INTRODUCTION
In marine fish larvae, vertical distribution governs the maintenance of geographic position (Pearre, Reference Pearre2003), opportunity to encounter prey (Murphy et al., Reference Murphy, Jenkins, Hamer and Swearer2011) and escape from predators (Yamashita et al., Reference Yamashita, Kitagawa and Aoyama1985; Scheuerell & Schindler, Reference Scheuerell and Schindler2003); it is one of the important ecological subjects related to the growth and survival of fish larvae. The diel vertical migrations (DVM) of larvae are roughly classified into two types: one in which the larvae move up to the surface at night, and the other in which they move down to the middle or bottom at night (Neilson & Perry, Reference Neilson and Perry1990). The former is noted in many fishes, including the anchovy Engraulis encrasicolus (Olivar et al., Reference Olivar, Salat and Palomera2001), red seabream Pagrus major (Tanaka, Reference Tanaka1985), haddock Melanogrammus aeglefinus (Perry & Neilson, Reference Perry and Neilson1988), walleye pollock Theragra chalcogramma (Olla & Davis, Reference Olla and Davis1990), and Japanese Spanish mackerel Scomberomorus niphonius (Shoji et al., Reference Shoji, Maehara and Tanaka1999). The latter is reported in black seabream Acanthopagrus schlegelii (Kinoshita & Tanaka, Reference Kinoshita and Tanaka1990), Japanese sand lance Ammodytes japonicus (formerly A. personatus; Yamashita et al., Reference Yamashita, Kitagawa and Aoyama1985) and pinfish Lagodon rhomboides (Lewis & Wilkens, Reference Lewis and Wilkens1971). Moreover, the day-night patterns in larval distribution vary in the same species of the pilchard Sardina pilchardus (Olivar et al., Reference Olivar, Salat and Palomera2001; Santos et al., Reference Santos, Ré, Dos Santos and Peliz2006). Thus, the patterns of larval DVM are variable and should be elucidated for deeper understanding of the early life history traits of each species.
The family Trichiuridae is widely distributed in oceans worldwide and includes many commercially important species. To our knowledge, no study has revealed the DVM of larvae of species belonging to this family, although some collection records of Trichiuridae larvae at various depths were observed only during the daytime (Loeb, Reference Loeb1979; Hayashi, Reference Hayashi1990).
This study aimed to elucidate diel patterns of the vertical distribution of the larvae of Trichiurus japonicus, the most dominant Trichiuridae species in the western North Pacific. This species had been confused with T. lepturus, but genetic difference between these two species was confirmed (Chakraborty et al., Reference Chakraborty, Aranishi and Iwatsuki2006a, Reference Chakraborty, Aranishi and Iwatsukib). Trichiurus japonicus spawns from June to September (Munekiyo, Reference Munekiyo1991) or May to October (Sakamoto, Reference Sakamoto1976). In this study, consecutive day–night collections were carried out in September and June.
MATERIALS AND METHODS
Study site and sample collection
