INTRODUCTION
Foraging is essential for the survival and reproduction of all animals. For endangered species, understanding when, where, what and how much food they acquire is critical to implement conservation measures. However, studies of the feeding behaviour of aquatic species in their natural environment are difficult, since direct observations are rarely possible. Stomach content analysis has been a common approach for studying the prey and feeding patterns of many cetaceans (Fitch, Reference Fitch1968; Perrin et al., Reference Perrin, Warner, Fiscus and Holts1973; Ohizumi et al., Reference Ohizumi, Yoshioka, Mori and Miyazaki1998; Tamura & Fujise, Reference Tamura and Fujise2002; Murase et al., Reference Murase, Tamura, Kiwada, Fujise, Watanabe, Ohizumi, Yonezaki, Okamura and Kawahara2007; Wang et al., Reference Wang, Shao, Huang and Chou2012) and sirenians (Mignucci-Giannoni & Beck, Reference Mignucci-Giannoni, Montoya-Ospina, Jimenez-Marrero, Rodriguez-Lopez, Williams and Bonde2000; Andre & Lawler, Reference André, Gyuris and Lawler2005; Marsh et al., Reference Marsh, O'Shea and Reynolds2012). However, this method does not provide information about the timing of food intakes. While measurements of stomach temperature using biologging techniques have allowed detection of prey ingestion time (Wilson et al., Reference Wilson, Cooper and Plotz1992; Gremillet & Plos, Reference Gremillet and Plos1994; Kato et al., Reference Kato, Naito, Watanuki and Shaughnessy1996; Andrews, Reference Andrews1998; Naito, Reference Naito2007; Horsburgh et al., Reference Horsburgh, Morrice, Lea and Hindell2008), they are only applicable in species that are relatively easy to capture and recapture, such as pinnipeds and nesting birds. In addition, water intake is not clearly differentiated from food intake, because both decrease stomach temperature. Even more, stomach temperature cannot be used as a prey event indicator when the ambient water temperature is similar to that of the stomach. Recently, small accelerometers have been attached to the cephalic region of marine birds and pinnipeds in order to record the jaw and head movement associated with feeding behaviours (Naito, Reference Naito2007, Reference Naito2010; Skinner et al., Reference Skinner, Norberg and Andrews2009; Suzuki et al., Reference Suzuki, Naito, Folkow, Miyazaki and Blix2009). This technique is a powerful tool for monitoring the time-series of feeding events, but it is limited to species that facilitate attachment of the recorder near the cephalic region. Captive Amazonian manatees (Trichechus inunguis) easily removed the acceleration data-loggers attached on the cephalic part by rubbing it against the enclosure wall (Kikuchi et al., unpublished data). In addition, it seems to be difficult to detect the feeding behaviour of manatees by the head movement, because of their gentle body movement during feeding. Naito (Reference Naito2007) summarized methods previously used for foraging studies of aquatic species and recommended further developments of methods for the monitoring of feeding events. One more major limitation is the identification of prey species. Animal-borne digital camera data loggers reveal prey species (Watanabe et al., Reference Watanabe, Mitani, Sato, Cameron and Naito2003, Reference Watanabe, Baranov, Sato, Naito and Miyazaki2004; Watanuki et al., Reference Watanuki, Daunt, Takahashi, Newei, Wanless, Sat and Miyazaki2008), but matching feeding events indicated by accelerometers and the captured image of prey was not easy (Watanabe & Takahashi, Reference Watanabe and Takahashi2013) due to the intermittent image capture and the limited angle of view, which caused some feeding events to be missed. In addition, the success of this technique depends on the clarity of the water and is not suited for tannin-stained or turbid environments such as rivers, lakes and estuaries. Ideally, the actual timing of feeding events needs to be recorded individually for a long duration with minimal effect on the behaviour of observed animals.
Sirenians (manatees and dugong) are the only extant herbivorous aquatic mammals. Despite utilizing coastal shallow habitats, much remains unknown about the feeding behaviour due to the limitations described above, especially for taxa that inhabit dark water habitats: Amazonian manatees, Trichechus inunguis (Rosas, Reference Rosas1994) and Antillean manatees, Trichechus manatus manatus (Olivera-Gomez & Mellink, Reference Olivera-Gomez and Mellink2005). To develop observation techniques in these limited situations, Tsutsumi et al. (Reference Tsutsumi, Ichikawa, Arai, Akamatsu, Shinke, Hara and Adulyanukosol2006) applied acoustic monitoring methods to identify feeding events for wild dugongs (Dugong dugon) by installing fixed acoustic recorders in seagrass areas. Feeding events were recorded as a sequence of mastication sounds, which provided the actual time of feeding. This technique is useful to monitor the feeding behaviour even during night-time or in turbid water. However, the monitoring system is fixed on the seabed, and thus is limited to dugongs that feed near the hydrophone, and does not allow for differentiation between individuals or the continuous monitoring of a particular individual. In order to continuously monitor the feeding sounds of objective animal, it is necessary to apply an animal-bone recording system. Only one previous study has attached sound recorders directly to manatees (Nowacek et al., Reference Nowacek, Casper, Wells, Nowacek and Mann2003). Nowacek et al. (Reference Nowacek, Casper, Wells, Nowacek and Mann2003) attached a sound recorder on Antillean manatees in Belize to compare the vocalizations with Florida manatees (Trichechus manatus latirostris). However, they did not mention the mastication sounds produced by feeding. In the present study, we focused on the direct recording of the manatee feeding sounds. We applied a newly developed animal-borne underwater sound recorder to captive Amazonian and Antillean manatees in order to detect manatee feeding events using mastication sounds.
