INTRODUCTION
Zooxanthellate stony corals have become increasingly popular as inhabitants of public and private show aquaria. The methodologies to breed and maintain zooxanthellate corals have improved substantially over the last 30 to 40 years, in particular techniques for illumination and water quality control (Borneman, Reference Borneman, Leewis and Janse2008; Carlson, Reference Carlson, Leewis and Janse2008).
More recently, nutrition has also been recognized as an important factor determining breeding success. Corals prey upon different types of plankton, detritus and dissolved organic matter, processes that have been described extensively in early studies on coral biology (e.g. Yonge, Reference Yonge1930; Goreau et al., Reference Goreau, Goreau and Yonge1971; Sorokin, Reference Sorokin1973). Heterotrophic feeding may provide the primarily autotrophic zooxanthellate corals with additional nutrition (Anthony & Fabricius, Reference Anthony and Fabricius2000) including nutritional supplements that cannot be obtained sufficiently from translocated photosynthetic products produced by the symbiotic zooxanthellae, such as nitrogen, phosphorous and essential organic compounds (Dubinsky & Jokiel, Reference Dubinsky and Jokiel1994; Allemand et al., Reference Allemand, Tambutte, Girard and Jaubert1998). Several studies indicated the benefits of heterotrophic feeding on coral performance (growth, calcification and photosynthesis: see reviews by Houlbrèque & Ferrier-Pagès, Reference Houlbrèque and Ferrier-Pagès2009; Osinga et al., Reference Osinga, Schutter, Griffioen, Wijffels, Verreth, Shafir, Henard, Taruffi, Lavorano and Gili2011). However, extensive feeding increases the costs for culturing corals (Lavorano et al., Reference Lavorano, Gili, Muti, Taruffi, Corsino, Gnone, Leewis and Janse2008). In addition, high loads of food supplied to aquaria will have a detrimental effect on water quality. It is therefore important to design optimal feeding regimes for aquarium corals. For this, detailed knowledge on the feeding behaviour of the targeted coral species is needed.
In this study, we investigated the feeding behaviour of the stony coral Galaxea fascicularis (Linnaeus 1767) under aquarium conditions, hereby focusing on zooplankton (nauplii of the brine shrimp Artemia sp.) as the targeted food item. Zooplankton is considered to be one of the main sources of food for stony corals (Houlbrèque & Ferrier-Pagès, Reference Houlbrèque and Ferrier-Pagès2009) and Artemia nauplii have been reported as a suitable prey item for several species of coral (Lavorano et al., Reference Lavorano, Gili, Muti, Taruffi, Corsino, Gnone, Leewis and Janse2008; Petersen & Laterveer, Reference Petersen, Laterveer, Leewis and Janse2008; Osinga et al., Reference Osinga, Schutter, Griffioen, Wijffels, Verreth, Shafir, Henard, Taruffi, Lavorano and Gili2011) including G. fascicularis (Hii et al., Reference Hii, Soo and Liew2008; Osinga et al., Reference Osinga, Charko, Cruzeiro, Janse, Grymonpre, Sorgeloos and Verreth2009). The study had the following objectives:
(1) to determine the maximal uptake of food particles per feeding event. It is common aquarist practice to feed batch wise; every day, one batch of food is added to the aquarium. Under such a feeding regime, the corals will be suddenly exposed to a large number of suspended food particles. Corals will capture the food, either until satiation occurs or until the available food is depleted. We studied the capture rate of G. fascicularis at different densities of available zooplankton prey in order to determine at which quantity satiation occurs;
(2) to investigate the ability of G. fascicularis to take up food particles when fed repeatedly with batches of prey. This information is needed to determine the maximal daily uptake of zooplankton prey by this species and hence, to determine the maximal contribution of heterotrophic feeding to its total carbon and energy budget; and
(3) to test whether the capture reflex of G. fascicularis is triggered by chemical cues originating from the prey. It has been reported that coral tentacles respond to the release of amino acids by prey items (e.g. Mariscal & Lenhoff, Reference Mariscal and Lenhoff1968). Mimicking this chemical triggering by adding prey extract prior to feeding may enhance the efficiency of food uptake in aquarium corals.
All experiments were conducted using prey clearance measurements as an indicator for zooplankton capture. The suitability of this method for feeding studies on passive filter feeders such as corals will be evaluated.
