INTRODUCTION
The veined rapa whelk is originally native to the Sea of Japan, Yellow Sea, Bohai Sea, and the East China Sea to Taiwan (Chung et al., Reference Chung, Kim and Kim1993; ICES, 2004). This species was transported to the Black Sea via the ballast waters of merchant ships, and was firstly observed in Novorossiysk Bay in 1947; then in Crimea in 1949, in Batumi in 1950, in Romania in 1955, in the Bosporus in 1960, and in the city of Trabzon in 1962 (Duzgunes et al., Reference Duzgunes, Sahin, Bascinar and Emiral1997). After 1969 they were distributed to the Sea of Marmara and the Aegean Sea via the Bosporus (Bilecik, Reference Bilecik1990). They can be found in all coastal waters of the Black Sea, in depths up to 60 m, in sandy, muddy and algal habitats around mussel banks, and feed on shellfish, such as the Mediterranean mussel (Mytilus galloprovincialis), oyster (Crassostrea gigas) and Venus clam (Chamelea gallina) (Duzgunes, Reference Duzgunes2001).
Whelks are harvested mainly by dredging and to a lesser extent by diving. In recent years, the use of traps has been recommended by scientists instead of dredging, which is very destructive to the sea bottom and demersal flora and fauna (Saglam et al., Reference Saglam, Kutlu, Bascinar, Dagtekin, Duzgunes and Sahin2007). The veined rapa whelk is one of the new stocks exploited by Turkish fishermen since 1986. At present, their total production is 6534 t, of which 6348 t were harvested from the Black Sea in 2011 (TUIK, 2012). The majority of production (97%) comes from the Black Sea, with the region east of Sinop city and the Georgian border accounting for 75% of the total (Figure 1) The production of the veined rapa whelk increased to reach a maximum level (14,034 t) in 2004 and gradually decreased to 6534 t in 2011 due to lack of a sufficient food supply from shellfish stocks and an increase in population; it has no conventional predators, such as starfish, in the Black Sea.
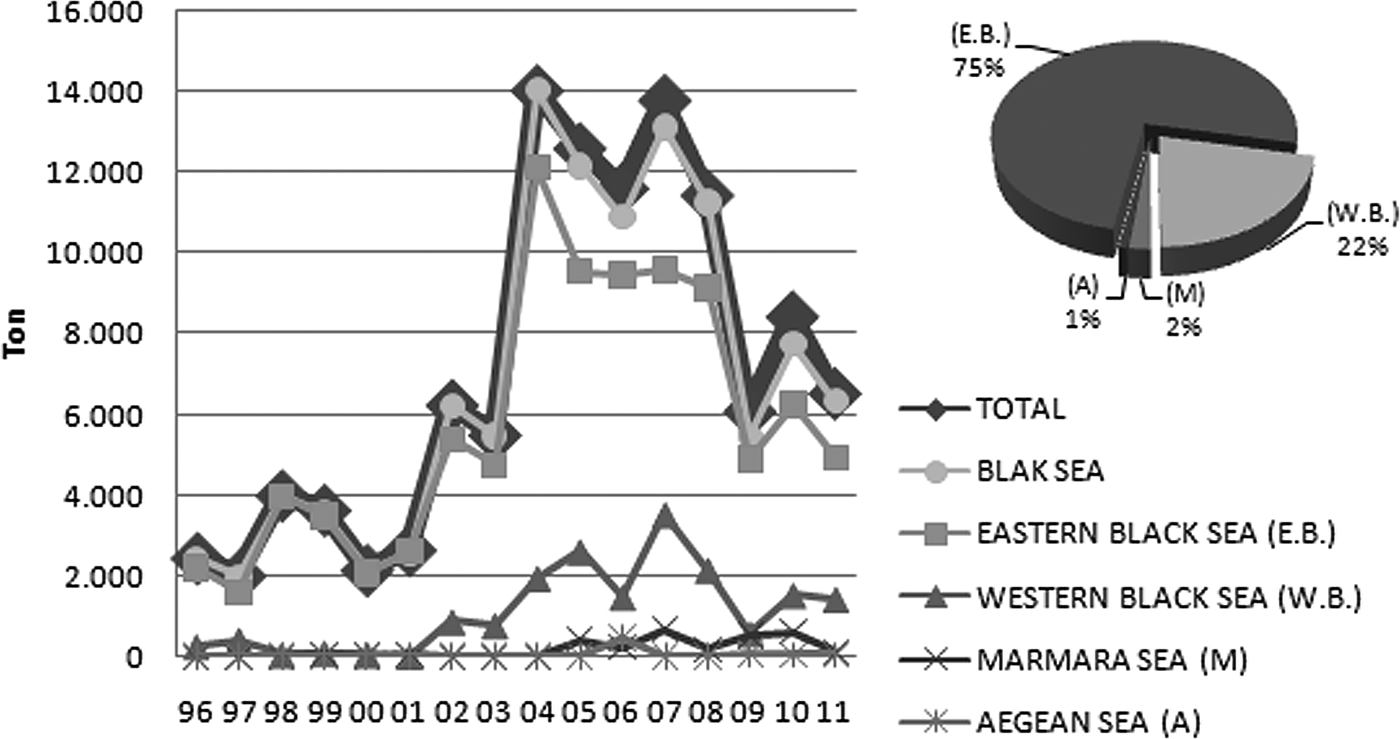
Fig. 1. Catch quantities of the veined rapa whelk in Turkey from 1996 to 2011.
According to observations among Riparian countries it is noted that there is a great scientific interest in carrying out research surveys on Rapana venosa (Valenciennes, Reference Duzgunes1846) stocks in the exclusive economic zones of each country to understand the impact of this invasive species on the Black Sea ecosystem. It is one of the most important research fields of interest of the General Fisheries Commission for Mediterranean (GFCM), the Black Sea Environmental Program (BSEP) and the European Union Scientific, Technical and Economical Committee for Fisheries (STECF). The results of this survey may provide information about the recent state of the veined rapa whelk population in order to inform further management decisions in the Black Sea.