MATERIALS AND METHODS
Study animals
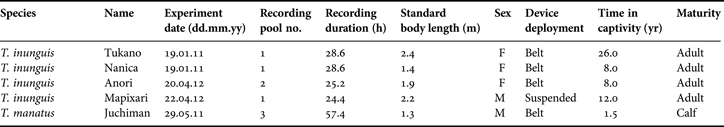
Experiments were conducted using four captive Amazonian manatees at the National Institute of Amazonian Research (INPA), Manaus, Brazil, in 2011 and 2012, and one captive Antillean manatee at the Autonomous University Juarez of Tabasco (UJAT), Mexico, in 2011. Study animals and recordings are summarized in Table 1. All study animals were rescued from the wild and were in captivity for rehabilitation purposes; with the objective of eventually releasing them back into the wild. In INPA, there were many other manatees being rehabilitated during the time of the study. Therefore, we did not have full control of where and how manatees were housed in this limited situation.
Table 1. Descriptive information of four Amazonian manatees and one Antillean manatee.

In 2011, two adult female Amazonian manatees (Tukano and Nanica) were kept in the outdoor pool no. 1 (10 m in diameter and 3.0 m deep) at the same time (Table 1). Visual observations were simultaneously conducted through an acrylic window, in order to record which manatee was foraging and what plant species was being consumed. We conducted the experiments only during the daytime. Daytime is defined as from sunrise to sunset and night-time from sunset to sunrise, respectively.
In 2012, an adult female and an adult male Amazonian manatee (Anori and Mapixari) were kept alone in the outdoor pools no. 1 and no. 2 (4.4 m long, 2.8 m wide, and 1.2 m deep), respectively (Table 1). Experiments were conducted on different days in each pool. The Antillean manatee calf (Juchiman) was kept alone in the outdoor pool no. 3 (5.0 m in diameter and 1.0 m in depth; Table 1).
The recording devices were attached to the manatees via a soft plastic belt around the caudal peduncle (Figure 1; Table 1). This type of belt has been mainly used to attach the devices to captive and wild manatees, and has a high safety and record of success with minimal impact on natural behaviour (e.g. Deutsch et al., Reference Deutsch, Bonde and Reid1998, Reference Deutsch, Reid, Bonde, Easton, Kochman and O'Shea2003; Weigle et al., Reference Weigle, Wright, Ross and Flamm2001; Marmonterl et al., Reference Marmontel, Reid, Sheppard, Morales-Vela, Hines, Reynolds, Aragones, Mignucci-Giannoni and Marmontel2012). In one Amazonian manatee in 2012, the recording device was suspended in the pool to compare the recorded sound between animal-borne and suspended recorders (Table 1).

Fig. 1. An Amazonian manatee equipped with an animal-borne sound recorder (AUSOMS-mini) by the caudal peduncle belt.
Animal-borne recorder
The animal-borne underwater sound recorder AUSOMS-mini (Automatic Underwater Sound Monitoring System; 51 mm in diameter, 193 mm in length, 350 g weight in air and slightly positive buoyancy in the water; Aqua Sound Co. Ltd, Kyoto, Japan) was used on all five manatees. The AUSOMS-mini consisted of a single omnidirectional hydrophone and a solid memory recorder housed in a water-resistant case. The frequency response of the hydrophone was between 40 and 13000 Hz. The dynamic range of the recording system was 70 and 160 dB re 1 μPa. The device possessed a 4 GB hard disc, which allowed monaural recordings in compressed format (Windows Media Audio; WMA) for up to 276 h. Present WMA format records up to 13 kHz, which fits for the hydrophone response of AUSOMS-mini and mastication sounds as well. In addition to the AUSOMS-mini, a hydrophone (Aquafeeler SH20k, System Intech Co. Ltd, Tokyo, Japan, flat frequency response up to 20 kHz within 3 dB) was suspended in the tank and connected to a linear PCM recorder (DS-750, Olympus, Tokyo, Japan).
Simultaneously, an M190L-D2GT acceleration data-logger (15 mm in diameter, 53 mm in length, 16 g weight in air: Little Leonardo Co., Tokyo, Japan) was attached on the same belt fixed on the Antillean manatee to investigate the day–night activity level. The M190L-D2GT logger recorded dive depth at 1 s intervals and longitudinal acceleration at 1/16 s intervals.