MATERIALS AND METHODS
Genetically identical colonies of G. fascicularis were prepared and maintained according to Schutter et al. (Reference Schutter, Kranenbarg, Wijffels, Verreth and Osinga2011) in a 600 dm3 aquarium system at Wageningen University (Experiments 1 and 3, six replicate colonies) and in the coral culture facility of Burgers’ Zoo (Arnhem, The Netherlands) as described by Schutter et al. (Reference Schutter, Van Velthoven, Janse, Osinga, Janssen, Wijffels and Verreth2008) (Experiment 1 and 2, nine replicate colonies). All colonies were captive bred and had been raised using Artemia nauplii as the main source of planktonic food. The number of polyps of each individual colony was counted. Numbers of polyps per colony ranged between 110 and 330. The colonies were nearly completely covered with living tissue, exhibiting only small stretches of uncovered skeleton at the bottom side of the colony, where the skeleton was attached to the substratum.
Experiment 1: prey capture at different densities of prey items
Coral colonies were incubated individually in 1500 cm3 incubation chambers as described by Osinga et al. (Reference Osinga, Charko, Cruzeiro, Janse, Grymonpre, Sorgeloos and Verreth2009). The chambers were filled with 1300 ml of seawater from the maintenance tanks in which the corals were kept in between measurements. Corals were put into the chambers at least 15 minutes prior to the start of each test. Freshly hatched Artemia nauplii (Great Salt Lake Artemia, Artemia International LLC, Fairview, USA) were enumerated (Osinga et al., Reference Osinga, Charko, Cruzeiro, Janse, Grymonpre, Sorgeloos and Verreth2009) and added to the incubation chambers. Numbers of nauplii added were normalized to polyp numbers to accomplish an equal availability of prey for each polyp. Hence, concentrations of prey items per dm3 were slightly different among replicate incubations. Densities applied ranged from 40 to 4000 nauplii polyp−1, which corresponds to start concentrations of nauplii in the incubation chambers (taking into account the number of polyps per colony) ranging from 4000 to 1,200,000 nauplii dm−3. All measurements were conducted at a photon flux density of 200 µE m−2 s−1. The incubation chambers were aerated with an air-stone to prevent under- or over-saturation of oxygen. The water in the chamber was continuously mixed throughout the incubations using a magnetic stirrer operated at a moderate speed, resulting in an average flow velocity in the chambers of approximately 10 cm s−1.
First, a series of 68 incubations was performed using the coral colonies maintained at Burgers’ Zoo with low numbers of prey items (40 to 350 nauplii polyp−1). Numbers of Artemia nauplii residing in the water column were quantified by counting the number of nauplii in three series of three replicate water samples of 20 cm3 taken from the incubation chamber after 15 and 60 minutes, respectively (Osinga et al., Reference Osinga, Charko, Cruzeiro, Janse, Grymonpre, Sorgeloos and Verreth2009). In order to increase sampling accuracy, the water in the chambers was gently mixed by hand prior to sampling, mainly to disturb vortex patterns that sometimes occurred as a result of the magnetic stirring. The nine experimental colonies were randomly assigned to the 68 incubations.
Second, a series of 17 incubations was performed using the coral colonies maintained at Wageningen University, hereby applying high numbers of prey items (400 to 4000 nauplii polyp−1). Numbers of Artemia nauplii residing in the water column were determined after 20, 40, 60, 80, 100 and 120 minutes by counting the number of nauplii in six series of three replicate water samples of 2, 5 or 10 cm3 (depending on the expected concentration) taken from the incubation chambers. Here, a longer incubation period of two hours was chosen, because in the previous study by Hii et al. (Reference Hii, Soo and Liew2008), satiation did not occur within one hour after feeding. The six colonies were randomly assigned to the incubations.
Corals were never used for incubations on two consecutive days, in order to prevent that previously used corals would still be satiated at the start of the next incubation. All incubations were performed at 26°C.
Artemia uptake was determined as follows:
where C0 = the initial concentration of Artemia nauplii (nauplii cm−3) at moment t0, Ct = the remaining concentration of Artemia nauplii (nauplii cm−3) at moment tt, Vwater = the volume of water added to the incubation chamber in cm3.