MATERIALS AND METHOD
This survey was carried out in the coastal waters of Samsun Province which is the major commercial fishing area for veined rapa whelk dredgers that are widely used to harvest this species. Samples were obtained directly from fishing vessels between June and November 2011 and transferred daily to the laboratory in the Faculty of Marine Sciences, Ordu University. When the number of samples was high they were frozen at −35°C to keep samples longer. The length and width of the samples were measured with callipers to the nearest 0.01 mm and weighed with a Prescia balance with 0.001 g sensitivity. Sex determination was done by observing gonads with the naked eye after breaking the shells. The yellow coloured tissue on the terminal of the internal organs is the indicator of a female and a brown colour indicates a male (Erik, Reference Erik2011). The age of samples was determined by the Bhattacharya method with FISAT software using length–frequency data (Bhattacharya, Reference Bhattacharya1967). Length–weight, width–weight and length–width relationships were derived as W = aL b , W = aL w b and L = bL w − a, respectively, in which the parameters of ‘a’ and ‘b’ were calculated by the least squares method (Schaeperclaus, Reference Schaeperclaus1967; Lagler, Reference Lagler1969; Ricker, Reference Ricker1975). In these equations W is total weight, L is total length and L w is width.
Von Bertalanffy growth equations of age–length and age–weight were derived as:
where L t is length at any age t, L ∞ is asymptotic length, k is Brody growth coefficient, t is age, t 0 is theoretical age when length of shellfish is zero, W t is weight at any age t, W ∞ is asymptotic weight and b is a regression coefficient for the length–weight relationship (Ricker, Reference Ricker1975).
Instantaneous total mortality rate (Z) was calculated from the slope (opposite sign) of the linear relationship between age converted length data (t 1 = t 0 − 1/k × ln(1 − L/L ∞) and frequencies (LnN/Δt) of each length group (Sparre & Venema, Reference Sparre and Venema1992) where L is length, t 1 is calculated age from mean of each length group, L is mean length, N is frequency and Δt are differences between adjacent two ages. Natural mortality rate was calculated by Pauly's (Reference Pauly1980) equation:
where M is natural mortality rate, L ∞ and K are Von Bertalanffy growth parameters and T is mean water temperature at the bottom in the Rapana habitat (15°C) (Sahin et al., Reference Sahin, Duzgunes, Engin, Mutlu and Hacimurtazaoglu2005).