Feeding procedure
Based on reports of the plants fed by wild Amazonian manatees (Colares & Colares, Reference Colares and Colares2002; Guterres & Marmontel, Reference Guterres and Marmontel2008), five different plant species were offered to the two Amazonian manatees in 2011, Eichornia crassipes (Ec), Pistia stratiotes (Ps), Echinochloa polystachya (Ep), Paspalum fasciculatum (Pf) and Paspalum repens (Pr). The two Amazonian manatees were fed one or two species of plants (Ep and Ec, Ps and Pf, or Pr), a total of three times. Plant species were separated using a floating fence in order to prevent mixing during feeding. Feeding times were 10:00 hours, 14:00 hours and 17:20 hours on the first day and 10:00 hours on the second day. In 2012, two or three different plant species were offered to the Amazonian manatees, Ec, Ps and Pr for Anori, Ec and Ps for Mapixari. Both manatees were fed only one plant species during each trial. Feeding times were 11:45 hours, 15:12 hours and 17:00 hours for Anori, 12:51 hours and 16:17 hours for Mapixari. The Antillean manatee was offered four plant species, Ec, Ps, Inga vera (Iv), and Calliandra belizensis (Cb). We fed the Antillean manatee a total of nine times with only one plant species during each trial. Feeding times were 12:30 hours and 15:10 hours on the first day, 8:30 hours, 10:10 hours, 15:20 hours, 18:20 hours and 19:50 hours on the second day and 12:10 hours and 15:40 hours on the third day. These plants were divided by trait as forb, grassy or foliage; Ec and Ps were forb, Ep, Pf, and Pr were grassy, Iv and Cb were foliage. We did not dictate when food was offered during our experiments, food was offered at the regularly scheduled feeding times. We retrieved the plants only when we introduced new plants. We confirmed that quite a lot of food was leftover in the pool, even during the night-time. Therefore, manatees had access to food both day and night.
Data analysis
We primarily used the recorded amplitude as the key for the detection of mastication sounds. WMA format enhances the spectrum component of received sound when it receives an intense signal. This means that higher amplitude sounds are shown more prominently in the spectrum analysis. Our approach to extract feeding events consisted of three steps that are outlined below.
In the first step, we obtained ground truth data of mastication events. The time of sound events recorded by the AUSOMS-mini was visualized and monitored using Adobe audition software (Adobe® Systems, Mountain View, CA, USA). We extracted a clear three-minute portion of mastication sounds for each manatee. The absolute times of each mastication sound were noted, which were used as ground truth data for the comparisons below.
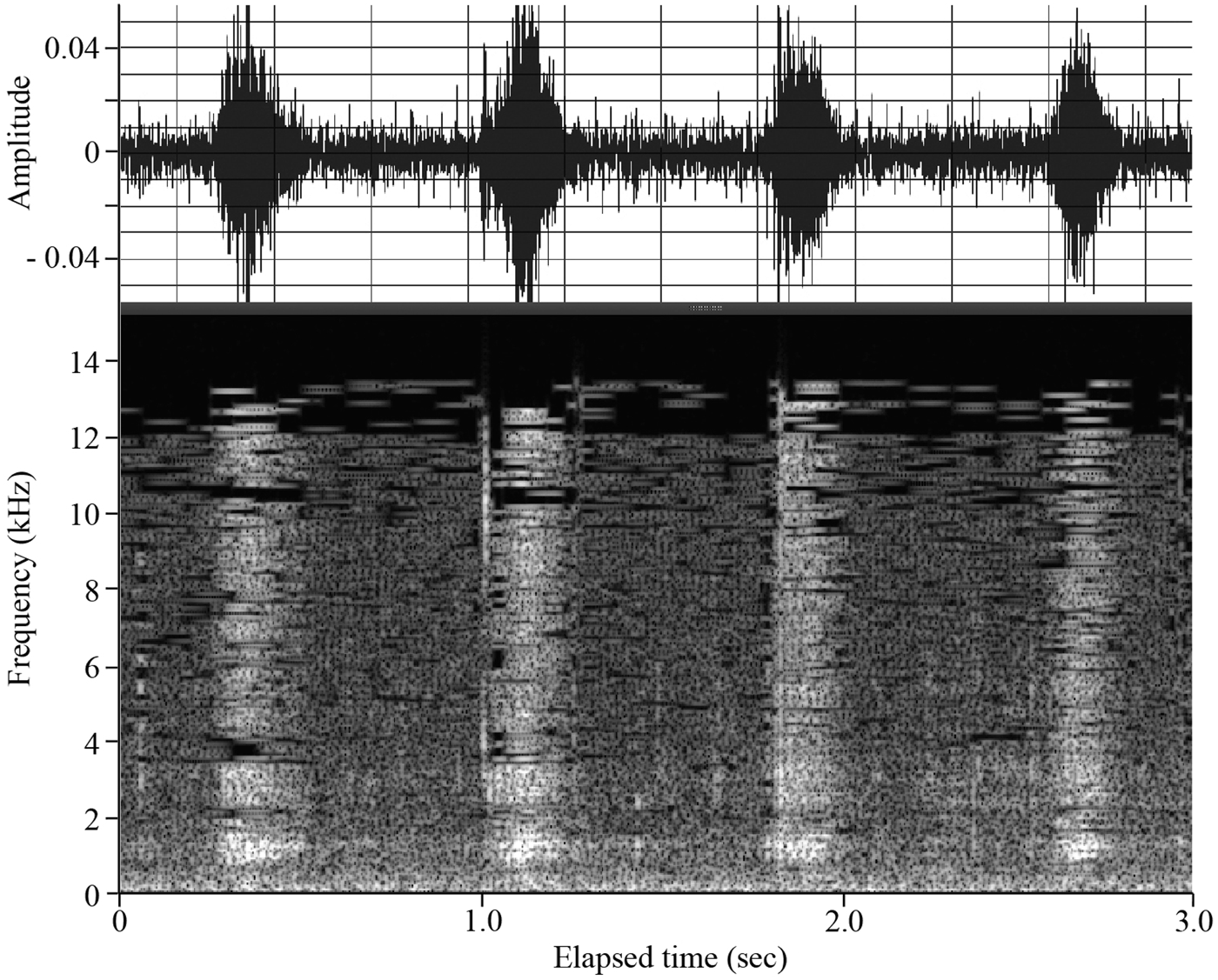