For some intervals, the clearance rate was determined. The clearance rate is equal to the amount of water that is completely cleared of Artemia nauplii per unit of coral biomass per unit of time. Clearance rate (Riisgård et al., Reference Riisgård, Thomassen, Jakobsen, Weeks and Larsen1993; Osinga et al., Reference Osinga, Kleijn, Groenendijk, Niesink, Tramper and Wijffels2001) is a concentration-independent measure for the efficiency of food capture; hence, a decrease in clearance rate indicates that satiation occurs. Clearance rate was calculated by the following equation, which describes the exponential reduction in Artemia nauplii concentrations as a function of time:
where Vwater = the volume of the incubation chamber (1300 cm3), N = the number of polyps, t = the measurement interval, i.e. the incubation time between two consecutive measurements of Artemia nauplii concentrations, C0 = the nauplii concentration at moment t0, and Ct = the nauplii concentration at moment tt.
Experiment 2: repeated batch feeding
Using the same setup as described above, we also subjected colonies of G. fascicularis to a series of four consecutive batch feeding events with freshly hatched Artemia nauplii. After each feeding event, corals were allowed to take up food for one hour, after which the decline in nauplii numbers was determined as described above. After taking the samples for nauplii enumeration, the water in the incubation chambers was refreshed (in 5 minutes) and the corals were given 10 minutes to acclimatize again before they received a new batch of Artemia nauplii. This resulted in a total incubation period of 285 minutes. The experiment was conducted with three densities of nauplii (50, 100 and 150 nauplii polyp−1). For each of these three experimental series, four colonies were randomly selected out of the nine experimental colonies maintained at Burgers’ Zoo. Hence, in total 12 incubations of 285 minutes were performed. All 12 incubations were performed at 26°C.
Experiment 3: testing the potential effect of chemical cues on feeding efficiency
Using the same equipment as described above, four colonies of G. fascicularis were each subjected to four different incubation treatments that all started with an initial feeding with a single batch of freshly hatched Artemia nauplii (quantity: 150 nauplii polyp−1). The four treatments were as follows: (1) repeated feeding (addition of a second batch of 150 nauplii polyp−1 after one hour); (2) repeated feeding (one hour after the initial feeding) with nauplii that had been pre-incubated at 26°C; this was done to test whether the nauplii would lose their attractiveness during the one-hour incubation period; (3) addition (one hour after the initial feeding) of an extract of Artemia nauplii, prepared by collecting the filtrate of an amount of freshly hatched nauplii reflecting 150 nauplii polyp−1, filtered over a 50 µm mesh; this was done to test if components leaking out of the fresh nauplii have the potential to increase the feeding efficiency of the coral on prey still residing in the incubation chamber after the first hour of the incubation; and (4) no addition after one hour (control). Artemia uptake was determined after 20, 40, 60, 80, 100, 120 and 360 minutes by counting the number of nauplii in three replicate water samples of 2, 5 or 10 cm3 (depending on the expected concentration) taken from the incubation chambers. All incubations were performed at 26°C.
RESULTS
At food densities ranging between 40 and 350 nauplii polyp−1, hourly prey capture showed a linear increase with food quantity (Figure 1; linear regression, R2 = 0.66, P = 0.00). There was no correlation between clearance rate and food quantity (data not shown), which indicates that satiation did not occur at the food densities supplied. However, when calculated over the first 15 minutes of the incubations, clearance rates appeared to be significantly higher than clearance rates calculated over the remaining 45 minutes of the incubations (paired samples t-test, P = 0.00; Figure 2). This indicates that satiation occurred during the course of the incubation regardless of the initial quantity that was supplied. In all incubations, substantial numbers of nauplii (>10% of the number that was initially supplied) were still found suspended in the water column after one hour.

Fig. 1. Prey capture in one hour by colonies of Galaxea fascicularis at prey densities between 40 and 350 nauplii polyp−1.

Fig. 2. Average clearance rates (error bars showing standard deviations) per time interval for Galaxea fascicularis colonies fed with 40 to 350 nauplii polyp−1.