The vertical distribution of T. japonicus larvae was investigated at a sampling station during 17–18 September 2015, 5–6 September 2016, 27–28 June 2017 and 20–21 September 2017. This station, located near the southern coast of Hiuchi-Nada in the central Seto Inland Sea (34°01′N 133°20′E; 25 m deep; Figure 1), Japan, was chosen because of the high concentration of larval fishes in this area (Shoji & Tanaka, Reference Shoji and Tanaka2005).
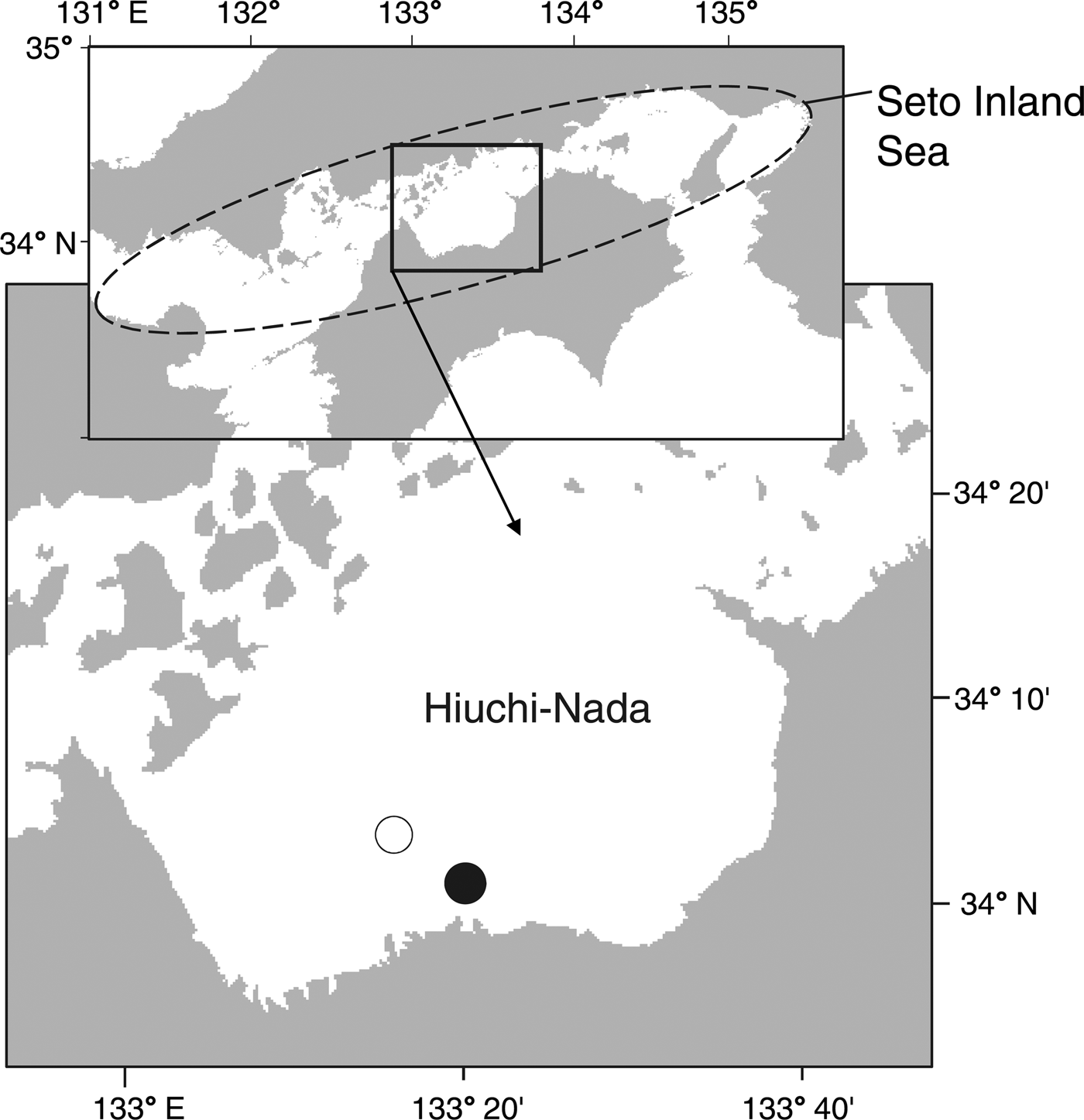
Fig. 1. Map of the study area in Hiuchi-Nada, the central Seto Inland Sea, Japan. Sample collections were conducted at stations shown by solid (in 2015–2017) and open (in 1997) circles.
In 2015, diurnal distribution patterns were investigated at 0900 and 1000 h on 17 September and at 1100 h on 18 September (Supplementary Table S1). In September 2016 and June 2017, diel distribution patterns were investigated by consecutive 24-h collections at 3-h intervals from 1500–1200 h in 2016 (eight collections in total) or from 1500–1500 h in 2017 (nine collections). In September 2017, larval collections were carried out six times (1500, 1800, 2100, 0000, 0600 and 0900 h). For larval collections, ring nets (called the MTD net; mouth diameter: 56 cm, mesh size: 335 µm; Horiki, Reference Horiki1981) were towed simultaneously at four depths (1, 6, 11 and 16 m) for 10 min at a speed over the water of 2 knots by the training and research vessel ‘Toyoshiomaru’ (256 tons). Water volume filtered per net was estimated to be 116 ± 36 m3 (mean ± SD, N = 35) and 128 ± 51 m3 (N = 24) in June and September 2017, respectively, by using a flow meter attached to the net. Samples collected were immediately preserved in 5% seawater formalin onboard.
Water temperature and salinity were measured at each collection by using a conductivity-temperature-depth sensor (SBE-9plus, SeaBird). The vertical structure of water temperature and salinity did not change greatly between day and night (Supplementary Figures S1–S3). Water temperature ranged from 26.0–27.4°C , 18.9–23.5°C and 25.4–26.2°C, and salinity ranged from 31.6–32.1, 31.9–33.1 and 30.1–32.3 in September 2016, June 2017 and September 2017, respectively. Times of sunset were 1828 h (5 September 2016), 1924 h (27 June 2017) and 1810 h (20 September 2017), and times of sunrise were 0543 h (6 September 2016), 0500 h (28 June 2017) and 0557 h (21 September 2017).
We also sorted T. japonicus larvae from specimens collected in the past 24-h survey (Shoji et al., Reference Shoji, Maehara and Tanaka1999). In this survey, larvae were collected from four depths (0, 5, 10 and 20 m) by using a net with 1.3-m mouth opening in Hiuchi-Nada on 3–4 June 1997. Larvae including T. japonicus were preserved in 10% seawater formalin (Shoji et al., Reference Shoji, Maehara and Tanaka1999). Water volume filtered per net was estimated to be 219 ± 93 m3 (mean ± SD, N = 22).
Laboratory observation and data analyses
Trichiurus japonicus larvae were sorted in the laboratory. The abundance of larvae at each depth was standardized to the number per 1000 m3. Because the water volume filtered by each net was not measured in 2015 and 2016, the average value in September 2017 (128 m3) was commonly used instead.