In the second step, automatic detection of mastication sounds was conducted using custom software developed on Matlab (Mathworks Inc., Natick, USA). According to the distinctive characteristics of typical mastication sounds (Figure 2), we used amplitude as the primary parameter for detection, as well as the index of spectrum level (spectrogram for the frequency of feeding sounds). The amplitude thresholds were changed from a relative value in Audition of 0.01 to 0.20 by increments of 0.01 for Amazonian manatees in 2011 and from a relative value in Audition of 0.001 to 0.01 by increments of 0.001 for Antillean and Amazonian manatees in 2012. The relative value 1 is the full range of the recorded file, which corresponds to the sound pressure level at 149 dB re V rms/1 µPa (Amazonian manatees in 2011) and 168 dB re V rms/1 µPa (Amazonian manatees in 2012 and Antillean manatee in 2011). 0.001 or 0.01 was chosen as the starting point because it was just above the noise level. In addition, an index of integrated spectrum level from 6 to 12 kHz was used for the Amazonian and Antillean manatee in 2011, and from 1 to 7 kHz for the Amazonian manatee in 2012. Note that this index does not correspond to the physical spectrum level due to the compressed format. According to the recordings from the separately fixed hydrophone and uncompressed linear PCM data, mastication sounds showed a broadband spectrum as described by Tsutsumi et al. (Reference Tsutsumi, Ichikawa, Arai, Akamatsu, Shinke, Hara and Adulyanukosol2006). Using the compressed data, the index was changed from a value of 0.001 to 0.027 by increments of 0.002 and from a value of 0.0001 to 0.001 by increments of 0.0001, in the Amazonian manatee in 2011, and Antillean manatee and Amazonian manatee in 2012, respectively.

Fig. 2. An example of a waveform (upper inset) and sonogram (bottom inset) of typical feeding sounds recorded by AUSOMS-mini for Amazonian manatee Tukano. Each feeding sound (higher amplitude and spectrum level than the ambient noise) corresponds to single mastication action.
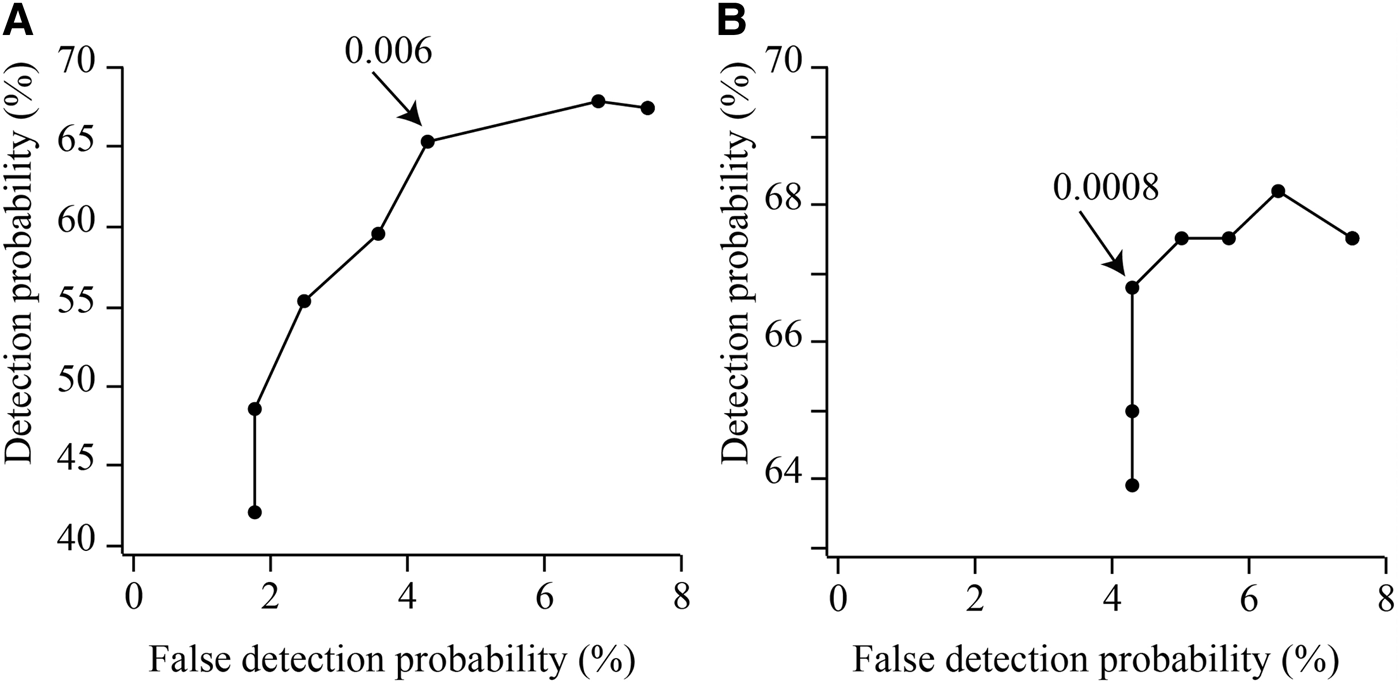
In the third step, performance of automatic detection was evaluated by calculating correct detection and false alarm. The extracted clear portion of mastication sounds was analysed for each manatee using automatic extraction software. Matched detections and unmatched detections between automatic extractions and ground truth data provide correct detection and false alarm rates. The relationship between the correct detection and false alarm ratio (e.g. receiver operating characteristic (ROC) curve) was calculated for each threshold of amplitude and index of spectrum level. The value of the parameter set at the inflection point of the ROC curve was adopted (Figure 3).