In the incubation series with food densities higher than 350 nauplii polyp−1, the prey capture within the first hour after addition of food increased with food quantity up till a food quantity of 2000 nauplii polyp−1 (Figure 3). At food densities higher than 2000 nauplii polyp−1, prey capture did not further increase. Prey capture at satiation was around 1200 nauplii polyp−1. Cumulative prey capture was calculated for each of the six sampling points in time (20, 40, 60, 80, 100 and 120 minutes after feeding). In the first 40 minutes, cumulative capture increased with concentration (Figure 4). After the first 40 minutes, cumulative capture tended to level off, particularly at the higher food densities. For 3000 and 4000 nauplii polyp−1, cumulative uptake even started to decrease after 40 minutes, indicating that the corals had started to release nauplii that had been captured during the first 40 minutes. During the incubations with high food densities it was frequently observed that aggregates of Artemia nauplii were formed on the coral polyps (Figure 5), which were apparently not ingested. At food densities higher than 1000 nauplii polyp−1, the corals started to excrete high amounts of mucus and a layer of Artemia nauplii trapped in mucus appeared on top of the coral surface.

Fig. 3. Prey capture in one hour by colonies of Galaxea fascicularis at prey densities between 400 and 4000 nauplii polyp−1.
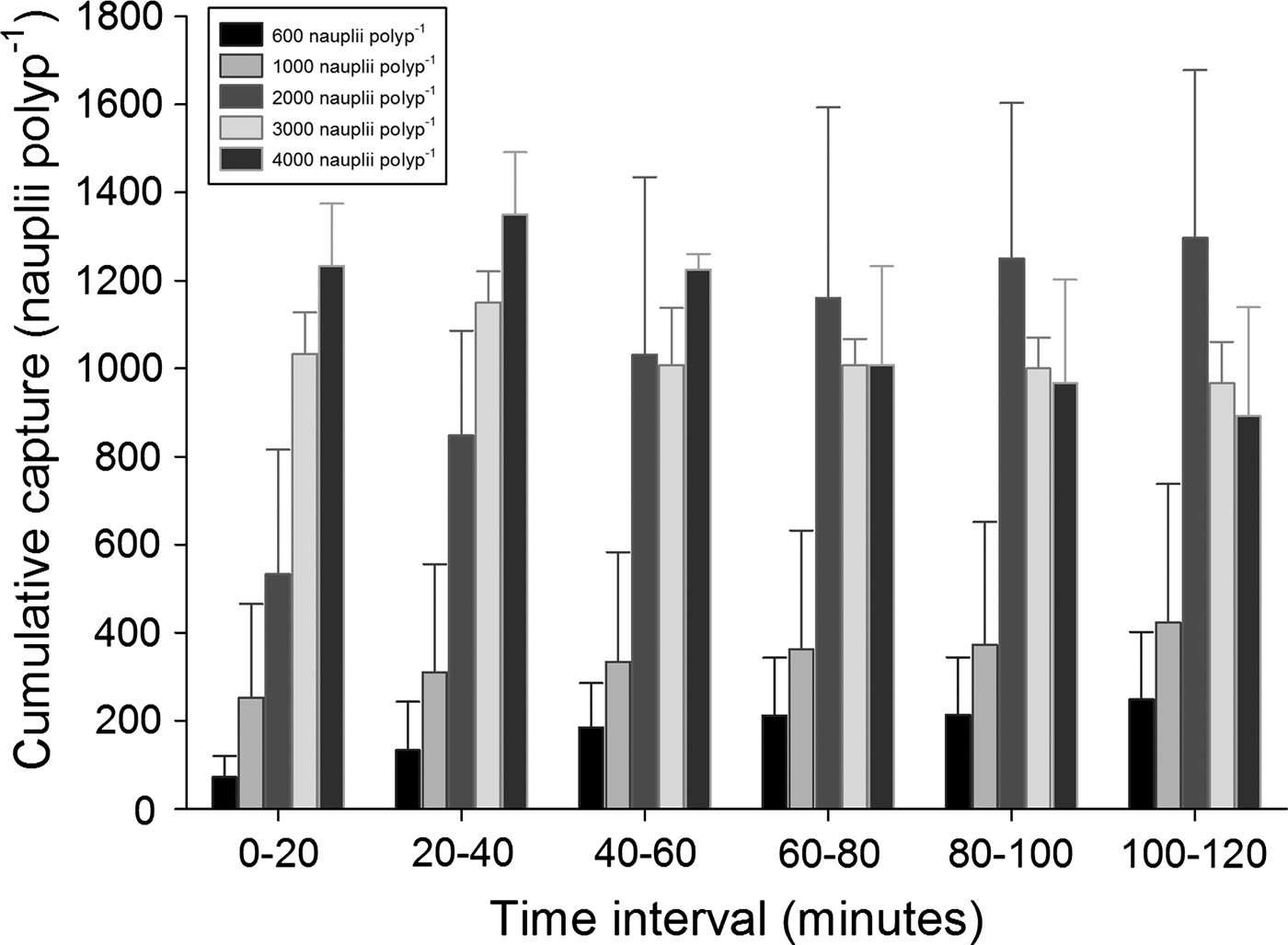
Fig. 4. Cumulative prey capture by colonies of Galaxea fascicularis (error bars showing standard deviations, N = 2) exposed to five different prey densities, calculated for six time-intervals.

