INTRODUCTION
Submersible operations in the deep water surrounding the Central Aleutian Islands led to the discovery of 20 new demosponge species in 2004 (Lehnert et al., Reference Lehnert, Stone and Heimler2005a, Reference Lehnert, Stone and Heimlerb, Reference Lehnert, Watling and Stonec, Reference Lehnert, Stone and Heimler2006a, Reference Lehnert, Stone and Heimlerb, Reference Lehnert, Stone and Heimlerc). While in the final process of archiving specimens from those collections and a few collected during an earlier cruise in 2003, we found one that turned out to be an undescribed species. Specimens described here exhibit the general shape and spicule complement of the genus Craniella, but the length and number of spines on the surface, lack of root tufts, and details of spicule categories clearly separate this new species from congeners.
The genus Craniella was erected by Schmidt, Reference Schmidt1870 for Craniella tethyoides from deep water near Florida, USA, belonging to the family Tetillidae Sollas, Reference Sollas1886, which contains mainly globular sponges. Characteristic megascleres are protriaenes which are totally lacking in a few species only (Van Soest & Rützler, Reference Van Soest, Rützler, Hooper and Van Soest2002). All have a strictly radial skeletal architecture and presently eight genera are accepted (Van Soest & Rützler, Reference Van Soest, Rützler, Hooper and Van Soest2002). Craniella by definition is included within the family Tetillidae because it lacks porocalices, is not stalked but may have root-like projections, has spicules that do not include amphiclads and has a collagenous cortical region. This cortical region is of special interest as it constitutes the main difference between the genera Craniella and Tetilla: present in the former and absent in the latter. However, authors in the past have not always clearly separated the two genera, so we decided to examine all northern hemisphere species from temperate waters described in both genera for conspecificity.
Presently the World Porifera Database (Van Soest et al., Reference Van Soest, Boury-Esnault, Hooper, Rützler, de Voogd, Alvarez, Hajdu, Pisera, Vacelet, Manconi, Schoenberg, Janussen, Tabachnik and Klautau2008) recognizes 42 species of Craniella. The present paper describes the new Craniella, compares it with relevant congeners, transfers two species and two subspecies from Craniella to Tetilla, returns one species, Tetilla ovata (Thiele, Reference Thiele1898) to its original allocation in Craniella and transfers Tetilla hamatum Koltun, Reference Koltun1966 to Craniella.
MATERIALS AND METHODS
Specimens were collected with a mechanical manipulator operated from the submersible ‘Delta’. Specimens were photographed both in situ and shortly after collection (Figure 1A, B) and were subsequently stored in 70% ethanol. For identification, fragments were boiled in nitric acid, transferred into distilled water and finally into ethanol using a centrifuge to isolate spicules. An Amray 1810Footnote 1 was used for scanning electron microscopy (SEM) investigations of the spicules that were cleaned, dried, and sputter coated with gold. For light microscopy, spicules and handmade semi-thin sections were embedded in Canada balsam. At least 20 spicules were measured of each category. The systematic hierarchy follows Hooper & Van Soest (Reference Hooper and Van Soest2002).