Total length (TL) was measured to the nearest 0.1 mm by using a digital calliper under a microscope. The developmental stages were determined according to Munekiyo (Reference Munekiyo1991) as follows (Supplementary Figure S4). No juveniles were collected.
Pr1 (Prelarva 1): yolksac is retained, the mouth is not opened, and the swim bladder is not formed.
Pr2 (Prelarva 2): yolksac is retained, the mouth is opened, and the swim bladder is formed.
Po (Postlarva): yolk is absorbed, and three serrated dorsal spines are present on the anterior dorsal fin.
To test the difference in the collection depth of the larvae between day and night, the Mann–Whitney U test was used for the data in September 2016, September 2017 and June 1997.
To test whether thermoclines and/or pycnoclines were formed at each survey, vertical profiles of average water temperature and average seawater density were described.
RESULTS
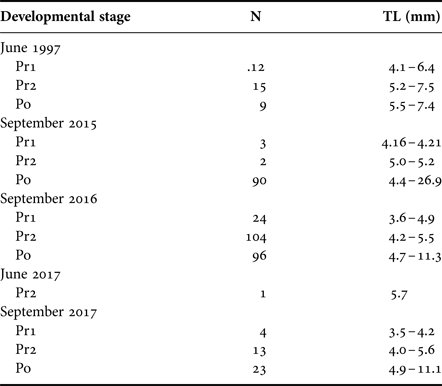