Fig. 3. Example of receiver operating characteristic curve for the automatic detection of mastication sounds of manatees. Arrows show the inflection point. We adopted a value of (A) 0.006 in amplitude threshold and (B) 0.0008 in spectrum-level threshold in this manatee, Anori.
Finally, the feeding sounds were automatically extracted from the rest of the data sets. We calculated the mastication cycle duration, which is the time between mastications. We also calculated the histogram of mastication cycle durations for each manatee. Separations of more than 0.7–1.2 s between feeding sounds were defined as cut-offs between different feeding events in each manatee.
Mastication rates during the day and night-time (s−1) were calculated for each manatee as the number of mastications during the day (or night-time) divided by the day (or night) duration. Furthermore, in the Antillean manatee, inactive behaviour on the bottom was classified from the recorded behavioural data by calculating the standard deviation (SD) of dive depth and longitudinal acceleration (Kikuchi et al., Reference Kikuchi, da Silva, Rosas and Miyazaki2010) using Igor Pro (WaveMetrics, Lake Oswego, USA). During inactive behaviour, the SD of dive depth and longitudinal acceleration ranged from 0.00 to 0.14 m and from 0.00 to 0.03 ms−2, respectively. Therefore, we used these values as definitions for classification of inactive behaviour.
To test the effect of plant species or day–night differences on the mastication cycle durations, we used the generalized linear mixed model (GLMM) with Gaussian errors and individual manatee was included as a random effect in the two Amazonian manatees in 2012. The dependent variable was mastication cycle duration, and the explanatory variable was plant species, plant trait (forb, grassy, or foliage) and day–night differences. In the Antillean manatee, we used the generalized linear model (GLM) with Gaussian errors. The dependent variable was mastication cycle duration, and the explanatory variables were plant species, plant trait and day–night differences. In the two Amazonian manatees in 2011, we used the GLMM with Gaussian errors in order to test the effect of plant species on the mastication cycle durations. The dependent variable was mastication cycle duration and the explanatory variables were plant species and plant trait. Because the two Amazonian manatees in 2011 were kept in the smaller pool with three other manatees during the night-time, we did not analyse the effect of day–night differences on the mastication cycle durations.
In order to analyse the relationship between standard body length and the mastication cycle durations, we used GLMM with Gaussian errors and an individual manatee was included as a random effect in the four Amazonian manatees in 2011 and 2012. The dependent variable was mastication cycle duration, and the explanatory variable was standard body length. The most porsimonious model was selected on the basis of the Akaike information criterion (AIC). For statistical analyses, we used software ‘R’ (www.r-project.org).
RESULTS
Detection performance
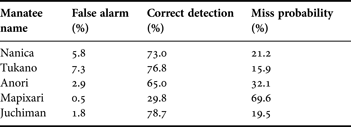
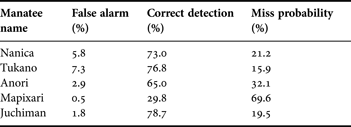
Total recording durations were from 24.4 to 28.6 h for the Amazonian manatees and 57.4 h for the Antillean manatee (Table 1). The value of the inflection point of the ROC curve for the amplitude and spectrum-level threshold differed for each individual. We adopted a value from 0.005 to 0.070 in amplitude threshold and from 0.0004 to 0.0230 in spectrum-level threshold for each manatee. This means we calibrated detection performance individually. The false alarm and correct detection probabilities were 0.5–7.3% and 29.8–78.7% (Table 2).
Table 2. False alarm, correct detection and miss probabilities in four Amazonian manatees and one Antillean manatee for the extracted data.
Despite the low false detection rate of automatic detection, broadband noise from rain, flatulence and pulse noise, thought to be created by the bumping of the hydrophone into a wall, remained. After the automatic extraction, the first author checked the sound data, and manually excluded the time of these noises. In addition to the noises, the sections without feeding sounds were removed. The total removed durations were 13603.5–41148.9 s, accounting for 2.9–46.8% of all data, depending on the individual.
