIM. Changes during this season were due to dominance of T. carolinus and A. brasiliensis during the day, and A. lyolepsis, U. coroides and S. janeiro during the night. Additionally, D. argenteus, C. latus and T. falcatus also contributed to the within similarity during the day, and O. ruber, G. luteus and E. gula, during the night (Table 4). In the winter, in spite of predominance of H. clupleola and S. janeiro during the night, the low average similarity in both, day (42.58%) and night (39.41%) contributed to non-significant diel differences in assemblage structure.
Table 4. Results of ANOSIM (R) and SIMPER to compare fish assemblages between day and night for each season. Those species contributing to 90% to the average similarity are listed. av sim, average similarity.
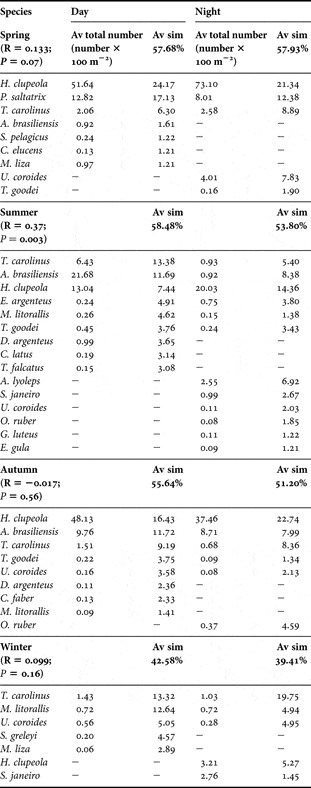
The most abundant species peaked at a particular season and changed abundance between day and night according to the season (Table 5; Figure 4). Trachinotus carolinus and A. brasiliensis tended to be more abundant during the day, peaking during summer (Figure 4). On the other hand, U. coroides, H. clupeola, A. lyoleps and S. janeiro tended to be more abundant during the night, peaking during spring (U. coroides and H. clupeola), summer (A. lyoleps) and winter (S. janeiro) (Figure 4). Pomatomus saltatrix occurred predominantly during the spring, and tended to be more abundant during the day.

Fig. 4. Seasonal catch variation in numbers of Pomatomus saltatrix, Trachinotus carolinus, Atherinella brasiliensis, Umbrina coroides, Harengula clupeola, Anchoa lyoleps and Sardinella janeiro during the day (open circles) and night (closed circles).
Table 5. Results of two-way ANOVA (seasons, day/night, seasons times day/night) for comparisons of abundances of fish species. df, degree of freedom; N, night; D, day; Seasons: 1, spring; 2, summer; 3, autumn; 4, winter. *P < 0.05; **P < 0.01.

The interaction between season and day/night catches was examined for the species given thus far in this section. The only species that did not show an interaction between season and day/night catches were H. clupeola and S. janeiro (Table 5), indicating that their diel patterns were consistent throughout the seasons.
DISCUSSION
The diel cycle plays an important role in fish richness and biomass at Flamengo beach, with a higher number of species and a larger biomass found during the night. The number of species was consistently higher at night for all seasons (especially during spring). Biomass was higher at night in all seasons except autumn. It was expected that higher abundances would occur at night, since behaviour and catchability or vulnerability of species differs according to the point in the day–night light cycle (Parsley et al., Reference Parsley, Palmer and Burkhardt1989). However, in this work, some abundant species had higher catches during the day, such as: P. saltatrix, T. carolinus and A. brasiliensis. As seen in this study and also, for example, by Wright (Reference Wright1989), some species are absent from catches during one time period or another. Similarly, the number of individuals can be different between day and night with many species occurring during both time periods, but being especially abundant only in one. The number of individuals was slightly more abundant during the day during the summer and autumn, and at night during the spring and winter. The greatest number of individuals to occur during the day was found by Nash & Santos (Reference Nash and Santos1998) for a fish assemblage from Porto Pim Bay (Azores) and Barreiros et al. (Reference Barreiros, Vicente, Mauricio and Ricardo2005) for a fish assemblage in a sandy beach in southern Brazil.