The fishing mortality and exploitation rates were calculated as F = Z − M and E = F/Z, respectively (Gulland, Reference Gulland1988; King, Reference King1995; Avsar, Reference Avsar1997; FAO, 2000).
Statistical analyses were done where necessary, i.e. the Chi square test for testing the differences between sex rates and the Student's t-test for comparing mean lengths of sex-ratios on different ages and time (Sokal & Rohlf, Reference Sokal and Rohlf1995).
RESULTS
The mean length, weight and width of 1704 specimens of Rapana venosa were calculated as 56.8 ± 0.36 mm, 45.7 ± 0.89 g and 42.5 ± 0.29 mm, respectively (Table 1; Figure 2). Females have slightly higher values than males but the difference is statistically insignificant. Minimum and maximum values of each parameter were also given in parentheses. The length–weight relationship was derived as W = 0.0006 L 2.719 for both sexes (N = 1704, R 2 = 0.826). According to the statistical analyses there is no difference between the regression coefficient of males and females. The relationship between length–width was found as L W = 0.793L − 2.601 (R 2 = 0.945). There is also a strong correlation between the weight and width of Rapana as R 2 = 0.811 in the equation of W = 0.004L W 2.437.
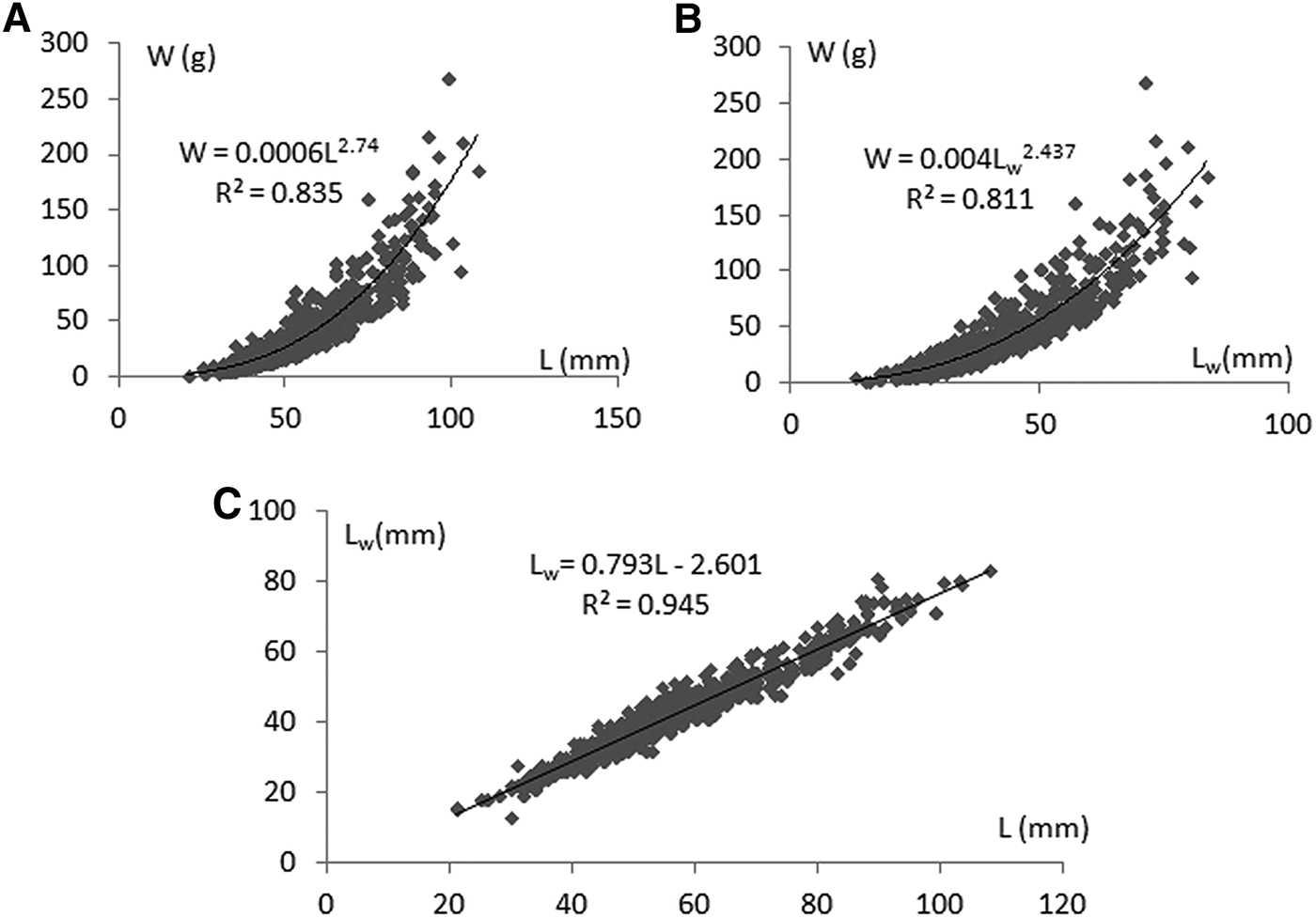
Fig. 2. Length–weight (A), width–weight (B) and length–width (C) relationships in Rapana venosa population (both sexes).