Plant species and feeding activity monitored by mastication sounds
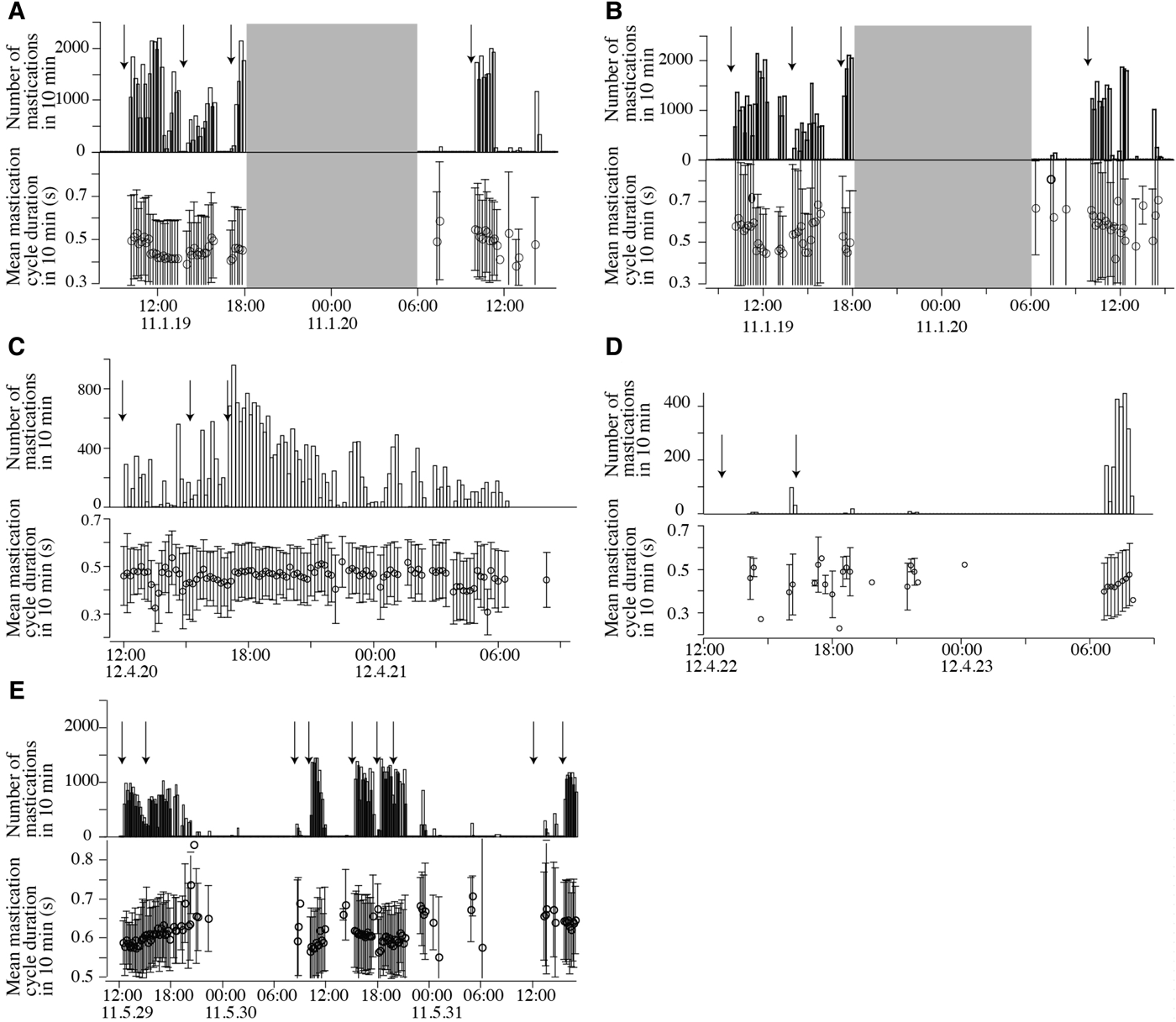
Day–night recording showed that all manatees fed during the day and night (Figure 4). Mean mastication rate ±SD was 0.31 ±0.17 s−1 and 0.35 ±0.32 s−1 during the day and night-time, respectively.

Fig. 4. Number of mastication and mean mastication cycle durations in 10 min, error bar shows standard deviation, arrows show the food deployed time in the Amazonian manatees: Tukano (A) and Nanica (B), Anori (C) and Mapixari (D); the Antillean manatee Juchiman (E). For Tukano (A) and Nanica (B), we did not record the feeding sounds during the night-time (grey square). For Mapixari (D), the recording device was suspended in the pool.