In 2015 and 2016, 98 and 224 T. japonicus larvae were collected, respectively. Their size ranged from 4.2–26.9 mm (stage: Pr1–Po) and 3.6–11.3 mm TL (stage: Pr1–Po) in 2015 and 2016, respectively (Table 1). In June 2017, only one larva (5.7 mm TL, stage Pr2) was collected from the 16 m layer at 0600 h. In September 2017, 40 T. japonicus larvae (stage: Pr1–Po; size: 3.5–11.1 mm) were collected.
Table 1. The number and body size (total length, TL) of collected larvae of Trichiurus japonicus at each developmental stage.
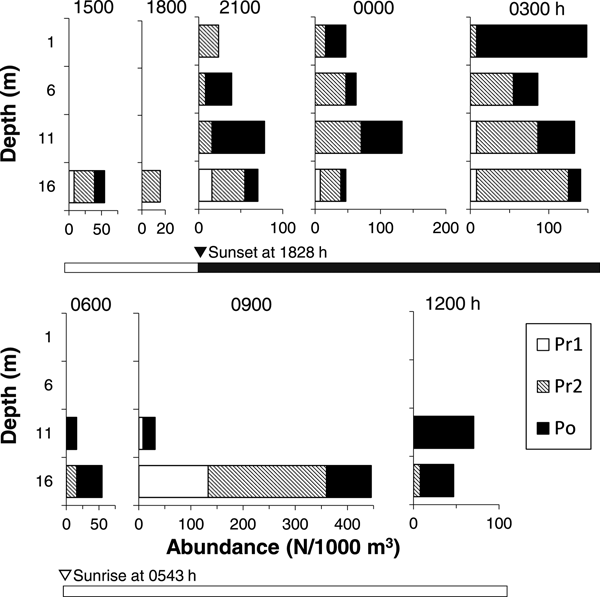
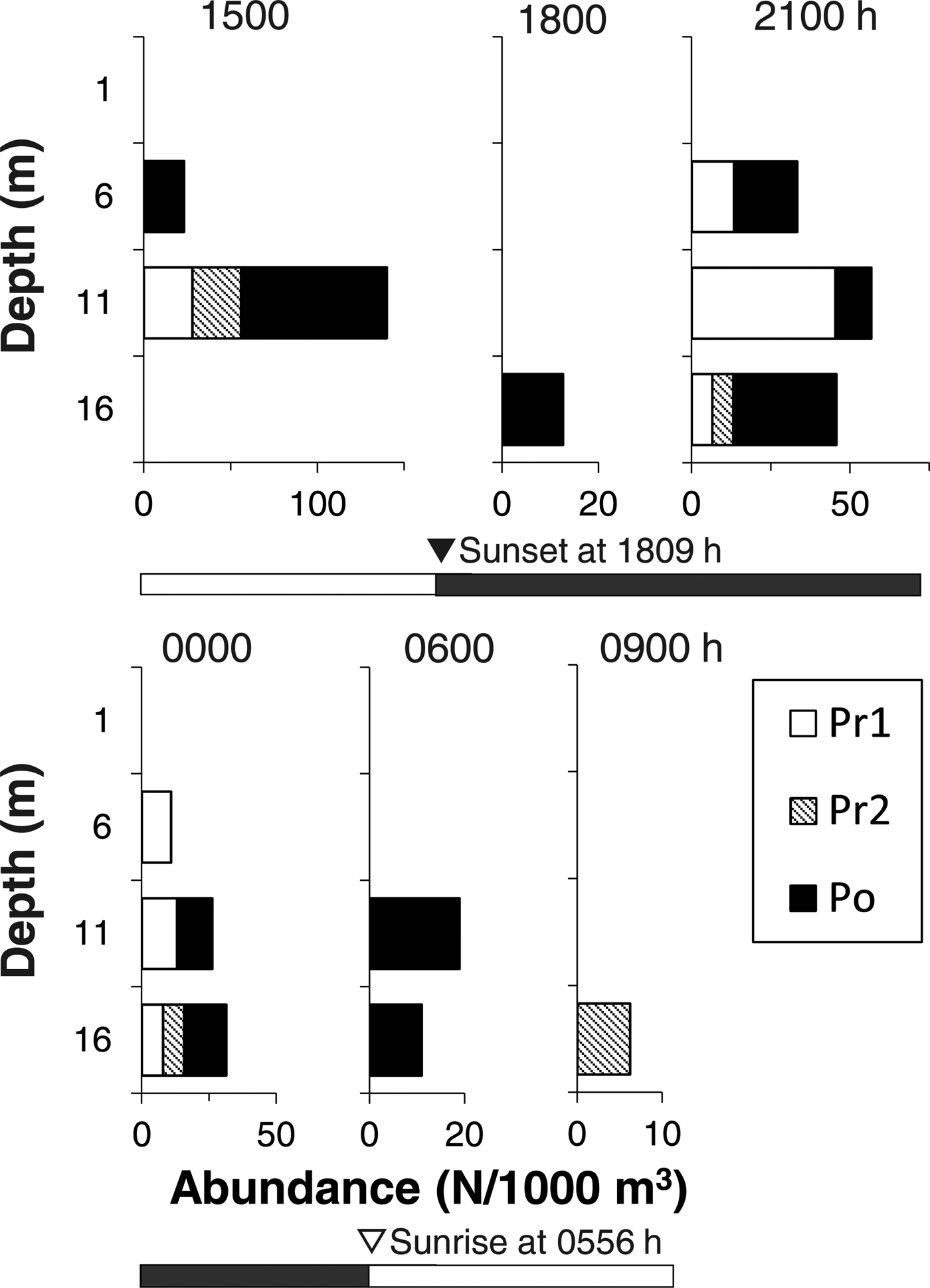
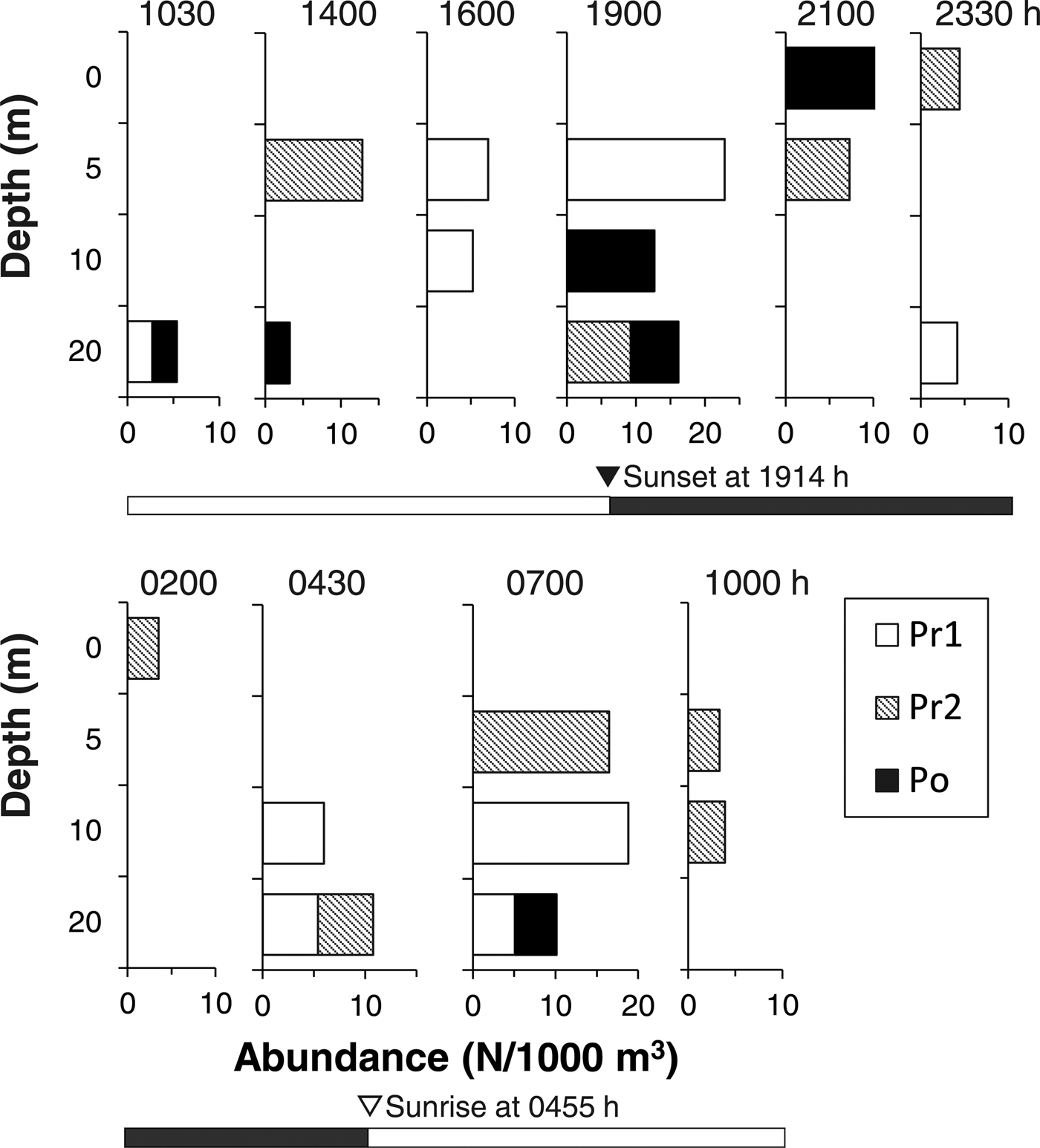
Larvae of T. japonicus were not observed at 1 and 6 m depths, but were collected at 11 and 16 m depths during the daytime (0900–1100 h) in 2015 (Figure 2). Similarly, the larvae were collected only at 16 m (at 1500 and 1800 h) or at 11 and 16 m depths (at 0600, 0900 and 1200 h) during the daytime in 2016 (Figure 3). In September 2017, one larva of T. japonicus at stage Po was collected at 6 m during the daytime (1500 h), but other individuals were collected at 11 and 16 m depths during the daytime (Figure 4). Conversely, T. japonicus larvae were collected from all depths during the night from 2100–0300 h in 2016 and from