Table 1. Mean total length (mm), weight (g), width (mm) and relationships between length–weight, length–width and width–weight of Rapana venosa population in the Central Black Sea in 2011 (±standard error, ranges in parentheses).

The sex-ratio was found as (F:M) 1:1.15. Chi square analyses show that the difference between females (46.42%) and males (53.58%) is not statistically significant. So, it can be said that male and female were equally represented in the R. venosa population in the Black Sea. The rates of females to males in the sampling period were derived as 1:1.16 in June, 1:1.21 in July, 1:1.23 in August, 1:1.09 in September, 1:1.10 in October and 1:1.17 in November. The total male to female ratio for 2011 was 1:1.15 (Figure 3). These monthly figures showed that the ratio of females is also less than males in monthly samplings but the differences were also found to be statistically insignificant.
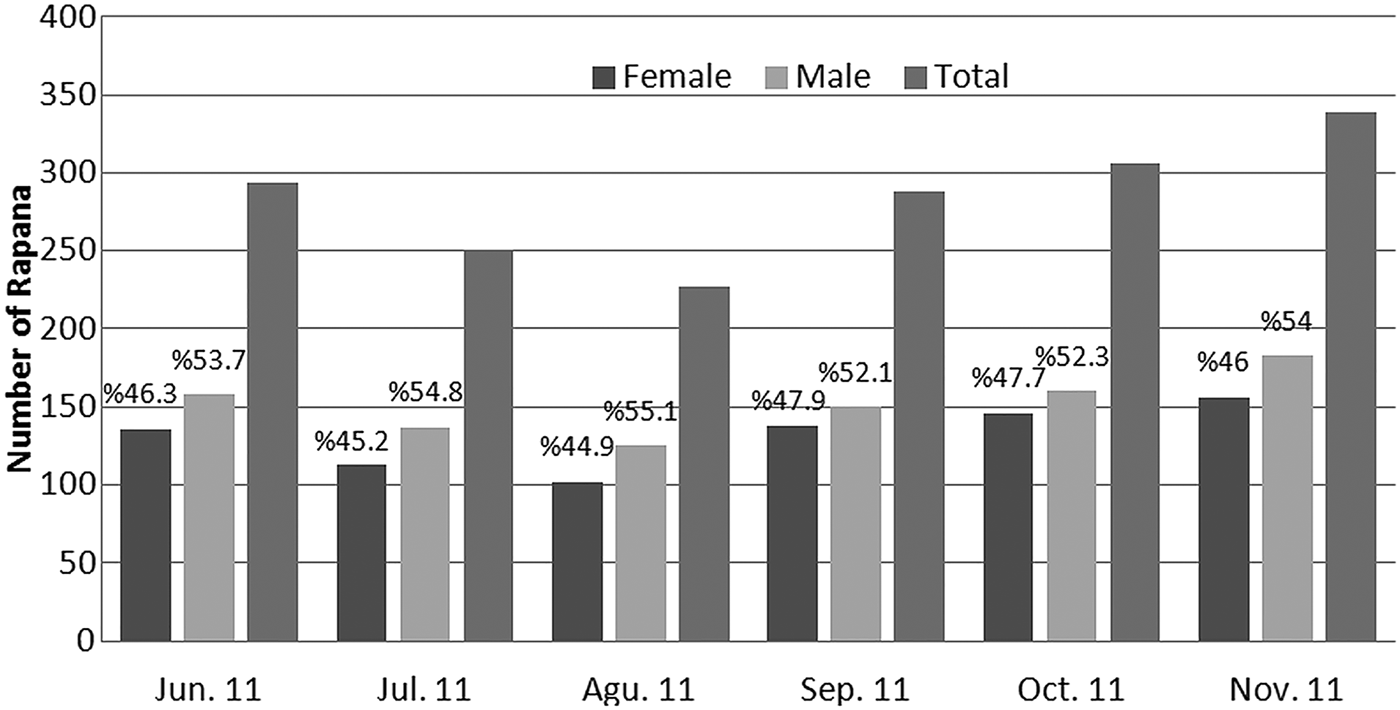
Fig. 3. Frequency distribution of veined rapa whelk by sexes and months in 2011.