The presence of fish schools, such as A. brasiliensis, strongly influenced sampling during the day. Schools are generally larger and more common during the day, probably because they offer protection against predators that hunt visually, such as P. saltatrix. Nocturnal fish may even rest in groups during the day for the same reason. At night, solitary fish or small schools are more common (Helfman, Reference Helfman and Pitcher1993). Pomatomus saltatrix are widely described as having nocturnal activities and using the shallows for feeding and growth (Kendall & Walfard, Reference Kendall and Walford1979; Wilber et al., Reference Wilber, Clarke, Burlas, Ruben and Will2003). Pomatomus saltatrix doubles in size (60 to 120 mm total length) and preys on small fish, mainly the shoals of Clupeiformes (Felix et al., Reference Félix, Spach, Moro, Schwarz, Santos, Hackradt and Hostim-Silva2007). Conversely, in the present work, this species was more abundant during the day, which could be attributed to the more common presence of competing predators at night or they are using shallow water as refuge from diurnal predators given their small size. Predators such us T. lepturus, D. volitans, and E. saurus were caught mostly, and in some cases exclusively, at night, which is compelling evidence that these species seek early juveniles at sandy beaches for prey, such as the abundant Clupeiformes H. clupeola and S. janeiro. Furthermore, adult T. lepturus that were larger than 50 cm in total length were also recorded in the samples taken at night, corroborating the hypothesis that predators are active at sandy beaches mainly during this period of the day.
Most juvenile fish come to shallower waters to feed on plankton or small invertebrates during the day (Wright, Reference Wright1989). This could be due to better visual acuity with high light conditions, or because fish are following the movements of phototactic prey organisms. The findings of the present work are not in accordance with these hypotheses, since most abundant plancktophagous species, such as H. clupeola, S. janeiro and A. lyoleps, were mainly found during the night. These species probably use senses other than vision to detect and capture prey. On the other hand, A. brasiliensis (preying mainly on plankton), and T. carolinus (preying on small invertebrates), probably use vision as their primary sense to capture prey during the day.
Biomass was significantly higher at night due to the nocturnal habits of adult species that are less numerous and less gregarious (larger individuals). This is the case of C. spinosus (autumn and winter) weighing 270 g, O. ruber (spring) at 116 g and M. furnieri (summer) at 514 g. In addition, the number of species found throughout the season was comparatively higher during the night. These findings are in accordance with Wright (Reference Wright1989) for an intertidal fish assemblage in Kuwait and Nash & Santos (Reference Nash and Santos1998) for a fish assemblage in Porto Pim Bay (Azores). In this work, increased richness at night was probably due to the larger number of rare species recorded during the nocturnal period (17) compared with the diurnal period (6).
This study examines the stability and persistence of the day and night assemblage structures over an intra-annual cycle. There are a number of variations between the day and night assemblages over seasonal cycles. However, the presence of similarities above 50% does suggest that many species occur in both time periods (Helfman, Reference Helfman and Pitcher1986). In the present work, spring, summer and autumn showed an average similarity above 50% for both day and night period. On the other hand, winter had the lowest number of species and the lowest similarity in both day and night, indicating that during this season most changes occurred at community level. The major winter disruptions of fish assemblages occurred when the assemblage underwent restructuring through periods of recruitment of new year-classes and as the assemblage switched from the winter to the spring/summer/autumn structure.