Fig. 5. Formation of aggregates of Artemia nauplii on the surface of a colony of Galaxea fascicularis.
Results of repeated batch feeding (Figure 6A) show that at the lowest food quantity applied (50 nauplii polyp−1), hourly prey capture did not change profoundly between feeding events, although a significant difference was observed between the first and the third feeding event (analysis of variance (ANOVA) for repeated measures, Greenhouse–Geisser correction, Bonferroni post-hoc test P = 0.01). When 100 nauplii polyp−1 were supplied, there was a considerable, significant decrease in prey capture between the first and the three subsequent feeding events, the prey capture rates being more than 50% reduced (ANOVA for repeated measures, Tukey's least significant difference (LSD) post-hoc test, P < 0.05). At the highest food quantity supplied (150 nauplii polyp−1), the decrease in prey capture after the first feeding event was less pronounced (30%) and not significant at the 0.05 probability level (ANOVA for repeated measures, Tukey's LSD post-hoc test). Overall prey capture in four hours (i.e. when the hourly uptake rates of the four subsequent feeding events were added; Figure 6B) was almost twice as high when compared to the two other food densities applied, which did not significantly differ from each other (ANOVA, P = 0.94; differences between 50 and 150 nauplii polyp−1 and between 100 and 150 nauplii polyp−1 were significant, P = 0.028 and P = 0.034, respectively).

Fig. 6. Results of repeated batch feeding with three prey densities: (A) prey capture per polyp of Galaxea fascicularis per feeding event; (B) cumulative prey capture per polyp after four consecutive feeding events. Error bars represent standard deviations, N = 4.
The effects of adding freshly hatched Artemia nauplii, pre-incubated Artemia nauplii and Artemia extract on prey capture one hour after an initial feeding with fresh nauplii are presented in Figure 7, expressed as clearance rates calculated over the sampled time intervals. All incubations, including the control, showed a decrease in clearance rate within the first hour (i.e. after the initial feeding) and an increase in clearance directly after the additions of new materials after one hour. In the subsequent time intervals, again a decrease in clearance rate was observed for all treatments. Hardly any prey was captured between 2 and 6 hours after the start of the incubations, although more than 10% of the nauplii that had been added were still present in the water column after 6 hours. No significant differences were found between the four treatments (ANOVA followed by Tukey LSD, applied to each of the seven time-intervals analysed; data had been tested for normality, homogeneity of variance and sphericity; 0.05 was taken as the probability level for significance).

Fig. 7. Prey clearance rates (expressed in ml water cleared per polyp per minute) for colonies of Galaxea fascicularis exposed to an initial feeding with 150 Artemia nauplii polyp−1 followed by the addition after one hour of: (a) a second batch of 150 nauplii polyp−1; (b) a second batch of 150 nauplii polyp−1, which had been pre-incubated at 26 °C; (c) an extract of Artemia nauplii; and (d) no addition (control). Error bars represent standard deviations, N = 4.
DISCUSSION
Prey capture: a critical reconsideration of the prey clearance technique
Our results suggest that polyps of G. fascicularis are able to capture large amounts of zooplankton. Satiation of prey capture occurred at prey densities higher than 2000 nauplii polyp−1. The maximal capture observed in this study (1200 nauplii polyp−1) is much higher than previously reported for this species (Hii et al., Reference Hii, Soo and Liew2008). The highest capture found by Hii et al. (Reference Hii, Soo and Liew2008) was 113.6 Artemia nauplii polyp−1 h−1, which was measured at a prey density of approximately 330 nauplii polyp−1, the highest prey density applied by these authors. Hii et al. (Reference Hii, Soo and Liew2008) did not find satiation within their range of applied prey densities (3–330 nauplii polyp−1), which is in good agreement with our results. The capture rates observed by Hii et al. (Reference Hii, Soo and Liew2008) match fairly close to our data obtained within that range of prey densities (~145 nauplii polyp−1 h−1).
