INTRODUCTION
The spatial distribution of demersal species has been widely studied (Cartes et al., Reference Cartes, Company and Maynou1994; Cartes & Sardà, Reference Cartes and Sardà1993; Moranta et al., Reference Moranta, Stefanescu, Massutí, Morales-Nin and Lloris1998; Blanchard, Reference Blanchard2001; Abelló et al., Reference Abelló, Carbonell and Torres2002; Cartes et al., Reference Cartes, Maynou, Moranta, Massutí, Lloris and Morales-Nin2004; Company et al., Reference Company, Maiorano, Tselepides, Politou, Plaity, Rotllant and Sardà2004; D'Onghia et al., Reference D'Onghia, Politou, Bozzano, Lloris, Rotllant, Sion and Mastrotaro2004; Massutí et al., Reference Massutí, Gordon, Moranta, Swan, Stefanescu and Merrett2004; Moranta et al., Reference Moranta, Quetglas, Massutí, Guijarro, Hidalgo and Diaz2008). In a broad sense, it refers to the dispersion of individuals within their area of distribution. Spatial distribution is usually considered to vary according to environmental features (e.g. depth, bathymetric boundaries, bottom type: Abelló et al., Reference Abelló, Carbonell and Torres2002), oceanographic conditions (e.g. characteristics of water masses: Carney, Reference Carney2005; Company et al., Reference Company, Puig, Sardà, Palanques, Latasa and Scharek2008; Canals et al., Reference Canals, Danovaro, Heussner, Lykousis, Puig, Trincardi, Calafat, Durrieu de Madron, Palanques and Sanchez-Vidal2009), biological factors (e.g. competition among species: Blanchard, Reference Blanchard2001, and references therein) and human influence (e.g. impact fisheries: Blanchard, Reference Blanchard2001). Among these, the depth gradient is generally considered to be the main factor affecting species associations (D'Onghia et al., Reference D'Onghia, Politou, Bozzano, Lloris, Rotllant, Sion and Mastrotaro2004; Massutí et al., Reference Massutí, Gordon, Moranta, Swan, Stefanescu and Merrett2004).
Bottom geomorphology can determine local hydrographic and substrate flows or facilitate species movements, thus contributing to the often unique spatial distributions observed, for example in submarine canyons (Orrù & Ulzega, Reference Orrù and Ulzega1988; Tobar & Sardà, Reference Tobar and Sardà1992; Sabatini et al., Reference Sabatini, Follesa, Locci, Pendugiu, Pesci and Cau2007). The varying mobility of particular animals can be very important in determining the spatial distribution of species, and can occasionally be an important cause of both seasonal and nycthemeral cycles (Cartes et al., 1993; Sabatini et al., Reference Sabatini, Follesa, Locci, Pendugiu, Pesci and Cau2007). In this context, sea bottoms with a particular geomorphological structure, such as canyons and seamounts, can be of ecological interest regarding the vertical migration of marine species. Submarine canyons are areas very rich in nutrients with strong turbidity currents (Shepard et al., Reference Shepard, Marshall and McLoughlin1974). They are complex environments and the species living there are usually more mobile than those of typical deep-sea assemblages (Rowe, Reference Rowe1971). The environmental influence on the movements of marine species has been studied (Sardà et al., Reference Sardà, Maynou and Tallò1997; Tudela et al., Reference Tudela, Sardà, Maynou and Demestre2003) as have the diurnal movements of the species living in these environments (Sabatini et al., Reference Sabatini, Follesa, Locci, Pendugiu, Pesci and Cau2007).
Submarine canyons, however, are not the only ecologically interesting seabed topographic feature influencing species' vertical migrations. Some seamounts far from the coast are characterized by a rising of the continental shelf leading to a decrease in water depth. Seamounts can be defined as isolated underwater features (mountains, terraces, ridges, banks, plateaux and shelves) less than 1000 m above the sea floor and peaking below sea level (WWF/IUCN, 2004). These features are often characterized by significant levels of endemism and relatively high primary production that could support productive fisheries (Rogers, Reference Rogers1994; Koslow et al., Reference Koslow, Gowlett-Holmes, Lowry, O'Hara, Poore and Williams2001). Seamounts can potentially enable vertical migration far from the coast and have been identified as diversity hotspots (Koslow, Reference Koslow1997). Given its distance from the coast, the first fishermen to fish close to the Baronie Seamount found many species, and more importantly they found economically important species such as the shrimps Aristaeomorpha foliacea (Risso, 1827) and Aristeus antennatus (Risso, 1816). According to local fishermen, this area has a higher biomass of commercial species than adjacent regions and hence represents a site of particular biological and economic interest.