Evidence for a distinct diurnal or nocturnal structure of fish assemblages at Flamengo beach was detected only in the summer. During this season, the relative abundance contribution to within similarity can also differ between the day and night for many species. Trachinotus carolinus and A. brasiliensis occurred in high abundance during the day. On the other hand H. clupeola and A. lyoleps occurred mainly during the night. Additionally, the comparatively less abundant D. argenteus, C. latus and T. falcatus occurred mainly during the day, while S. janeiro, U. coroides, O. ruber, G. luteus and E. gula, during the night. Diel variation in fish assemblages is an indicator of species separation and a partitioning of the habitat along a photoperiod axis. The selective advantage of such a partitioning can be manyfold including reductions in competition for food and/or space and avoidance of predation (Ross, Reference Ross1986). The reasons for diel changes in fish assemblages during the summer found in this study need further understanding. The temperature stress during the summer could be reflected in the differential susceptibility and species-specific changes in behaviour increasing their preference for either day or night. Behaviours such as foraging may have contributed to the diel patterns (Hagan & Able, Reference Hagan and Able2008). The summer dominant A. brasiliensis and T. carolinus could have preference for foraging during the day and show territorialism behaviour, with other abundant species adapting their foraging behaviour for the night period, thus decreasing competition.
In spite of a particular diel preference, the same species have been frequently recorded during diurnal and/or nocturnal periods by different authors, due to different net sizes or because these variations are more associated with the behaviour (horizontal and vertical migrations) of some species for feeding and protection (Albert & Bergstad, Reference Albert and Bergstad1993; Gibson et al., Reference Gibson, Robb, Burrows and Ansell1996). There is no previous information available on the diel periodicity in the catches of many of the fish species found in Guanabara Bay. However, behavioural traits may not be similar at the species level in areas close to the Bay.
Comparisons between the findings of the present work in the Guanabara Bay (latitude = 22°50′S) with the available information on the shallow fish community of the Sepetiba Bay (23°S), a nearby embayment in south-eastern Brazil, can be done. For example, A. brasiliensis, in the Sepetiba Bay, was recorded throughout the year, except for autumn, and occurred in both periods (day and night) in similar abundances (Pessanha & Araújo, Reference Pessanha and Araújo2003). In Guanabara Bay (the present study), this species showed a seasonal peak in summer and was more abundant during the day. Harengula clupeola, for Sepetiba Bay, occurred in autumn–winter only, with abundances peaking at sunset and during the night (Pessanha et al., Reference Pessanha and Araújo2003). For Guanabara Bay, the abundance was lowest during the winter. Anchoa tricolor was recorded primarily during the day in winter and spring for the Sepetiba Bay, while con-generic Anchoa lyoleps was absent for Guanabara Bay in winter and peaked during summer nights. Therefore, it is reasonable to suppose that the diel activity is strongly influenced by the local habitat constraints, even when comparisons are performed between similar closed systems, such as Sepetiba and Guanabara Bays. Habitat structure, which is unique to each beach, as well as biotic interactions, could be the underlying factors influencing the use of the tropical beaches by juvenile fish. Additionally, competitive interactions may be a dominant feature shaping juvenile fish assemblages since resource partitioning describes the limits that interspecific competition imposes on the number of species that may stably coexist (MacArthur, Reference MacArthur1965; Schoener, Reference Schoener1974; Roughgarden, Reference Roughgarden1976, Reference Roughgarden1983).
Some species appear to switch from day to night, or vice versa, during the year. Care must be taken when describing assemblage dynamics to include this factor if only one time frame is being considered. As seen here, abundance fluctuations may not be as severe as first perceived because species can switch their behavioural habits. Some studies that have considered changes in spatial distribution are possibly documenting changes in diel behaviour, either alone or in combination with spatial changes (Rainer, Reference Rainer1984). This study confirms that changes in diel activity in juvenile fish species can differ from other areas, even for a given species. The potential causes of such changes need to be thoroughly investigated.
ACKNOWLEDGEMENTS
We thank A.G. Cruz-Filho, for his help in the fieldwork and A.L.M. Pessanha for his helpful suggestions about species identification. This study was partially financed by the Brazilian National Council for Research Development (CNPq).