It was observed that the corals started to excrete large amounts of mucus at high prey densities. The coral mucus may have trapped many of the Artemia nauplii that were considered as captured in the prey clearance measurements. However, this process of entrapment in mucus should be regarded as passive sedimentation rather than active food capture and resembles the response of corals that suffer from high sedimentation loads (Fabricius & Wolanski, Reference Fabricius and Wolanski2000; Golbuu et al., Reference Golbuu, Victor, Wolanski and Richmond2003; Fabricius, Reference Fabricius2005). Indeed, colonies of G. fascicularis that were kept for more than 24 hours under these high prey densities started to show signs of necrosis after 24 hours and died completely after 48 hours. These observations show that prey clearance techniques do not enable a clear distinction between active food capture and passive sedimentation at high prey densities. It must be noted here that the high prey densities applied in this study do not reflect common practice in coral husbandry, where prey densities after batch feeding are not likely to exceed 100 prey items per coral polyp. However, when optimization of coral culture is concerned, food levels for optimal growth may be much higher than what is currently being applied in most coral aquaria (Osinga et al., Reference Osinga, Schutter, Griffioen, Wijffels, Verreth, Shafir, Henard, Taruffi, Lavorano and Gili2011). Since heterotrophic feeding adds to coral growth (Ferrier-Pagès et al., Reference Ferrier-Pagès, Witting, Tambutté and Sebens2003), it is of interest for coral culture to define the quantitative limits of food uptake by corals.
Some further inconsistencies were found when analysing the data. The observed decrease in clearance rate between the first 15 minutes and the subsequent 45 minutes of the incubations suggests that the capture efficiency of the corals decreased, which usually reflects the occurrence of satiation. It is, however, unlikely that satiation already occurred at the lowest prey density that was applied, because the absolute number of nauplii captured was much higher at higher prey densities. If it would indeed have been satiation that occurred after 15 minutes, it is also not logical that the corals always increased their capture efficiency immediately upon the addition of new food during repeated batch feeding experiments. Furthermore, it remains unexplained why cumulative uptake after four repeated feeding events did not increase between food densities of 50 and 100 nauplii polyp−1, while providing 150 nauplii polyp−1 led to a doubling of the cumulative capture over four hours. A critical reconsideration of the prey clearance method is required. Prey clearance has been widely used to quantify food uptake in actively pumping filter feeders such as sponges and bivalves (e.g. Jørgensen, Reference Jørgensen1955; Frost, Reference Frost1978; Riisgård et al., Reference Riisgård, Thomassen, Jakobsen, Weeks and Larsen1993; Clausen & Riisgård, Reference Clausen and Riisgård1996; Pile et al., Reference Pile, Patterson and Witman1996; Osinga et al., Reference Osinga, Kleijn, Groenendijk, Niesink, Tramper and Wijffels2001) and the method has been applied to passive filter feeders such as corals as well (Leversee, Reference Leversee1976; Dai & Lin, Reference Dai and Lin1993; Hii et al., Reference Hii, Soo and Liew2008; Osinga et al., Reference Osinga, Charko, Cruzeiro, Janse, Grymonpre, Sorgeloos and Verreth2009; Purser et al., Reference Purser, Larsson, Thomsen and Van Oevelen2010). The method is appropriate for sponges, because these organisms often have a prey retention efficiency that approaches 100% (Reiswig, Reference Reiswig1971; Pile et al., Reference Pile, Patterson and Witman1996). For cnidarians (such as corals) feeding on zooplankton, retention efficiency may be lower: life zooplankton can swim and actively escape when approaching a predator tentacle (Heidelberg et al., Reference Heidelberg, Sebens and Purcell1997). Prey initially counted as captured may be released again into the surrounding water and may be captured for a second time within the same incubation period. Hence, although prey clearance measurements may estimate net total food uptake within the incubation period accurately, actual prey capture efficiency may be much higher than assumed.