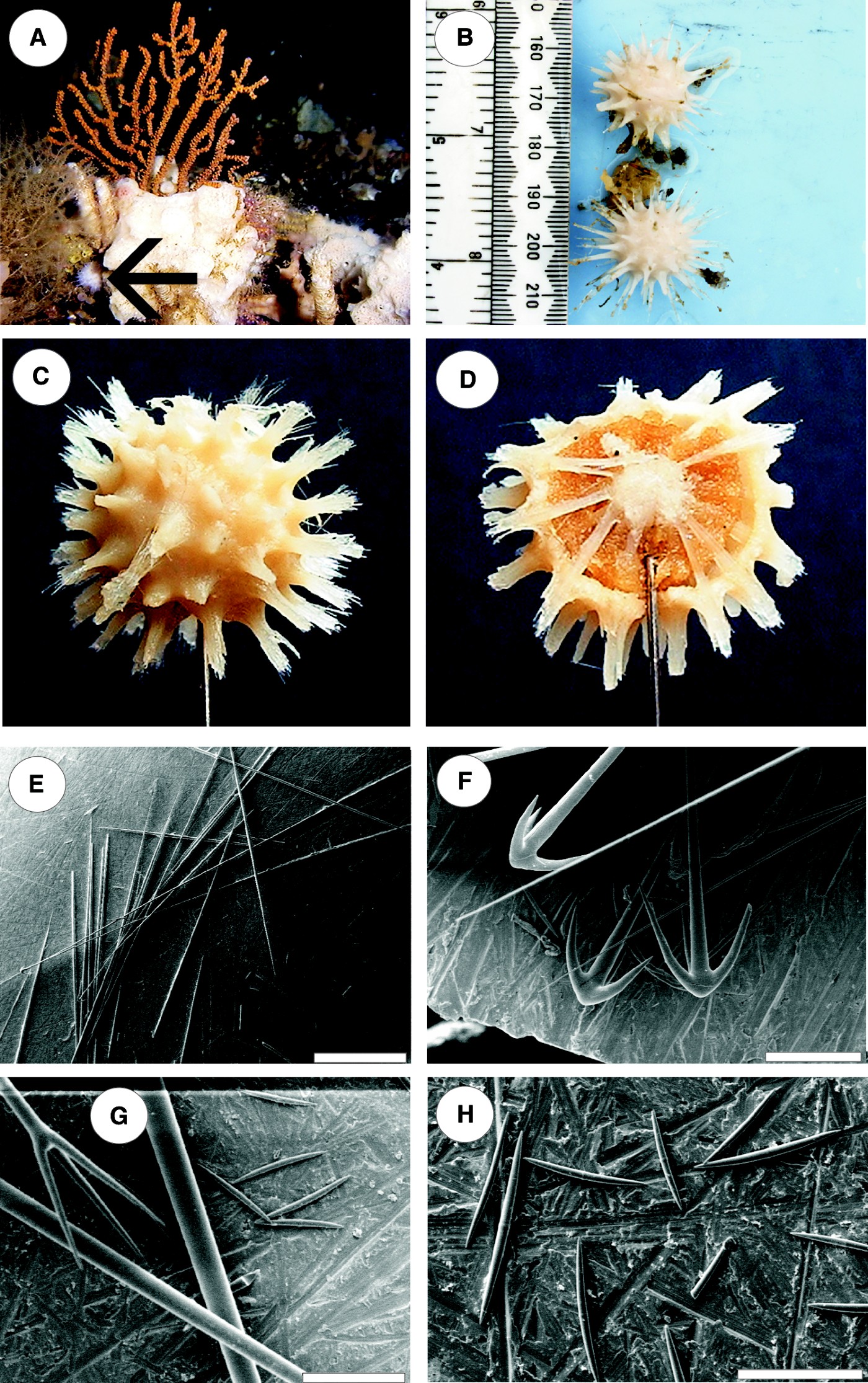
Fig. 1. Craniella sputnika sp. nov. (A) In situ, position indicated by black arrow, attached to the calcareous sponge Clathrina sp. at a depth of 119 m in Kanaga Sound, Aleutian Islands; (B) holotype and paratype shortly after collection; (C) preserved holotype, note that acute spines have become frayed if compared with B; (D) section through paratype showing visible radial arrangement of spicule tracts which run into the spines; (E) overview, scale bar is 2 mm, oxeas and triaenes are not distinguishable at this magnification; (F) clads of anatriaenes and filament-like end of anatriaene, scale bar is 200 µm; (G) upper left clad of protriaene, upper right small category of cortical oxeas, scale bar is 200 µm; (H) large and small categories of cortical oxeas, scale bar is 200 µm.
TYPE MATERIAL
Two specimens were collected on 4 July 2003 by Patrick Malecha at a depth of 115 m and 26.3 km west–north-west of Amatignak Island, Delarof Islands, South Amchitka Pass, Aleutian Islands, Alaska, USA, coordinates: 51°21.042′N 179°30.483′W. Holotype (Figure 1C) and paratype (Figure 1D) are deposited at the Zoologische Staatssammlung München (ZSM) registration numbers: holotype: 20100004, paratype: 20100007.
DESCRIPTION
Globular sponge with numerous acute spines (holotype with 74 spines) distributed over the surface. The surface between the spines is smooth, no apertures visible. The diameter of the specimens including the spines is 2.1–2.4 cm, without spines 1.4–1.6 cm. The spines covering the surface are 0.8–1.3 mm in diameter and the spaces in between vary from 2 to 3 mm. Cross-sections show a clear cortical region and a strict radial arrangement of the spicule tracts. Spicule tracts start from the centre of the globular sponge and extend out to the cortex and into the spines. The cortex and the spines are creamy white in life and in ethanol; the choanosome between the white spicule tracts is yellowish and much softer than the rest of the sponge. Number of spicules per cross-section diminishes towards the point of the spines. Due to the high spicule content of the sponge the consistency is rather hard, only slightly elastic. Spicules are anatriaenes (Figure 1E, F), 3430–8820 × 12–21 µm, clads (Figure 1F) are 48–154 × 17–22 µm per ray; protriaenes (Figure 1G) 4520–8960 × 12–40 µm. Clads (Figure 1G) 50–250 × 5–17 µm per ray; the long axis of the pro- and anatriaenes is thinning out and becomes filament-like towards the tip; choano-somal oxeas (Figure 1E) 4530–5425 × 50–75 µm; two categories of cortical oxeas (Figure 1H): small, centrotylote oxeas 97–372 × 8–17 µm, tyle in the centre more like a ring than a tyle, and larger ones, 540–987 × 28–63 µm. No sigmaspires or similar sigmoid spicules were observed. Root tufts or special root spicules were not present but one of the specimens had a tiny fragment of another sponge adhering to it. Fragments were too small for a definitive identification of the species but allowed assignment to the genus Myxilla. The Myxilla fragments were attached to several spines of the Craniella with some spicule tracts of the spines extending into the Myxilla fragments, attaching both specimens to each other. Whether the second Craniella specimen was also attached to another sponge is unknown.