We studied the Baronie Seamount located 25 miles off the north-eastern coast of Sardinia (Italy), called also ‘K’ bank by local fishermen. This is the first study concerning the demersal assemblages of this area, which started to be economically exploited only few years ago. The aim of this paper is to study the different species assemblages found at different times of the day, and to highlight the potential vertical movements of these species, as observed in another Sardinian area, the Quirra Canyon (Sabatini et al., Reference Sabatini, Follesa, Locci, Pendugiu, Pesci and Cau2007). Juveniles and adults of some very important species such as the shrimps, showed variations in diet, bathymetric distribution and, even, daily migratory behaviour (Cartes et al., Reference Cartes, Maynou, Moranta, Massutí, Lloris and Morales-Nin2004, Reference Cartes, Papiol and Guijarro2008). In addition, the daily timing of changes in depth occurrence and size composition of the giant red shrimp, A. foliacea and the blue and red shrimp A. antennatus was studied.
MATERIALS AND METHODS
Study area
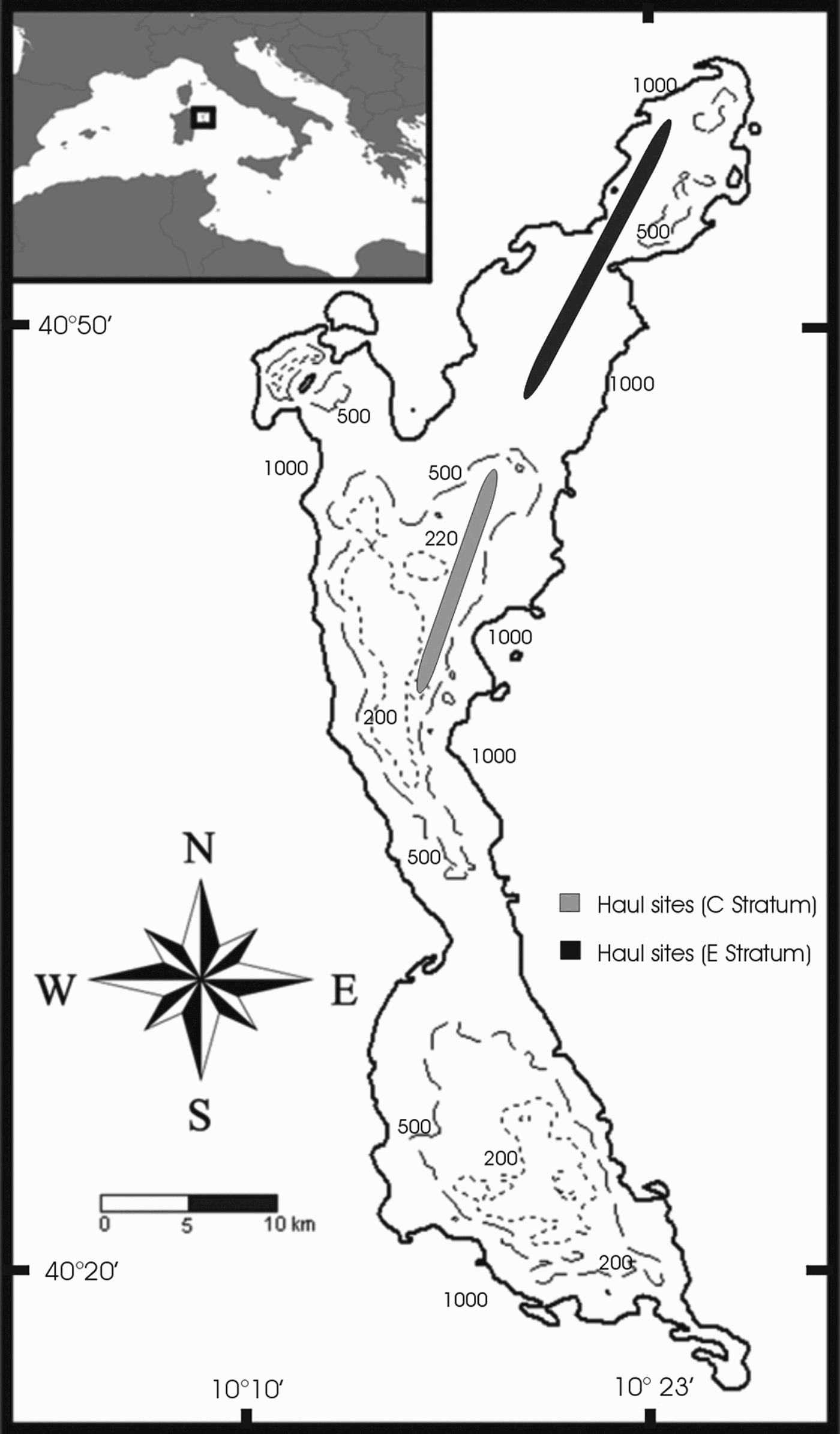
The data analysed in this work were collected on the Baronie Seamount, which is located off the north-eastern coast of Sardinia (in the western part of the central Tyrrhenian Sea) (Figure 1). It is characterized by a rise in the continental shelf, which results in a decrease in sea depth. The seamount rises from the sea bottom to two levels lying at 