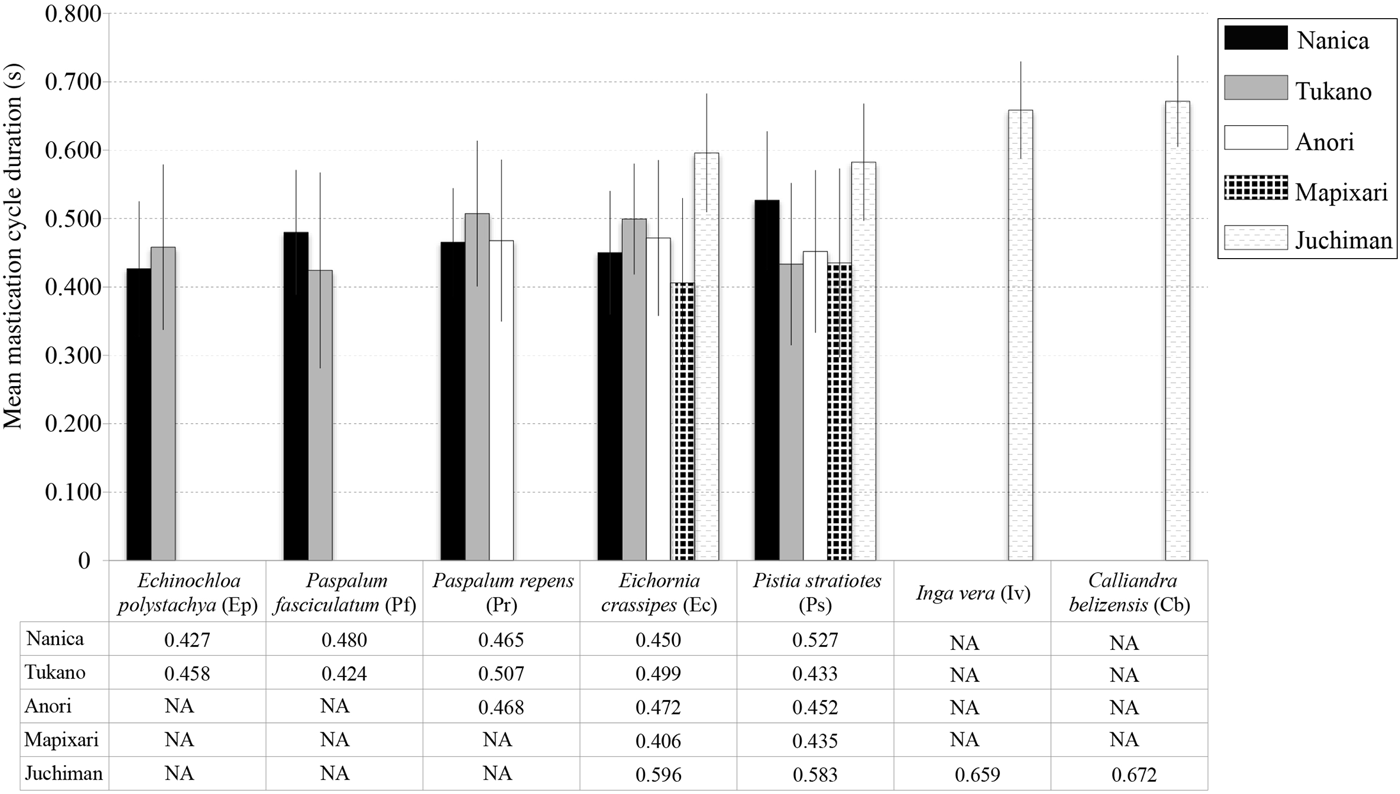
In the two Amazonian manatees in 2012, the GLMM for the mastication cycle durations including plant species and day–night differences as an explanatory variable was the best model with the lowest AIC. The model had an AIC value 7.0 lower than the second best model, which included only day–night differences. It showed that mastication cycle durations were affected by plant species (Figure 5) and day–night differences. Mastication cycle durations during the day and night were 0.459 ± 0.011 s (estimated value ±SE) and 0.472 ±0.002 s, respectively. In the Antillean manatee, the GLM for mastication cycle durations including plant species and day–night differences as an explanatory variable was the best model with the lowest AIC. The model had an AIC value 26.0 lower than the second best model, which included only plant species. The GLM for mastication cycle durations revealed that it was affected by plant species (Figure 5) and day–night differences (estimated value ±SE = 0.605 ± 0.091 s and 0.606 ±0.093 s in the day and the night, respectively). These results revealed that mastication cycle durations differed by species of plants consumed and the time of day they were consumed (day–night). And mastication cycle durations were shorter during the day than during the night in these three manatees. In the two Amazonian manatees in 2011, the GLMM for mastication cycle durations including species of plants as an explanatory variable was the best model with the lowest AIC. The model had an AIC value 128.0 lower than the second best model, which included the dependent variable only, revealing that mastication cycle durations were affected by plant species (Figure 5).

Fig. 5. Mean mastication cycle durations for each plant for four Amazonian manatees (Nanica, Tukano, Anori, Mapixari) and one Antillean manatee (Juchiman). Error bar shows the standard deviation. NA, not applicable.
In the four Amazonian manatees in 2011 and 2012, the GLMM for mastication cycle durations including the dependent variable only was the best model with the lowest AIC. The model had an AIC value 6.0 lower than the second best model, which included standard body length, revealing that mastication cycle durations did not differ with standard body length.
In the Antillean manatee, the percentage of inactive behaviour was 9.6 and 53.2% in the day and the night, respectively. Mean duration of each period of inactive behaviour was 165.1 ± 64.5 and 176.5 ± 64.5 s in the day and the night, respectively. A previous study showed that captive Amazonian manatees at INPA were more inactive during the night, and the duration of each period of inactive behaviour was longer during the night than during the day, which indicated these manatees rested during the night (Kikuchi et al., Reference Kikuchi, da Silva, Rosas and Miyazaki2010). As is the case with captive Amazonian manatees, the captive Antillean manatee seemed to rest more during the night than during day.