depths of 6–16 m during the night in September 2017. However, larvae of stage Pr1 were not present in samples collected from depths of 1 or 6 m irrespective of day and night in 2015 and 2016 (Figures 2 & 3). Similarly, the larvae of stage Pr1 were not collected from 1 m depth in 2017, although four individuals were collected from a depth of 6 m during the night (Figure 4). In September 2016, significant differences in the collection depth between day and night were observed for stages Pr2 (U = 2033, P < 0.001) and Po (U = 1901.5, P < 0.001), while no significant differences were observed for stage Pr1 (U = 54.5, P = 0.33). In September 2017, no significant differences in the collection depth between day and night were observed for each stage (Pr1: U = 1, P = 0.62; Pr2: U = 7, P = 0.88; Po: U = 67, P = 0.92).
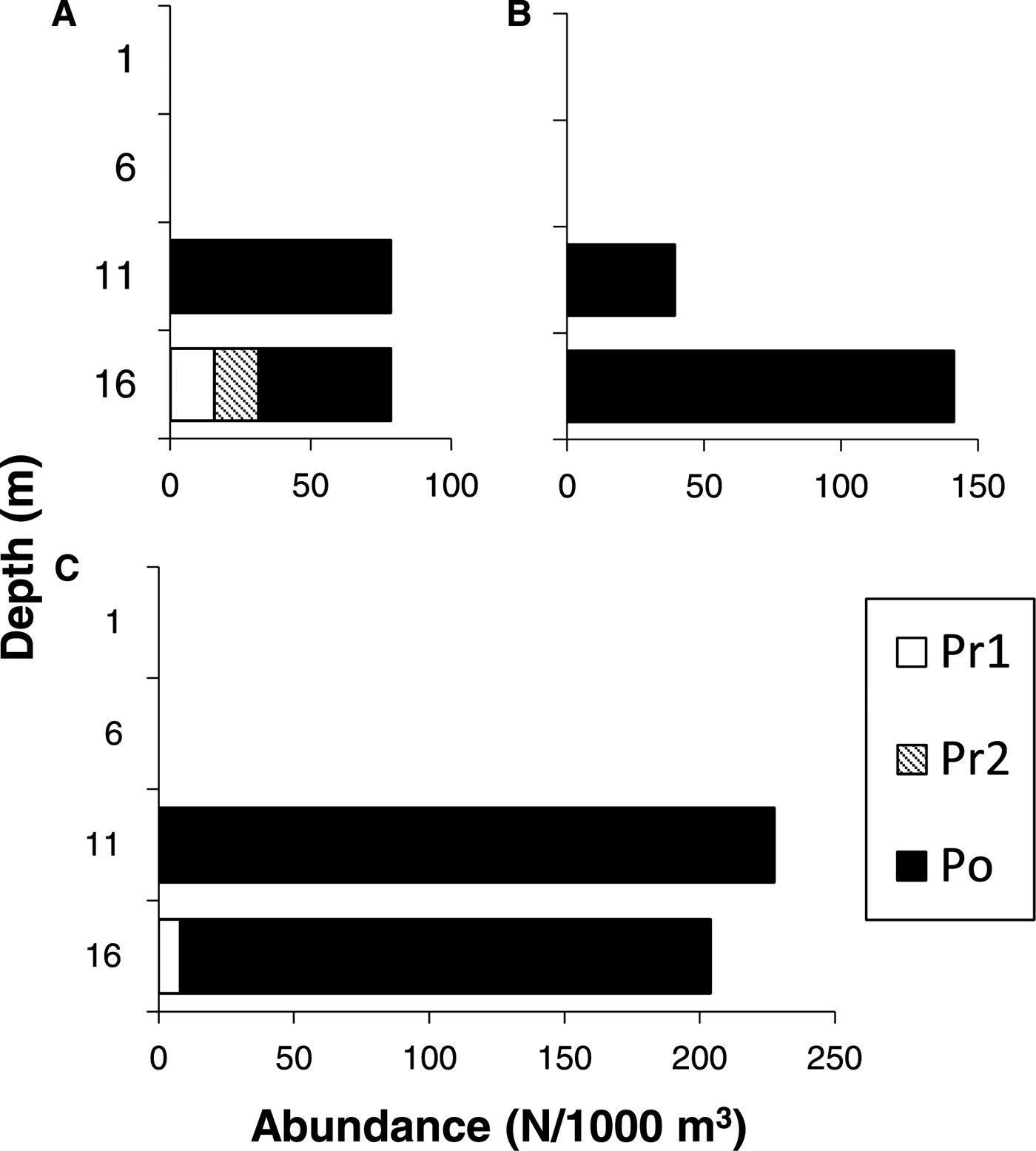
Fig. 2. Diurnal distribution of Trichiurus japonicus larvae at (A) 0900 h on 17 September, (B) 1000 h on 17 September, and (C) 1100 h on 18 September 2015. The number of collected larvae per 1000 m3 is shown. Developmental stages (Pr1–Po) were identified.