162 m and 168 m characterized by a flat morphology and a thin sedimentary cover (Bellagamba et al., Reference Bellagamba, Napoleone and Tramontana1979). It has an elongate shape, running from north to south, parallel to the coast line.
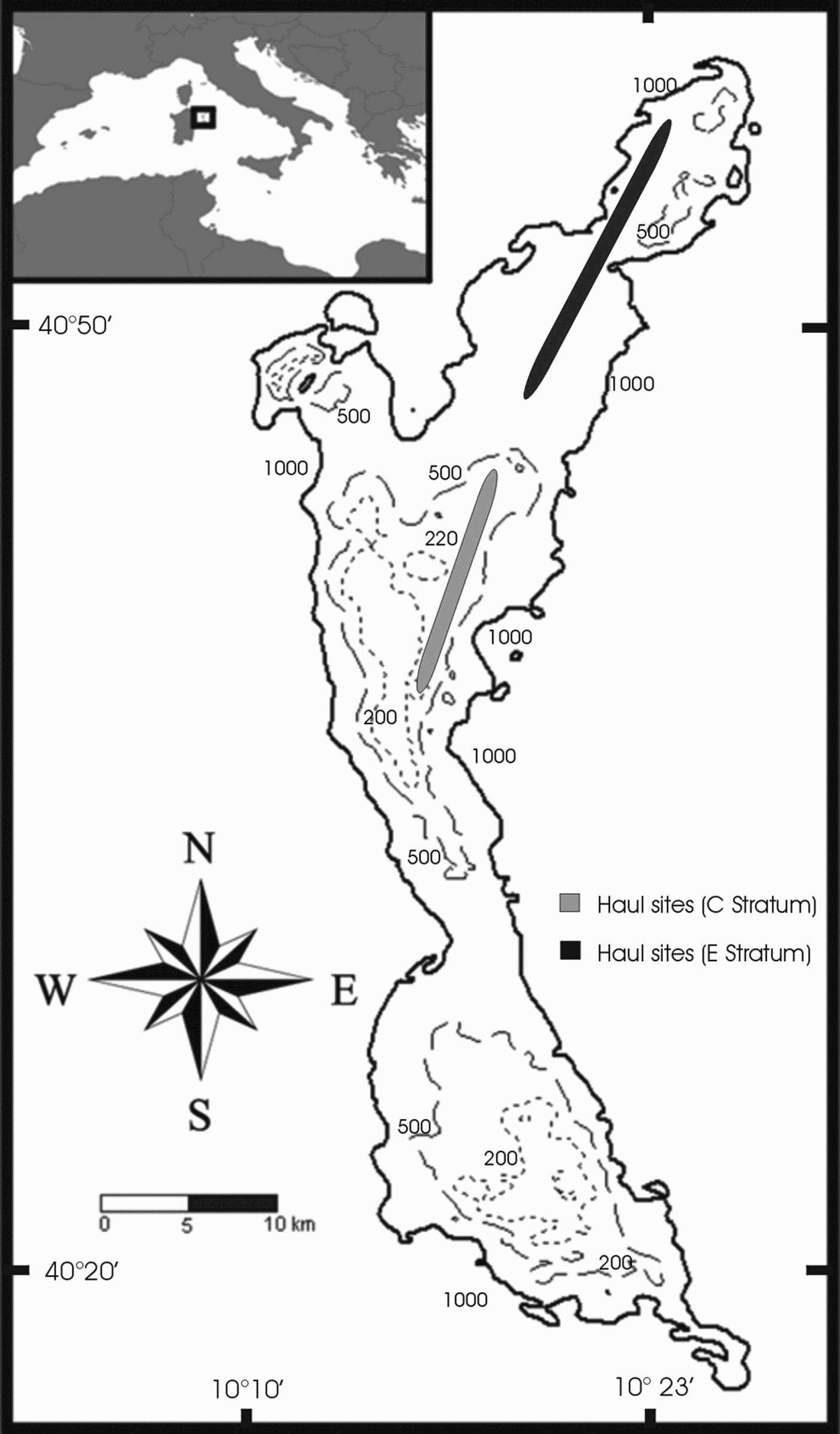
Fig. 1. Map of the study area. Continuous lines, 1000 m depth; hatched lines, 500 m; dotted lines, 200 m. In the upper left rectangle, the geographical position of the Baronie Seamount is indicated.
Samples were taken on the eastern side of the seamount, where a substantial variation in depth occurs. Depth varies from 180 m to over 1000 m in only 2 nautical miles. This is one of the few areas in the Baronie Seamount that can be exploited by bottom fishing trawl.
Data collection
Data were collected between January and April 2003. The survey vessel (80 tons and 338 kw) was equipped with a gear commonly used by local fishermen (trawl net, 40 mm stretched mesh size in the cod end). The trawl samples were collected throughout the entire 24-hour period and categorized as to time of day and depth, as reported in Sabatini et al. (Reference Sabatini, Follesa, Locci, Pendugiu, Pesci and Cau2007). A total of 22 samples were collected from depths ranging between 176 m and 745 m and from 110 to 334 minutes in duration. Samples coded ‘C’ were for hauls made above 350 m (14 hauls) and ‘E’ for hauls deeper than 500 m (8 hauls). The geomorphological features of the Baronie Seamount's bottom prevent trawling at intermediate depths (350–500 m).
Thirteen of the 22 hauls were performed during daylight (‘L’, from 1 hour before sunrise to 1 hour after sunset), 5 during the first part of the night (‘N1’, from 1 hour after sunset to 01:00 a.m.) and 4 during the second part (‘N2’, from 01:00 a.m. to 1 hour before sunrise). The night was divided into two time intervals to distinguish the different species caught during the first and the second part (Table 1).
Table 1. Code, date, season, start and end time (solar time), duration and depth for each haul studied.

We noted the yield in weight and the catch in number of individuals of fish and shellfish for each sample. These data were used to generate biomass indices which were standardized per 1 hour of trawl (kg/h).
For the red shrimps A. antennatus and A. foliacea only, the carapace length (CL) was measured by sex to the nearest millimetre, from the posterior margin of the eyestalk to the posterior mid-dorsal edge of the carapace, in order to obtain length–frequency distributions.
Statistical analysis
Cluster analysis was used to study the species assemblages, carried out using the biomass data, standardized per hour of trawling. Data were analysed in relation to depth (C and E depth levels) and the time of day (L, N1 and N2). The data were root-root transformed to reduce the influence of the dominant species and to support the importance of the rare ones (Somerfield & Clarke, Reference Somerfield and Clarke1997).