Finally, a technical consideration has to be made. During incubations, zooplankters may be removed from the water column due to processes other than active capture, for example: sedimentation on spots in the incubation chambers where mixing is suboptimal, and sticking of zooplankters onto non-active parts of the corals; in addition, aggregation of zooplankters in the water column may obfuscate the accuracy of sampling and enumeration. Blank controls are not easy to design. A control without a coral colony will exhibit a different flow pattern in the incubation chamber, whereas passive adhesion of zooplankters onto a dummy colony may be very different from passive adhesion to a live colony.
The above mentioned uncertainties that are associated with the prey clearance method (no distinction between active food capture and passive sedimentation; the difficulties to provide accurate blank controls; prey clearance not being a good estimator for prey capture efficiency) suggest that results obtained with this method for passive filter feeders such as corals should be interpreted with caution. To circumvent the uncertainties associated with prey clearance data, it is strongly recommended to execute direct prey capture measurements by video analysis. Heidelberg et al. (Reference Heidelberg, Sebens and Purcell1997) used such an approach to look at avoidance and escape behaviour of zooplankton prey in short term (10–15 minutes) incubations and found that 67% of the nauplii of copepods escaped shortly after the initial encounter with a coral tentacle. Direct observations will provide better insight into the dynamics of capture and release of prey at the polyp level.
Provoking prey capture: the role of chemical cues
The results on repeated feeding presented above show that prey capture efficiency (clearance rate) always increased immediately after addition of a new batch of Artemia nauplii. Our experiment on the effect of chemical cues was designed to test the hypothesis that chemical components residing in freshly hatched Artemia nauplii provoke this increase in clearance. Prey capture by coral polyps may be induced by the presence of extremely small amounts of some amino acids excreted into the water by the future prey (Titlyanov & Titlyanova, Reference Titlyanov and Titlyanova2002). Several studies presented evidence for this proposed role of dissolved free amino acids as stimulating agents for prey capture activity (Mariscal & Lenhoff, Reference Mariscal and Lenhoff1968; Goreau et al., Reference Goreau, Goreau and Yonge1971; Lehman & Porter, Reference Lehman and Porter1973). Goreau et al. (Reference Goreau, Goreau and Yonge1971) reported that low concentrations of glycine, alanine, phenylalanine and leucine trigger a typical feeding response, including extension of tentacles, swelling of the coenosarc tissue, and sometimes extrusion of mesenterial filaments, in several Caribbean coral species. Lehman & Porter (Reference Lehman and Porter1973) found that for the massive reef building coral Montastrea cavernosa, glutamic acid is by far the most successful feeding activator, promoting tentacle extension. In the current study, we found no evidence that addition of Artemia extract provoked the observed increase in prey capture in colonies of G. fascicularis during repeated feeding. Hence, for G. fascicularis feeding on Artemia nauplii, potential involvement of amino acids in provoking repeated prey capture mechanisms could not be mimicked by adding Artemia extract. Our results do not confirm the anecdotal information that claims that prey capture efficiency by aquarium corals can be stimulated by so-termed ‘priming’—the addition of food extract prior to adding the life food itself—but this conclusion is limited to priming of repeated feeding events. It remains to be studied to what extent priming aids in provoking feeding responses in corals that receive their first batch of food.
Clearance rate increased both after the addition of freshly hatched nauplii and after the addition of nauplii that had been pre-incubated at 26°C, showing that the incubation period of one hour did not cause a decrease in attractiveness of the prey. An increase in clearance rate after one hour was also observed in the control series. Probably, feeding efficiency is subjected to an endogenous rhythm controlled by the time needed by the coral polyp to ingest digest the captured prey. According to Hii et al. (Reference Hii, Soo and Liew2008), Artemia digestion by G. fascicularis is completed after 180 minutes, indicating that ingestion of the first batch of nauplii, which are being captured primarily within the first 20 to 40 minutes of the incubations, may have been completed after one hour. Notwithstanding the potential existence of endogenous rhythms in prey capture, the results of our experiment on the effects of chemical cues further support the conclusion presented above that the dynamics of capture and release of prey should be better understood to quantify food uptake by stony corals.
ACKNOWLEDGEMENTS
The authors wish to thank the staff of Burgers’ Ocean and the staff of De Haar Vissen for technical support. Johan Schrama and Tim Wijgerde are acknowledged for fruitful discussions regarding experimental design and data analysis. The project CORALZOO was funded by the FP6 programme of the European Community, grant 012547.