Table 2 and Figure 4 show variations of length, width, weight and sex-ratios by months. The bigger sized individuals were observed in June, then gradually decreased till November. Similar evaluations can be done for mean weight and mean width values for both sexes. The reason for this reduction can be explained by the recruitment of the population after spawning (which starts in May and peaks in June to August) and the addition of younger cohorts to the population. The age of R. venosa was determined by the Bhattacharya method based on length–frequency distribution of the samples. Figure 5 shows seven age groups derived by this method. The first year-class is the age of 0+ with a mean length of 22.67 ± 0.66 mm. Other mean lengths for successive ages are given in Table 3. These data were also used in order to estimate Von Bertalanffy growth parameters; L ∞, k and t 0 with the Ford–Walford method which were also used to compute calculated lengths. According to the regression between L t and L t+1 the Ford–Walford method gives the estimation of L ∞ and k as, 112.35 mm and 0.310, respectively. The parameter of t 0 is −0.486 computed from the length converted catch curve (Pauly, Reference Pauly1979) (Figure 6). The differences between lengths derived by two methods were found to be statistically insignificant. Asymptotic length was also estimated as 243.94 g from the length–weight relationship using the asymptotic length computed. Age–length and age–weight equations can be summarized as follows (Figure 7):

Fig. 4. Mean length, width and weight of Rapana venosa by months.

Fig. 5. Composite distributions of the pooled length–frequency composition of veined rapa whelk identified by the Bhattacharya (Reference Bhattacharya1967) method.
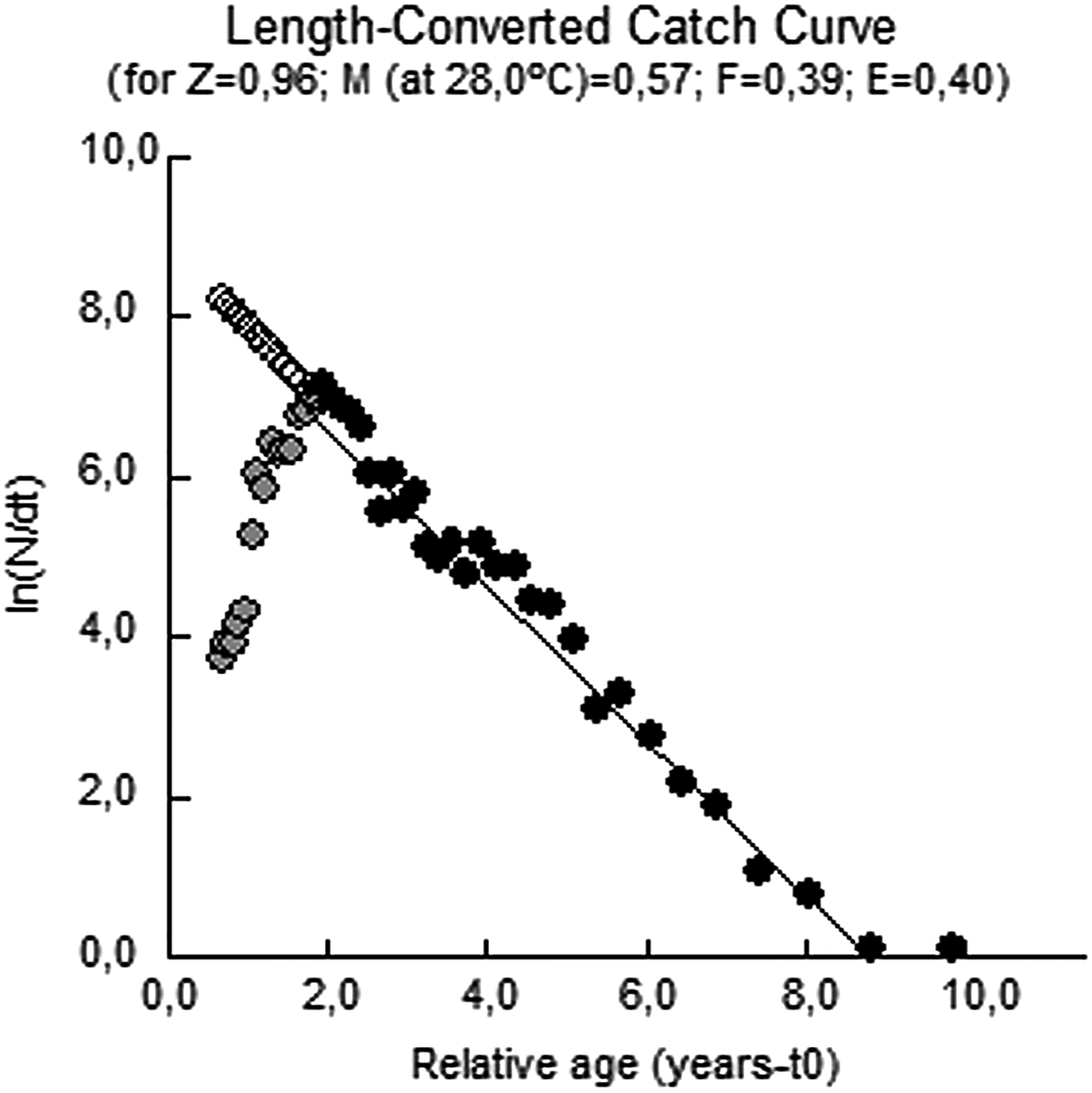
Fig. 6. Length converted catch curve of veined rapa whelk from the Central Black Sea.

Fig. 7. Age–length and age–weight relationship of Rapana venosa population in the Central Black Sea.
Table 2. Mean length, weight, width (±standard error) and sex-ratio of Rapana venosa by months.
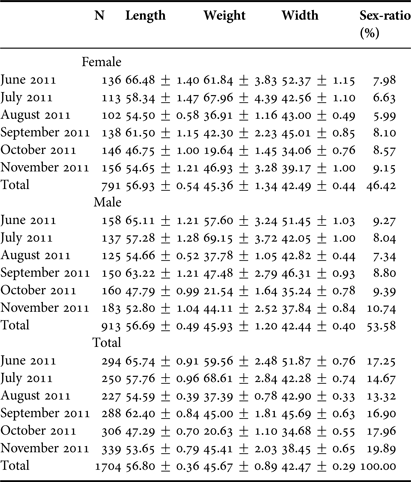
Table 3. Mean length (cm) of Rapana venosa computed from Bhattacharya method and Von Bertalannfy growth equation (VBGE) at corresponding ages.

Mortality rates (Z, M, F) and the exploitation rate were all derived by the catch curve method as Z = 0.96, M = 0.57 and F = 0.39. Annual mortality and survival rates were calculated as 62 and 38% in relation to Z, respectively. The exploitation rate (E) was 0.40 for the 2011 fishing season.