The similarity among hauls was calculated using the Bray–Curtis index (Bray & Curtis, Reference Bray and Curtis1957; Field et al., Reference Field, Clarke and Warwick1982). This is generally considered an excellent similarity measure since it preserves the ecological distance among the studied communities (Clarke & Warwick, 1994).
Samples were then classified by hierarchical agglomerative cluster analysis, using the group average linking method. A SIMPER (SIMilarity PERcentage) analysis was performed (Clarke, Reference Clarke1993; Clarke & Warwick, Reference Clarke and Warwick1994) in order to quantify the percentage contribution of each species to the average similarity/dissimilarity between samples. Statistical analyses were all performed using the PRIMER package (release 6.0) (Clarke & Gorley, Reference Clarke and Gorley2006). The Kolmogorov–Smirnov test (KS) was performed on pairs of size–frequency distributions for depth, time and sex to detect significant differences (P = 0.05) (Conover, Reference Conover1980).
RESULTS
On the Baronie Seamount, a total of 94 species were collected, including 57 fish (48 bony fish, 9 cartilaginous), 13 molluscs and 24 crustaceans (Table 2). Most of them were mainly found at their typical depth-range (as reported in the literature). It was therefore possible to subdivide the species into four groups according to their depth habits (Table 2).
Table 2. Sampled species by depth level (C < 350 m, E >500 m) and time period (L, daylight; N1, first part of the night; N2, second part of the night) with the corresponding standardized abundance (ind/h) and biomass (kg/h) estimates. The depth-range (m) found in the literature is also indicated: outside parentheses, the preferential depth-range at which the species is more abundant; within parentheses, the depth-range at which the species can be found in the Mediterranean Sea (Tortonese, Reference Tortonese1970, Reference Tortonese1975; Relini et al., Reference Relini, Bertrand and Zamboni1999; Serena, Reference Serena2005; www.fishbase.org, www.sealifebase.org).

The first two groups represent the species found during the entire 24-hours period at their typical depth-range (C species or E species). They were never found at different depth levels, indicating that they do not move up and down the water column.
The third group is represented by the ubiquitous species, found within a wide depth-range (Tortonese, Reference Tortonese1970, Reference Tortonese1975; Relini et al., Reference Relini, Bertrand and Zamboni1999). Some species, such as Phycis blennoides (Brünnich, 1758), were found in both depth levels, during the entire day. Others, such as Helicolenus dactylopterus (Delaroche, 1809), were found at all times in the C depth level, while only during daylight hours in the E depth level. They were put into the third group on the basis of their ecologically known depth-range from the literature (Tortonese, Reference Tortonese1970, Reference Tortonese1975; Relini et al., 1999; Serena, 2005; www.fishbase.org; www.sealifebase.org).
The fourth group includes all the species that typically live at the E depth level, but which were put into a separate group since they were also found at depths of less than 350 m, but only during the night. Their typical depth-range, according to published information and experience, is deeper than 400–500 m but they are known to make some migrations to shallower depths (Cartes et al., 1993) like the shrimp A. antennatus or the bony fish Mora moro (Risso, 1810). These species appear to rise up the seamount during the night, reaching 350 m and less. During daylight hours, they stay at deeper levels. In fact, they were never found at the shallower depth level (C) during daylight hours (L) (Table 2).
Cluster analysis describes the relationship between depth and time of the day, in the various samples (Figure 2). We found three groups: the first (•) consists of all hauls made at depths below 500 m, both during daylight and during the night. The second (■) comprises the hauls made in the C depth level, but only during the night (N1 and N2), while the third (▴) is composed of hauls made in the C depth level as well, but only during daylight hours (L).

Fig. 2. Classification (cluster analysis) of species assemblages in the Baronie Seamount. Clusters at 43% similarity are indicated by the dashed line. •, 1st group; ■, 2nd group; ▴, 3rd group.
Each group contains some indicator species which typify and characterizes it. For example, deep species (E depth level, clustering group 1) such as A. foliacea were clearly separated from shallow ones (C depth level, clustering groups 2 and 3) such as C. agassizii or H. dactylopterus.
The SIMPER analyses (Table 3) showed the species with the highest contribution to the similarity between the three groups. The main indicator species of the first group was A. foliacea (14.6%), while for the second group, it was Loligo forbesi (Steenstrup, 1856) (11.5%) and for the third one, it was Capros aper (Linnaeus, 1758) (10.5%).