Fig. 3. Diel changes in the vertical distribution of Trichiurus japonicus larvae in September 2016. The number of collected larvae per 1000 m3 is shown. Detailed information on larval total length by year and developmental stage is shown in Table 1. Developmental stages (Pr1–Po) were identified. Open and closed bars indicate daylight (day) and dark (night) periods, respectively.

Fig. 4. Diel changes in the vertical distribution of Trichiurus japonicus larvae in September 2017. The number of collected larvae per 1000 m3 is shown. Detailed information on larval total length by year and developmental stage is shown in Table 1. Developmental stages (Pr1–Po) were identified. Open and closed bars indicate daylight (day) and dark (night) periods, respectively.
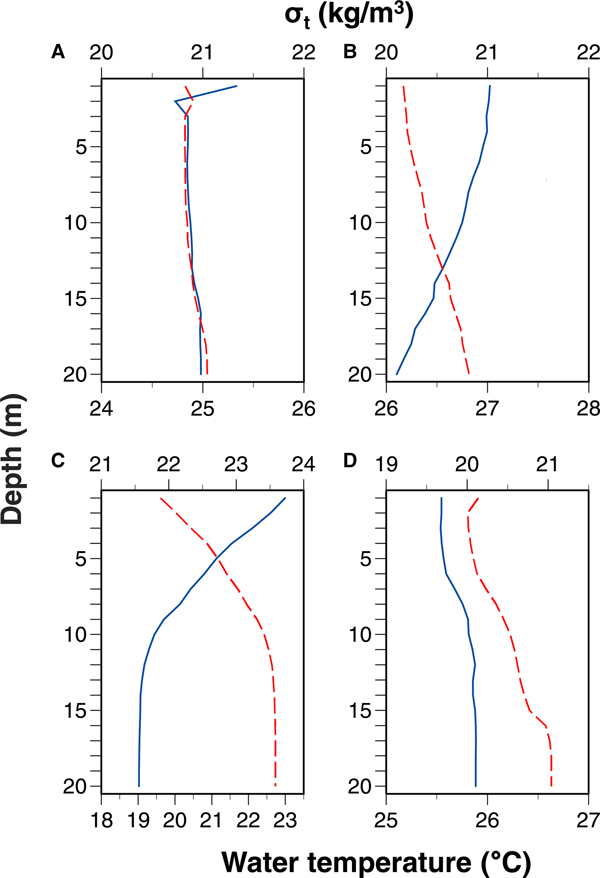
Water temperature was higher at shallower depths except for in September 2017 (Figure 5). Seawater density was lower at shallower depths. Differences in both water temperature and seawater density between 1 and 20 m depths were the greatest in June 2017. No clear thermoclines or pycnoclines were observed.