ECOLOGY
Specimens were collected in a habitat of boulders, cobbles and sand at a depth of 115 m. Since the specimens lack root tufts and one specimen appeared to not be attached to anything, we hypothesize that the species can live unattached on the seafloor, perhaps drifting along until it attaches to something. Whether the species preferentially attaches to certain substrates is unknown. Observations made from submersibles (by R.P.S.) indicate that it can attach to the calcareous sponge Clathrina sp., to unidentified demosponges and primnoid gorgonians. Additional submersible observations along the Aleutian Islands indicate a similar lifestyle of attachment to other biota for both C. sigmoancoratum (Koltun, Reference Koltun1966) and C. spinosa Lambe, Reference Lambe1893, and possibly C. arb (de Laubenfels, Reference de Laubenfels1930).
ETYMOLOGY
‘Sputnik’ was the working name for this new species when taxonomic investigations began due to its small size, globular body and numerous ‘antennae’ (although the sponge clearly has many more spines than the satellite Sputnik had antennae). The launch marked the start of the space age and we name the species in honour of Earth's first artificial satellite.
DISCUSSION
Van Soest & Rützler (Reference Van Soest, Rützler, Hooper and Van Soest2002) describe the genus Craniella as ‘globular sponges with a conulose but optically smooth surface over most of the body; at the base there are bundles of spicules acting as a root.’ The species described in this paper is globular, it lacks the root tufts and the surface is covered with long spines. However, it is not the only Craniella species without root tufts and with long spines. We decided to compare the new species with all northern hemisphere species of the genus from temperate waters listed in the World Porifera Database (Van Soest et al., Reference Van Soest, Boury-Esnault, Hooper, Rützler, de Voogd, Alvarez, Hajdu, Pisera, Vacelet, Manconi, Schoenberg, Janussen, Tabachnik and Klautau2008). In the past many authors used the genera Craniella and Tetilla more or less as synonyms, causing ongoing confusion about the identity of several species. In our opinion there are two species and two subspecies that should be transferred from Craniella to the genus Tetilla and two species, Tetilla ovata and Tetilla hamatum, that should be transferred from Tetilla to Craniella. Craniella ellipsoidea Hoshino, Reference Hoshino1982 appears to be a Tetilla as the original species description does not mention a cortex nor cortical spicules and the author writes that (p. 143) ‘the species resembles Crniella [sic] globosa var. anamonaene [sic] Tanita… .’ Craniella globosa anamonaena Tanita, Reference Tanita1968 should also be transferred to Tetilla as the original species description states (p. 55) ‘there is no cortex and no visible distinction between the ectosome and choanosome.’ Consequently Tanita's specimen is not a subspecies of C. globosa (a true Craniella with a cortex). If Craniella globosa anamonaena Tanita, Reference Tanita1968 is transferred to the genus Tetilla we suggest raising the subspecies to species rank as Tetilla anamonaena (Tanita, Reference Tanita1968). Craniella laminaris (George & Wilson, Reference George and Wilson1919) should return to the genus Tetilla as the authors describe (p. 142) ‘no part of the ectosome is differentiated to form a fibrous layer.’ Consequently the subspecies Craniella laminaris symmetrica (Wilson, Reference Wilson1931), which differs in growth form only, should be moved to the genus Tetilla also.
Additionally, Tetilla ovata (Thiele, Reference Thiele1898) should return to the genus Craniella where it was originally placed, as Thiele explicitly describes cortical oxeas as a major character of the species (p. 27: ‘Das Hauptmerkmal der Art dürften die grossen corticalen Amphioxe darstellen’). Though he does not describe a cortex, this structure seems to be present as he describes cortical oxeas. The description of Tetilla hamatum by Koltun, Reference Koltun1966 includes cortical oxeas and therefore this species should also go to Craniella. With these changes we now have 19 species of Craniella in temperate waters of the northern hemisphere to compare with Craniella sputnika sp. nov. Table 1 summarizes the main characters of these 19 species.
Table 1. Members of northern hemisphere species of Craniella in bold letters. Species for which a transfer to Tetilla is suggested are mentioned as Tetilla and do not appear in bold letters.