Table 3. Results of the SIMPER analysis; species are listed in order of their contribution to the average similarity within their own group, with a cut-off when the cumulative percentage contribution reaches 90%.

This analysis also showed that the first and third groups are the most diverse (average dissimilarity 91.5%), indicating that the species caught at the C depth level during daylight hours (3rd group) are very different from those caught in the E depth level (1st group). The most species which most discriminates between these two groups is A. foliacea (5.2%), never being found at a depth of less than 350 m during daylight hours. The 2nd and the 3rd group are the least diverse (average dissimilarity 62.1%). The discriminating species between them is L. vulgaris (5.4%). The dissimilarity between the 1st and the 2nd group is 78.1%, which is intermediate compared to the previously observed dissimilarities. The species that most discriminates between these two groups was found to be L. forbesi (4.7%).
The length–frequency distribution of both red shrimps in the catches varied according to time, depth and sex (Figure 3). The above analysis indicated that their bathymetric distribution is correlated to the size and sex of the individuals. For each temporal and spatial set of hauls analysed, the percentage of females was greater than that of males.

Fig. 3. Length–frequency distributions by time, depth and sex in Aristaeomorpha foliacea and Aristeus antennatus sampled in the Baronie Seamount.
Regarding A. foliacea, the KS test showed significant statistical differences (P < 0.05) between the females caught at different depths (C and E depth levels) and periods of time. On the contrary, no significant differences were found between N1-E and N2-E samples indicating a similar size composition within the population both for females (D = 0.188 P = 0.243) and for males (D = 0.170 P = 0.596). The analysis of N1-C samples showed the exclusive presence of females which were statistically smaller than those caught at greater depths (L-E, N1-E, N2-E).
The analysis of A. antennatus catches showed a similar predominance of females, like A. foliacea. In contrast however, the mean of the CL increased progressively during hauls N1-C and N2-C, and decreased during the day samplings (Figure 3). In fact, for A. antennatus females, the KS test showed significant differences in the composition of the population between samples caught at different depths (C, E), while no significant differences were found between the hauls L-E and N2-E (D = 0.095 P = 0.423) and N1-C versus N2-C (D = 0.119 P = 0.246). For males, no significant differences were found between the hauls carried out at shallower depths and those below 500 m during the day or night.
DISCUSSION
These analyses allowed a first overview of the species distribution on the Baronie Seamount and to study the species assemblages found there. The results strongly suggest that the spatial distribution patterns of the demersal species of the Baronie Seamount vary according to both depth and time of day. Most of the species seem to be sedentary or limited to a particular depth-range and it was possible to clearly distinguish the fauna of the deeper waters from those living at shallower depths. Groups 2 and 3, both related to the C depth level, had many species in common, all typical of shallow waters. However, some species were found in both groups 1 and 2 as well. These are the typically deep water species found between 500 and 800 m.
This work clearly shows that both red shrimps, A. foliacea and A. antennatus, adapt their life cycle to the geomorphology of the seamount. Their daily and nocturnal movements from the base of the seamount to the edge of the continental shelf increase the range of their distribution. These two populations of crustacean decapods exhibit an uneven distribution and their diurnal movements appear to involve only the females. In fact, the presence of males in shallower waters (depth level C) was minimal when compared to those fished at the same time of day at the E depth level. Particularly for the red shrimp A. foliacea, the smallest individuals were usually found at a depth of less than 350 m (depth level C) compared to those of larger size, which showed a lesser migratory tendency. The smallest individuals reach the first upper edge of the seamount immediately after sunset (N1-C) and then disappear completely during the second part of the night (N2-C). The smallest females seem to rise to shallower depths than those where they usually live and then, during the last hours of the night, they descend again to greater depth. Moreover in the N2-C samples only four individuals were caught and the KS test was not applied to these samples. We can suppose that the individuals with a greater size do not reach depths lower than 350 m during the night, but we cannot confirm this because we were not able to sample the intermediate depth levels (350–500 m).