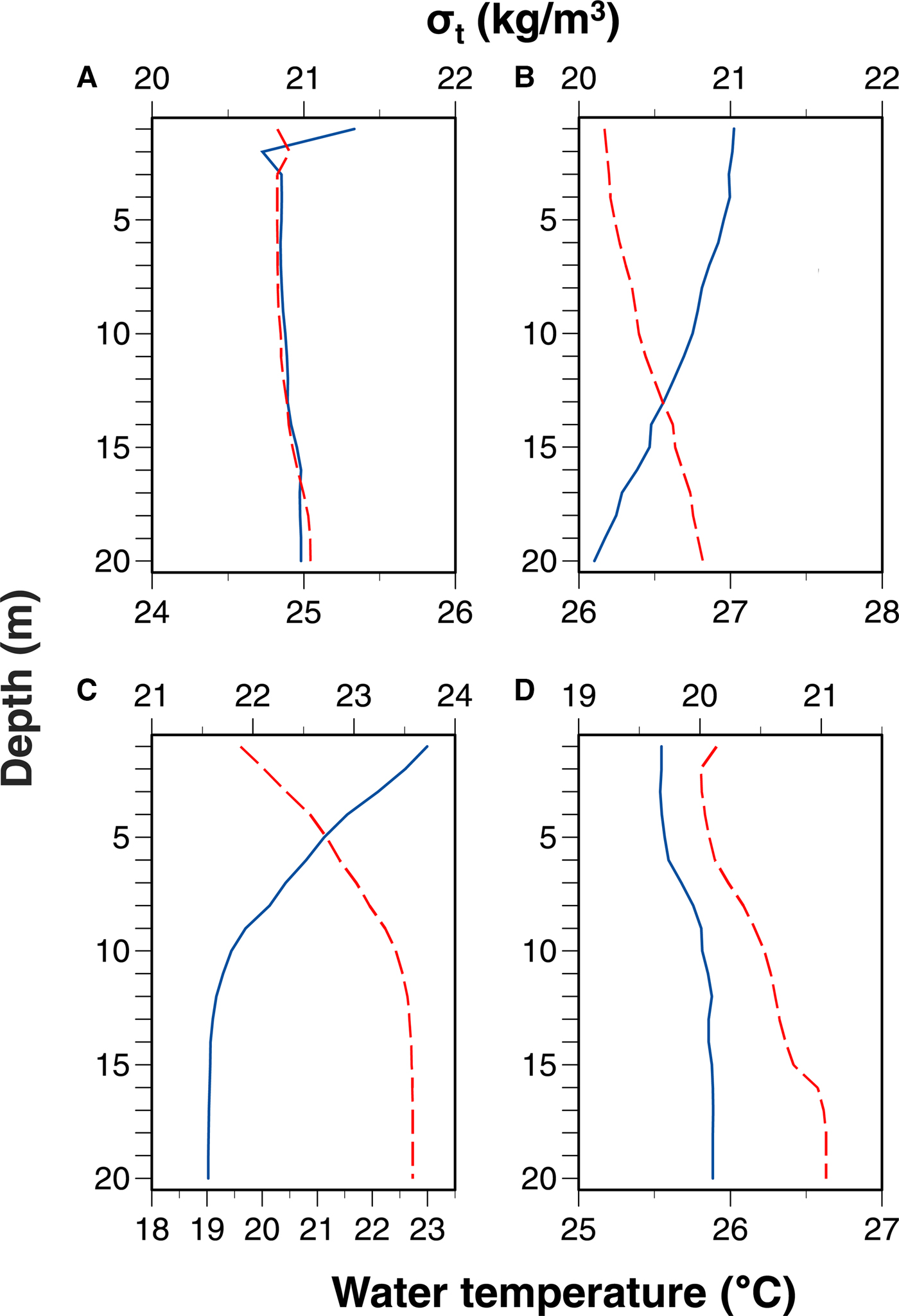
Fig. 5. Vertical profiles in average water temperature and seawater density (σ t) in (A) September 2015, (B) September 2016, (C) June 2017 and (D) September 2017. Solid and dashed lines indicate water temperature and σt, respectively. Diel changes in vertical profiles in temperature and salinity are shown in the supplemental figures.
In 1997, 36 T. japonicus larvae were collected. Their size range was 4.1–7.5 mm TL (stage: Pr1–Po). The larvae were collected from 5–20 m depths during the daytime, while some larvae except for stage Pr1 were collected from the surface layer (0 m) during the night (Figure 6). No significant difference in the collection depth between day and night was observed (Pr1: U = 6, P = 0.17; Pr2: U = 32, P = 0.18; Po: not applicable because of small sample sizes), although presence of larvae at surface was observed only during the night.

Fig. 6. Diel changes in the vertical distribution of Trichiurus japonicus larvae in June 1997. The number of collected larvae per 1000 m3 is shown. Detailed information on larval total length by year and developmental stage is shown in Table 1. Developmental stages (Pr1–Po) were identified. Open and closed bars indicate daylight (day) and dark (night) periods, respectively.
DISCUSSION
This is the first study to describe the vertical distribution of Trichiuridae larvae during the night and to clarify their DVM. The daytime patterns in the vertical distribution of T. japonicus larvae corresponded with those reported previously: the larvae did not appear at the surface, but were mostly found at depths of around 50 m (Okiyama, Reference Okiyama1965; Horiki, Reference Horiki1981; Munekiyo & Kuwahara, Reference Munekiyo and Kuwahara1986; Hayashi, Reference Hayashi1990).