DISCUSSION
By focusing on the direct recording of mastication sounds, feeding events could be detected with a high detection probability in captive manatees (Table 2). Our results support the conclusions of Tsutsumi et al. (Reference Tsutsumi, Ichikawa, Arai, Akamatsu, Shinke, Hara and Adulyanukosol2006), that the mastication sounds can be used as the actual index of feeding events of herbivorous aquatic animals. The correct detection probabilities ranged up to 78.7 whereas the false alarm rate was below 7.3%. We also suspended the same recorder in the pool (Table 1; Mapixari) to compare the sound recorded by animal-borne recorders. Because of the distance between the hydrophone and the objective animal during feeding, recorded mastication sounds were occasionally very weak. In order to detect the mastication sounds with a low false alarm, we set conservatively high thresholds. This caused low correct detection and negligible level of false alarm at 0.5% (Table 2). Unlike fixed acoustic monitoring systems, animal-borne recorders are a more useful way to detect the feeding sounds because the device is always located near the sound source. Low frequency sound propagates nearly omni-directionally, which is advantageous because any location on the body can be used to attach the tag, even though the actual sound source is the jaw of the tagged animal. Compared with the previous methods, recording mastication sounds is a reliable way to detect feeding behaviours. It was not affected by the position of the tag on the animal. Low false alarm and fairly high detection probabilities were confirmed.
We found that the mastication cycle durations depended on plant species rather than the plant trait (forb, grassy and foliage) (Figure 5). Constraints could be the ease of mastication, such as characteristics of caulome, leaves and roots, which have not been identified yet. Consumption rates of terrestrial herbivores are reported to be influenced by morphological characteristics of plants (Cooper & Owensmith Reference Cooper and Owensmith1986; Spalinger et al., Reference Spalinger, Hanley and Robbins1988). Marshall et al. (Reference Marshall, Kubilis, Huth, Edmonds, Halin and Reep2000) suggested that other factors, such as fibre content, plant anatomy and material properties, also may affect handling time and mastication rates. During feeding on grassy plants, multiple distinctive pulse sounds were also recorded in a single bite event, which seemed to come from cutting of thick caulomes. These acoustic characteristics could potentially allow us to detect not only exact time of feeding, but also the species of plants.
Day–night recordings showed that all manatees fed on plants during the day and night. All manatees tended to start feeding soon after food was deployed, but they tended to stop feeding before finishing all plants. In this study, the percentage of inactive behaviour in the Antillean manatee increased during the night, and a previous study showed that captive Amazonian manatees in INPA also rested primarily at night (Kikuchi et al., Reference Kikuchi, da Silva, Rosas and Miyazaki2010), thus we considered that all of the studied manatees were more inactive at night than during the day. In combination with the result that mastication cycle durations were shorter during the day than the night in the three manatees (two Amazonian manatees in 2012 and the Antillean manatee), this suggested that they seemed to focus on feeding during the day.
In this study, there is no definite relationship between mastication cycle duration and standard body length in the Amazonian manatees. Previous studies reported a positive relationship between Florida manatee mastication cycle duration and body length; small manatees masticated faster than adults (Etheridge et al., Reference Etheridge, Rathbun, Powell and Kochman1985, Marshall et al., Reference Marshall, Kubilis, Huth, Edmonds, Halin and Reep2000). Etheridge et al. (Reference Etheridge, Rathbun, Powell and Kochman1985) considered that the differences in mastication cycle durations were attributed to calves having a smaller grinding surface area and smaller mouths. Marshall et al. (Reference Marshall, Kubilis, Huth, Edmonds, Halin and Reep2000) suggested that the difference in mastication cycle durations between body sizes is likely due to normal allometric and physiological changes in the manatee feeding apparatus (lips, bristles, tongue, jaw, and associated musculature) during growth, which explains the correlation between mastication and body length. The increase in size of the oral disc, distance between perioral bristles and gape and larger facial and masticatory musculature likely act in concert to alter timing of feeding mechanics (Marshall et al., Reference Marshall, Kubilis, Huth, Edmonds, Halin and Reep2000). In order to confirm the relationships between mastication cycle duration and manatee body size, further experimental data are needed from a larger sample size with wide range of body sizes.
The direct acoustic monitoring system allows us to understand the time-series feeding events of studied manatees, even in the night-time or in turbid and tannin-stained environments which restrict visual observation. By coupling this system with a time-scheduled release device (Watanabe et al., Reference Watanabe, Baranov, Sato, Naito and Miyazaki2004), which releases the devices from the animals at a scheduled time, and which can be retrieved via VHF radio signals, we would be able to apply this animal-borne recorder to wild manatees without the need to recapture them. In addition to the mastication sounds, the acoustic data included insect and birdcalls, rainfall and conspecific vocalizations, which could be useful to understand habitat selection, weather and the presence of other manatees in the natural environment.
ACKNOWLEDGEMENTS
We thank Y. Sasaki and Y. Yoshida for their assistance in this study. We thank all INPA staff, UJAT students, I. Aoki and T. Imaizumi for supporting this study, and two anonymous referees for helpful comments on the draft.
FINANCIAL SUPPORT
This work was supported by Research and Development Program for New Bio-industry Initiatives, the program ‘Bio-Logging Science of the University of Tokyo (UTBLS)’, JSPS Grant-in-Aid for Young Scientists (B) (grant number 24710272), the Mohamed bin Zayed Species Conservation Fund (grant number 10051229), the Inui Memorial Trust for Research on Animal Science, Japan Science and Technology Agency CREST and JSPS Core-to-Core Program A. Advanced Research Networks.