Regarding the blue and red shrimp A. antennatus, the data showed a greater presence of smaller individuals at the E depth level. The population does not have a uniform distribution, and during the migration individuals of different sizes behave differently. The largest individuals were found at the C depth level during the first part of the night (N1-C) and the small individuals were only found during the second part of the night (N2-C).
In the Baronie Seamount 7 other species were found performing this migration pattern in addition to the two red shrimps: C. granulosus, C. agassizii, E. spinax, G. melastomus, M. moro, P. multidentata and T. scabrus.
Some species are able to adapt their normal cycles near topographic structures such as submarine canyons and can make some vertical migrations within these structures (Cartes et al., 1993; Tudela et al., Reference Tudela, Sardà, Maynou and Demestre2003; Sabatini et al., Reference Sabatini, Follesa, Locci, Pendugiu, Pesci and Cau2007). The seamounts that can occasionally be found far from the coast are so proven to allow movements as submarine canyons do. Quirra Canyon is one example of these. It is located in central-eastern Sardinia where day–night cycle migrations were also observed (Sabatini et al., Reference Sabatini, Follesa, Locci, Pendugiu, Pesci and Cau2007). Comparison of the two sites, the Baronie Seamount and the Quirra Canyon, demonstrates some interesting differences. Firstly, the number of species found in the two areas was different. In Quirra Canyon, a great number of species were typically coastal, such as Mullus barbatus (Linnaeus, 1758), Mullus surmuletus (Linnaeus, 1758) and Boops boops (Linnaeus, 1758). The Quirra Canyon is in fact closer to the coast (2 nautical miles) than the Baronie Seamount (25 nautical miles from the north-eastern Sardinian coast). Both in Quirra Canyon and on the Baronie Seamount, vertical diurnal migrations were recorded for the species living there. These are probably linked to trophic requirements, as suggested for benthopelagic decapods (Cartes et al., 1993) and for demersal fish (Blaber & Bulman, Reference Blaber and Bulman1987 and references therein; Madurell et al., Reference Madurell, Cartes and Labropoulou2004 and references therein; Cartes et al., Reference Cartes, Papiol and Guijarro2008; Preciado et al., Reference Preciado, Velasco and Olaso2008). However, some differences in migration patterns were observed between the two sites. Mobile species were noted in the C depth level both during N1 and N2 on the Baronie Seamount, while only during N1 in the Quirra Canyon. The different patterns of migration can probably be linked to the geomorphological and bottom features of the two sites. In the Quirra Canyon the horizontal distance that migrating species have to cover between the depth levels C and E, where the species have their usual haunts, is about three times greater than that on the Baronie Seamount. On the seamount, species can cover the shorter distances in 1/3 of the time compared to the canyon. The different bottom features probably allow migrating species on the Baronie Seamount to stop for longer times (N1 and N2) at shallower depths compared to those in the Quirra Canyon, which should move toward depths of 500 m during the latter part of the night (N2), before the first hours of the day.
Nycthemeral movements have been investigated in Sardinian seas by Cau & Deiana (Reference Cau and Deiana1982) who highlighted daily and nocturnal movements of red shrimps which were strictly linked to the substratum. In the same way, Maurin (Reference Maurin1960), Bombace (Reference Bombace1975) and Matarrese et al. (Reference Matarrese, D'Onghia, Deflorio, Panza and Costantino1995) observed similar situations in the Corsican, Sicilian and Ionian Seas, respectively. The size and sex frequency distributions described in these studies, seem to follow similar trends to the crustacean populations that we studied. In fact, the larger individuals are found at greater depths, while smaller ones are captured at lower depths. In addition, at greater depths we found a greater number of females larger than males.
The pattern of diurnal movements is protracted on the seamount and can be studied more easily than in the generally surrounding continental shelf and the same is true for the submarine canyon. Areas such as submarine canyons, or seamounts far from the coast, make it possible to study phenomena such as the diurnal movements of the species found there which cannot be so easily observed in other areas with different geomorphological conformations.
ACKNOWLEDGEMENTS
We are particularly grateful to Nino Pinto, the captain of the FV ‘Marisanto’, and all the crew for their help during the sampling activities. Also we wish to thank the two anonymous referees for their very useful suggestions to improve the paper.