The absence of stage Pr1 larvae near the surface throughout the surveys indicates that the DVM of T. japonicus larvae depends on the developmental stage. The formation of the swim bladder, as observed at stage Pr2, is likely related to the vertical migration, because the swim bladder plays important roles in changing depth or acquiring neutral buoyancy (Kanwisher & Ebeling, Reference Kanwisher and Ebeling1957). Swim bladder inflation at night has been observed in larvae of other species, including Clupeidae (Uotani, Reference Uotani1973; Hoss & Phonlor, Reference Hoss and Phonlor1984; Landaeta & Castro, Reference Landaeta and Castro2013), the red seabream P. major (Kitajima et al., Reference Kitajima, Tsukashima and Tanaka1985) and Japanese Spanish mackerel (Shoji et al., Reference Shoji, Maehara and Tanaka1999). Thus, the formation of the swim bladder enables the larvae to adjust their buoyancy and to move up towards the surface at night, as well as to reduce offshore advection (Landaeta & Castro, Reference Landaeta and Castro2013). However, another possibility should be considered for the timing of swim bladder inflation. The swim bladder seemed to connect to the alimentary canal in the larvae at stages Pr2 and Po, indicating that they are physostomous, at least during larval stages. If so, the larvae have to ascend to the surface to ingest gas bubbles to inflate their swim bladder. Further observation is necessary to elucidate this.
The swim bladder development begins before the yolk-sac absorption in T. japonicus (Munekiyo, Reference Munekiyo1991). It is different from other species. For example, the swim bladder begins to inflate from the larvae after yolk-sac absorption in Pacific bluefin tuna Thunnus orientalis (Ina et al., Reference Ina, Sakamoto, Miyashita, Fukuda, Torisawa and Takagi2014). The yolk-sac larvae of Pacific bluefin tuna use yolk-sac and oil globule to acquire buoyancy. Similarly, Japanese Spanish mackerel larvae that complete yolk absorption show DVM, but yolk-sac larvae do not (Shoji et al., Reference Shoji, Maehara and Tanaka1999). The absence of DVM was also observed in preflexion (yolk-sac) larvae of many other species (Rodríguez et al., Reference Rodríguez, Cabrero, Gago, Guevara-Fletcher, Herrero, Hernandez de Rojas, Garcia, Laiz-Carrion, Vergara, Alvarez, Piñeiro and Saborido-Rey2015). Relationships between the presence/absence of DVM and the larval developmental stages in relation to yolk absorption and swim bladder formation should be further investigated in other species.
The stage Pr1 larvae appeared chiefly around the bottom layers, although T. japonicus spawns pelagic eggs. It is likely to be related to the egg distribution of this species. Eggs of early developmental stages are scarce around the surface and are abundant around middle layers ≥10 m depth (Horiki, Reference Horiki1981; Munekiyo & Kuwahara, Reference Munekiyo and Kuwahara1986). Developed eggs are more abundant at deeper layers around 50 m compared with eggs of early developmental stages observed at a depth of 20 m (Horiki, Reference Horiki1981). Thus, larvae would hatch around middle-bottom layers and spend a little time there until their swim bladders are formed.
The vertical distribution of larvae is often affected by the hydrographic stratification. Thermoclines and/or pycnoclines may limit the distribution of larvae (Olivar et al., Reference Olivar, Sabatés, Alemany, Balbín, Fernández de Puelles and Torres2014). In an offshore environment, larvae of some species show diel movement between above and below the pycnocline formed at depths ranging from 36–108 m, and others remained above the pycnocline (Sakuma et al., Reference Sakuma, Ralston and Roberts1999). However, the DVM is often not observed in well-mixed environments (Rodríguez et al., Reference Rodríguez, Cabrero, Gago, Guevara-Fletcher, Herrero, Hernandez de Rojas, Garcia, Laiz-Carrion, Vergara, Alvarez, Piñeiro and Saborido-Rey2015). Clear thermoclines or pycnoclines were absent, especially in September, in our study (Figure 5) probably because of the shallow water depth of the sampling site. Nonetheless, larval appearance at depths with relatively low seawater density during the night may reflect the DVM.
The ecological significance of the nocturnal migration of T. japonicus larvae is unclear. Since these larvae feed during the day (Munekiyo & Kuwahara, Reference Munekiyo and Kuwahara1985), their upward movement to the surface is not likely to increase their foraging success at night. One possible explanation is that inflation of the swim bladder at night reduces the energy required for maintaining a position in the water column and also reduces predation by predators responding to prey activity (Hunter & Sanchez, Reference Hunter and Sanchez1976). However, it is not yet known whether the upward movement of larvae is advantageous or not. Because not all larvae of T. japonicus at stages Pr2 and Po moved up to the surface, other factors such as the condition of larvae (Sclafani et al., Reference Sclafani, Taggart and Thompson1993) could affect the larval distribution. Further observations on the energy expense or survival at various depths are necessary to elucidate the role of the vertical migration of T. japonicus larvae.
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at https://doi.org/10.1017/S002531541800019X
ACKNOWLEDGEMENTS
The authors are grateful to the students of the Laboratory of Biology of Aquatic Resources, Hiroshima University, and the staff of the training and research vessel ‘Toyoshiomaru’ for their help in sample collections. We thank Dr S. Harada, Wakayama Prefectural Fisheries Experimental Station, for his advice on larval collection. We also thank anonymous reviewers for their critical comments on the manuscript.
FINANCIAL SUPPORT
This work was partly supported by the Environment Research and Technology Development Fund (S-13) granted by the Ministry of the Environment, Japan.