DISCUSSION
In this study, the sex-ratio in the Rapana venosa population was found to be 1:1.15; the number of males in the samples was slightly higher than females, though this difference is not statistically important. Saglam (Reference Saglam2003) reported the F:M ratio as 1:1.6 for the samples obtained from the Eastern Black Sea; mainly in the length groups of 50–60 and 60–70 mm males were dominant. There are also regional differences in the sex composition of the samples taken from Samsun (Central Black Sea), Ordu and Trabzon (both in the Eastern Black Sea) which were 1:1, 1:1.4 and 1:1.6, respectively, in the surveys carried out between April 2006 and February 2007 (Saglam et al., Reference Saglam, Duzgunes, Kutlu, Dagtekin, Bascinar, Selen and Sahin2008). A similar slight difference was reported by Samsun et al. (Reference Samsun, Erik, Kalayci and Dalgic2008) in Sinop (Central Black Sea) as 1.15:1. Major differences were reported by Erik (Reference Erik2011) in the same area as 1:1.8 which is significantly different from the previous findings. All these figures on slight abundance on behalf of males may be the indication of possible imposex, as reported by Micu et al. (Reference Micu, Comanescu and Kelemen2009) in Romanian Black Sea costs. However, more scientific research is needed in the survey area for imposex.
The size of R. venosa in Chesapeake Bay, which is the most recently invaded area, is between 102.7–149.0 mm (Harding & Mann, Reference Harding and Mann1999) while the size range is reported as 67–136.7 mm in the Adriatic Sea (Savini et al., Reference Savini, Castellazzi, Favruzza and Occhipinti-Ambrogi2004). In the case of the Black Sea, Prodanov et al. (Reference Prodanov, Konsulova and Todorova1995) reported that the size range of the samples showed a variation between 70–92 mm and 80–172 g off the Bulgarian coast. The mean length and weight of R. venosa in the Eastern Black Sea (Trabzon) was reported as 62.25 ± 0.19 mm and 47.22 ± 0.45 g (Duzgunes et al., Reference Duzgunes, Unsal and Feyzioglu1992), and 55.96 ± 0.41 mm and 32.01 ± 0.9 g in the Western Black Sea (Sinop) (Samsun et al., Reference Samsun, Erik, Kalayci and Dalgic2008). In the same period, Saglam et al. (Reference Saglam, Duzgunes, Kutlu, Dagtekin, Bascinar, Selen and Sahin2008) reported the mean length and weight of the samples collected from Samsun, Ordu and Trabzon as 75.5 ± 0.64 mm, 65.9 ± 1.84 g; 48.7 ± 1.93 mm, 36.4 ± 6.33 g and 61.5 ± 0.45 mm, 48.2 ± 1.61 g, respectively. In the recent survey carried out by Erik (Reference Erik2011) in Sinop the calculated mean length and weight of R. venosa was 64.9 ± 0.23 mm and 46.1 ± 0.51 g. Finally, in the present study, we found that the mean size of R. venosa was 56.80 ± 0.36 mm and 45.55 ± 0.89 g in the Central Black Sea area in 2011. All these figures show that the mean sizes of R. venosa are similar in the Black Sea countries. The size of the individual is strongly influenced by environmental conditions, such as temperature and food supply (Yankova et al., Reference Koutrakis, Tsikliras and Sinis2013). The reason for these variations can be explained by the changes in demand from importing countries, reduction in prey quantities in some locations and different exploitation levels performed in certain locations.
There was a slight increase in mean values of all three parameters in September due to growth. In this period, trawling is legally permitted after mid-September and the majority of R. venosa fishermen use trawl nets to catch demersal fish species outside of a 3 mile zone, which causes a decrease in fishing efforts with regards to R. venosa. Thus, small scale dredgers are able to find large-sized R. venosa in this area in even smaller quantities, although they command higher prices.