Geographically proximate records of the genus include six species in total. These include two species described by Lambe, Reference Lambe1893, C. spinosa and C. villosa. Craniella spinosa is a small species with relatively long spines and is similar to the new species in habitus and also in several spicule categories, as it has very long pro- and anatriaenes, choanosomal oxeas of similar size and even one category of cortical oxeas of comparable size. It differs in having sigmaspires, absent in C. sputnika, and in lacking the second category of cortical, centrotylote oxeas, present in C. sputnika. Craniella villosa while considerably larger than C. sputnika is the only other Craniella possessing two categories of cortical oxeas (e.g. Lambe distinguished between cortical and dermal oxeas). Craniella villosa differs from C. sputnika in having sigmaspires and in the dimensions of the spicule types present. Craniella craniana de Laubenfels, Reference de Laubenfels1953 has a diameter of 4–8 cm and is therefore considerably larger than C. sputnika. The spicules are insufficiently described so comparison is only possible for the sigmaspires which measure 17–22 µm in C. craniana and are lacking in C. sputnika, which separates these two species. Craniella sigmoancoratum differs from C. sputnika in being larger (up to 5 cm in diameter), the presence of sigmaspires, and in having only one category of cortical oxeas. Craniella hamatum (Koltun, Reference Koltun1966) differs in growth form, it has more blunt spines, and it again differs in the smaller sizes of most occurring spicule categories (Table 1). Additionally, C. hamatum differs in the possession of sigmaspires and in having anamonaenas. Craniella arb differs in having longer ana- and protriaenes and extremely long choanosomal oxeas, it lacks cortical oxeas but possesses sigmaspires which means that both species have a quite different set of spicules and different dimensions in the shared spicule types.
Proceeding geographically there are seven species known from the Sea of Japan: C. globosa, C. lentiformis, C. ovata and C. varians described by Thiele, Reference Thiele1898; C. serica Lebwohl, Reference Lebwohl1914 and C. lentisimilis and C. prosperiaradix described by Tanita & Hoshino (1990) (Table 1). All but one of these species has sigmaspires and therefore differs from C. sputnika. The other species lacking sigmaspires is C. lentiformis which differs from C. sputnika in habitus, the smaller size of spicules in all categories, and in lacking a second category of cortical oxeas (Table 1). Craniella cranium, a North Atlantic species, has much shorter ana- and protriaenes; it has only one category of cortical oxeas with dimensions not corresponding to any of the two categories of cortical oxeas of C. sputnika and it possesses sigmaspires. Craniella disigma, known from deep water of the North Atlantic off the Azores has shorter choanosomal oxeas which are anisoxeas, it has one category of cortical oxeas not corresponding to one of the categories of C. sputnika but has two categories of sigmaspires. Craniella longipilis, another North Atlantic species known from the Azores differs in having anisoxeas as choanosomal oxeas, in having only one category of cortical oxeas and in possessing sigmaspires. Craniella polyura, is a North Atlantic species described from Iceland by Schmidt, Reference Schmidt1870 who did not provide spicule dimensions except for the sigmaspires. Schmidt did not mention the presence of anatriaenes or cortical oxeas in his species which occur in C. sputnika. He did describe sigmaspires in C. polyura which are missing in C. sputnika. Though sigmaspires can be lost in the genus Craniella (Rützler, Reference Rützler, Vacelet and Boury-Esnault1987; Van Soest & Rützler, Reference Van Soest, Rützler, Hooper and Van Soest2002) and are therefore not a safe tool to separate species, the presence of several different characters allows ruling out conspecifity of C. polyura and C. sputnika.
In summary (Table 1), only four species aside from C. sputnika lack sigmaspires (C. azorica, C. ellipsoida, C. lentiformis and C. zetlandica), but none of these and only one other listed species (C. villosa), a much larger species, have the two categories of cortical oxeas present in C. sputnika. Additionally, no species of Craniella is reported to have centrotylote cortical oxeas. Craniella sputnika is a small species with relatively long spines, characteristics it shares with C. spinosa and C. sigmoancoratum (Koltun, Reference Koltun1966) the former from the same immediate region in the central Aleutian Islands, the latter from the Kurile Islands. Together these features support recognizing C. sputnika as a new species.
ACKNOWLEDGEMENTS
Thanks to the crew of the RV ‘Velero’ and the submersible ‘Delta’ for friendly and professional support during the field trip. Thanks also to Wolfgang Heimler, Institute für Entwicklungsbiologie, Erlangen University and Wolfgang Christel, IPAT-Uni-Erlangen, Germany, who operated the SEM. Thanks to three anonymous referees who helped to improve the manuscript; special thanks to Tom Hourigan (NOAA) for his continued support of deep-sea research in the North Pacific Ocean and for making funds for this project available through the NOAA Deep Sea Coral Research and Technology Program. H.L. was funded by a contract from NOAA/NMFS.