Length–weight relationship parameters also show spatial and temporal variations according to the results of the surveys carried out on R. venosa populations (Table 4). Saglam et al. (Reference Saglam, Duzgunes, Kutlu, Dagtekin, Bascinar, Selen and Sahin2008) derived length–weight equations in Trabzon, Samsun and Ordu provinces as W = 0.0006 × L 2.712, W = 0.0011 × L 2.2560, W = 0.0002 × L 2.933, respectively, while Duzgunes et al. (Reference Duzgunes, Unsal and Feyzioglu1992) reported the relationship 20 years ago as W = 0.0004 × L 2.7716 (R 2 = 0.93). Both of the parameters of ‘a’ and ‘b’ are bigger than in the study carried out by Saglam et al. (Reference Saglam, Duzgunes, Kutlu, Dagtekin, Bascinar, Selen and Sahin2008). A similar trend can be seen in the previous studies of Saglam (Reference Saglam2004), Sahin et al. (Reference Sahin, Duzgunes, Engin, Mutlu and Hacimurtazaoglu2005) and later in Saglam et al. (Reference Saglam, Duzgunes, Kutlu, Dagtekin, Bascinar, Selen and Sahin2008) by comparing the ‘b’ coefficient calculated in the surveys. It shows that R. venosa had a higher growth rate in the past. The relationship between a specimen length and its weight varies too over time and between locations, depending on the abundance of food, competitors and reproductive activity (Yankova et al., Reference Koutrakis, Tsikliras and Sinis2013).
Table 4. Mean length, width, weight, sex-ratio and length–weight parameters of Rapana venosa populations in previous studies.

The findings of Wu (Reference Wu1988) in Laizhou Bay (W = 0.0001 × L 2.933) have shown that the ‘b’ coefficient is rather high compared to the Black Sea. The parameters in Table 4 indicate that the ‘b’ values of the samples from the west–east direction in the Black Sea are decreasing spatially. The minimum determination coefficient (R 2) was observed in our present study as 0.84 (Table 4).
In the present study, seven age groups were identified in the samples. In the previous study of Sahin et al. (Reference Sahin, Duzgunes, Engin, Mutlu and Hacimurtazaoglu2005) 0–5-year age groups were reported with population parameters of L ∞ = 103.97 mm, k = 0.345, t 0 = −0.310 and W ∞ = 213.52 g. In the present study, the same parameters were derived as L ∞ = 112.35 mm, k = 0.310, t 0 = −0.486 and W ∞ = 243.94 g although the growth rate is decreasing by years. A comparison of the condition coefficient ‘K’ also shows a slight decrease in R. venosa growth performance. Exploitation rate and fishing mortality are also in a decreasing trend in the region. Natural mortality is decreasing while total mortality rate seems stable over years (Table 5).
Table 5. Some population parameters of Rapana venosa.

This study contains findings about the recent population parameters in the Black Sea. It is the second survey giving age, length and weight data for R. venosa populations after 2005. From this point of view it is very important for the scientists working on R. venosa not only in the Black Sea but also in other locations in the world.
Rapana venosa is one of the important invasive species in the Black Sea. It became a dominant mollusc widely distributed all over the Black Sea due to a lack of natural predators such as starfish in the Black Sea ecosystem (Thomas & Himmelman, Reference Thomas and Himmelman1988; Bilecik, Reference Bilecik1990; Harding & Mann, Reference Harding and Mann1999). The fecundity of R. venosa is very high and their egg capsules are very resistant to all negative environmental conditions. As a result of these facts, all the coastal waters in the Black Sea were invaded by this species with a high growth rate at the beginning. Following this, the population biomass reached a maximum level in 2007 in line with the prey abundance in its habitat, but later the growth rate started to decrease, with specimens having smaller length and weight. At present, stocks in the Eastern Black Sea are smaller in size than in the Central and Western Black Sea. This is why the main commercial fishing effort is concentrated in a very limited area in the Central and Western Black Sea. This species is still threatening the existence of mollusc stocks in the Black Sea, i.e. the Mediterranean mussel (Mytilus galloprovincialis), oyster (Crassostrea gigas) and Venus clam (Chamelea galin). Also, the harvesting method of R. venosa by dredges is very harmful for the bottom substrate in the locations where bottom trawling is forbidden due to a narrow continental shelf area in the Eastern Black Sea. It would be useful to change the harvesting method to traps and pots, allowing R. venosa fishermen to catch throughout the whole year in order to reduce the population. By this measure, fishermen may benefit more from the R. venosa by catching bigger sized whelks which can grow better by utilizing the existing prey due to a reduced population in the sea.
More scientific surveys on R. venosa population are needed for assessment of the stock size, reproduction physiology (mainly imposex) and behaviour, and prey–predator relations in the whole Black Sea area.














